Abstract
Transcatheter aortic valve replacement (TAVR) is a treatment option in high‐risk patients with severe aortic stenosis who are not surgical candidates. In light of emerging evidence, it is being increasingly performed even in intermediate‐risk patients in recent years. Patients who develop acute kidney injury (AKI) following TAVR are known to have worse outcomes. The objective of this concise review was to identify the prevalence and the impact of AKI following TAVR on patient outcomes by including the most recent literature in our search. After a thorough search on MEDLINE, Google Scholar, and PubMed, we included all literature relevant to AKI following TAVR. We found that AKI was caused by a variety of reasons, such as hemodynamic instability during rapid pacing, blood transfusion, periprocedural embolization, and use of contrast medium, to name a few. In patients who developed AKI following TAVR, 30‐day and 1‐year mortality were increased. Further, in these patients, length and cost of hospital stay were increased as well. Preventive measures such as optimal periprocedural hydration, careful contrast use, and techniques to prevent embolization during device implantation have been tried with limited success. Given that TAVR is expected to be increasingly performed, this review aimed to summarize the rapidly expanding currently available literature in an effort to reduce procedural complications and thereby improve patient outcomes.
Keywords: Aortic Disease, Kidney Disease, Valvular Heart Disease
1. INTRODUCTION
Since the time of its inception in the 1960s, surgical aortic valve replacement (SAVR) has remained the gold standard for the management of severe aortic stenosis. Despite low complication rates in high‐volume centers with surgical expertise, approximately 30% of patients are not considered surgical candidates due to underlying comorbidities.1 In the last decade, transcatheter aortic valve replacement (TAVR) has emerged as an alternative in selected patients.2 This procedure has gained popularity since the landmark Placement of Aortic Transcatheter Valves (PARTNER) trial showed improved outcomes in high‐risk or inoperable patients who otherwise would have been managed medically.3
Though initially conceived with the idea of reducing patient morbidity and mortality by utilizing a less invasive approach, TAVR has been associated with complications that need careful consideration when weighing risks and benefits. Potential major complications include stroke, heart block, paravalvular aortic regurgitation, and acute kidney injury (AKI).4, 5 Given that patients undergoing TAVR are generally elderly and often have preexisting chronic kidney disease (CKD), the impact of postprocedure AKI can be significant, as even subtle decreases in glomerular filtration rate (GFR) are associated with increased mortality.6, 7 Over the last few years, the body of literature in support of TAVR has strengthened greatly, and TAVR procedures are being performed more frequently in intermediate‐risk patients.8
In this review we will focus on AKI following TAVR, describing its prevalence, risk factors, and prognostic significance. All work was conducted in accordance with the Declaration of Helsinki (1964).
2. IMPORTANCE AND DEFINITION
The reported prevalence of AKI after TAVR ranges from 5% to as high as 57%.4, 5, 9 AKI following TAVR is independently associated with a 5‐ to 8‐fold increase in 30‐day mortality and a > 3‐fold increase in 1‐year mortality.4, 5, 9, 10, 11 Although few studies have looked at AKI following SAVR specifically,12, 13 30‐day mortality for patients with AKI following other cardiac surgery (most commonly coronary artery bypass grafting) has ranged from 6% to 45%, compared with 1% to 3% for patients without AKI.10, 11, 12, 13, 14 This wide range can be largely attributed to the lack of a standard definition for AKI.
In addition to the lack of a standard definition that caused different studies to use different definitions, factors such as varying sample sizes of single‐center experiences, different valve prostheses under use, and varied inclusion criteria were responsible for the dramatically different reported incidences of AKI following TAVR. A few important experiences are highlighted in Table 1.15, 16, 17, 18, 19
Table 1.
Incidence of AKI and criteria used
| Study, Year | Incidence Rate of AKI, % | Criteria Used |
|---|---|---|
| Strauch et al, 201015 | 57 | RIFLE |
| Barbash et al, 201216 | 15 | RIFLE |
| Sinning et al, 201017 | 26 | AKIN |
| Khawaja et al, 201218 | 36 | VARC |
| Yamamoto et al, 201319 | 15 | VARC |
Abbreviations: AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; RIFLE, Risk, Injury, Failure, Loss, and End‐Stage; VARC, Valve Academic Research Consortium.
In 2004, the Acute Dialysis Quality Initiative Group proposed the Risk, Injury, Failure, Loss, and End‐Stage (RIFLE) criteria to standardize the definition of kidney injury.20 AKI was defined based on a 150% to 200% increase in serum creatinine (sCr) from baseline, by a decrease in estimated GFR of 25% to 75% from baseline, or by time of onset of oliguria (6, 12, or 24 hours; Table 2). Further outcomes such as complete loss of kidney function and the development of end‐stage renal disease (ESRD) were included in this definition.
Table 2.
AKI as defined by RIFLE, AKIN, and KDIGO
| RIFLE | AKIN | KDIGO | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage | Cr | Urine Output | Stage | Cr | Urine Output | Stage | Cr | Urine Output |
| Risk | sCr increase to 1.5‐fold OR GFR decrease >25% from baseline | <0.5 mL/kg/h for 6 h | 1 | sCr increase ≥26.5 μmol/L (≥0.3 mg/dL) OR increase of 1.5‐fold to 2.0‐fold from baseline | <0.5 mL/kg/h for 6 h | 1 | Increase in sCr 1.5× to 1.9× baseline that is known or presumed to have occurred within 7 d | <0.5 mL/kg/h for 6 h |
| Injury | sCr increase to 2.0‐fold OR GFR decrease >50% from baseline | <0.5 mL/kg/h for 12 h | 2 | sCr increase >2.0‐ to 3.0‐fold from baseline | <0.5 mL/kg/h for 12 h | 2 | Increase in sCr 2.0× to 2.9× baseline | <0.5 mL/kg/h for 12 h |
| Failure | sCr increase to 3.0‐fold OR GFR decrease >75% from baseline OR sCr ≥354 μmol/L (≥4 mg/dL) with an acute increase of ≥44 μmol/L (0.5 mg/dL) | Anuria for 12 h | 3 | sCr increase >3.0‐fold from baseline OR sCr ≥354 μmol/L (≥4.0 mg/dL) with an acute increase of ≥44 μmol/L (0.5 mg/dL) OR need for RRT | <0.3 mL/kg/h for 24 h OR anuria for 12 h OR need for RRT | 3 | Increase in sCr to 3.0× baseline or increase in sCr to ≥4.0 mg/dL OR initiation of RRT | Anuria for 12 h |
Abbreviations: AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; Cr, creatinine; GFR, glomerular filtration rate; KDIGO, Kidney Disease Improving Global Outcomes Foundation; RIFLE, Risk, Injury, Failure, Loss, and End‐Stage; RRT, renal replacement therapy; sCr, serum creatinine; VARC, Valve Academic Research Consortium.
The Acute Kidney Injury Network (AKIN) modified the RIFLE criteria by staging the Risk, Injury, and Failure components as stage 1 to stage 3. Further, they added an absolute change in sCr of >0.3 mg/dL to the definition (Table 2).
The Kidney Disease Improving Global Outcomes Foundation (KDIGO) merged aspects of the RIFLE and AKIN definitions in 2012; AKI was defined as an increase in sCr of >0.3 mg/dL within the previous 48 hours, a relative increase of >50% from baseline within the previous 7 days, or a decrease in urine volume to <0.5 mL/kg/h. Of note, urine output was included in their definition, but GFR was not (Table 2).
In 2011, the Valve Academic Research Consortium (VARC) introduced standardized endpoints into the classification of AKI in TAVR.11 These definitions were modified in the VARC‐2 criteria, published in 2014.21 VARC‐2 recommended that the AKIN system be followed in making a diagnosis of AKI following TAVR (Table 3). In comparison with the original VARC criteria, the timing and diagnosis of AKI was extended from 72 hours to 7 days postprocedure.
Table 3.
AKI (AKIN criteria) based on the VARC‐2 Guidelines
| Stage | sCr | Urine Output |
|---|---|---|
| 1 | Increase in sCr to 150%–199% (1.5–1.99× increase compared with baseline) OR increase of ≥0.3 mg/dL (≥26.4 mmol/L) | <0.5 mL/kg/h for >6 but <12 h |
| 2 | <0.3 mL/kg/h for ≥24 h OR anuria for ≥12 h | <0.5 mL/kg/h for >12 but <24 h |
| 3 | Increase in sCr to ≥300% (>3× increase compared with baseline) OR sCr of ≥4.0 mg/dL (≥354 mmol/L) with an acute increase of ≥0.5 mg/dL (44 mmol/L) | <0.3 mL/kg/h for ≥24 h OR anuria for ≥12 h |
Abbreviations: AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; sCr, serum creatinine; VARC, Valve Academic Research Consortium.
Further, in light of emerging research, it is important to summarize the evolving patterns of AKI temporally, because with the currently available long‐term follow‐up data, unexpected possible benefits such as cognitive‐function improvement are being reported despite the presence of subclinical ischemia in patients who have undergone TAVR.22, 23
3. PATHOGENESIS AND RISK FACTORS
The pathogenesis of AKI following TAVR is multifactorial (Figure 1). It is thought to develop from a combination of hemodynamic instability and the use of contrast medium, which is nephrotoxic.24 Athero‐embolization of cholesterol debris to the renal vasculature during cannulation is also thought to cause injury, as evidenced by reports of eosinophils appearing in the urine.5 Risk factors can be classified as preoperative, perioperative, or postoperative.
Figure 1.
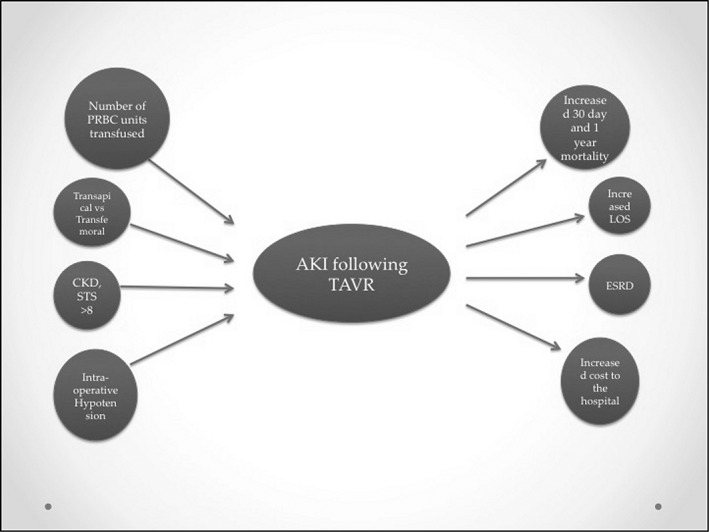
Factors responsible for and consequences after AKI post‐TAVR. Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; ESRD, end‐stage renal disease; LOS, length of stay; PRBC, packed red blood cells; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement
3.1. Preoperative risk factors
Preoperative or patient‐related factors associated with AKI are the presence of CKD (elevated baseline Cr or reduced GFR), hypertension, higher Society of Thoracic Surgeons (STS) or EuroSCORE risk score, diabetes mellitus, and the presence of peripheral arterial disease or chronic obstructive pulmonary disease (Figure 1).14, 25 A review of the literature shows an inverse correlation between baseline GFR and risk of AKI.10 Further, a recent substudy from the PARTNER 1 registry showed that in patients with baseline renal impairment, those with worsening GFR had an increased all‐cause mortality at 1 year. However, it is also interesting to note that 76% of the patients did not have their baseline renal impairment exacerbated following TAVR.26 Conflicting reports exist with regard to sex‐based risk, with some studies showing higher risk in males whereas others find females to be at greater risk of AKI.14, 16, 27 Age is an additional independent risk factor for the development of AKI.4, 5
These risk factors are additive, and caution must be exercised prior to intervention in patients with multiple risk factors.
3.2. Intraoperative risk factors
Intraoperative factors are among the most significant for development of AKI, with the number of units of blood transfused being the strongest. In patients undergoing SAVR, use of cardiopulmonary bypass and blood transfusion result in platelet activation, inflammation, and free‐radical generation, all of which contribute to the development of AKI.4, 16, 25 Some attribute this association to the increased inflammatory milieu perioperatively, accentuated by concomitant blood products administration; others have suggested that a greater transfusion requirement is a surrogate for intraoperative hemodynamic instability, which is known to increase AKI risk. Although this seems logically appealing, no data are available to support or refute either hypothesis. In patients undergoing SAVR or TAVR, among those who developed stage 2 or stage 3 (severe) AKI, granular casts have been noted, suggestive of acute tubular necrosis. Athero‐emboli are another potential source of AKI during aortic valve replacement.4, 5 Cholesterol embolization during cannulation and vascular instrumentation can cause renal injury, though the exact incidence of this important complication is unknown.28
The transfemoral approach is most commonly employed for TAVR, with the transapical and transaortic approaches used less often. The incidence of AKI appears to be increased when using nonfemoral approaches. However, it is important to note that peripheral arterial disease (which may necessitate an alternative approach) is itself an independent and important predictor of AKI. Thus, its presence confounds the risk for AKI of these alternative access methods. It is also known that in high‐volume centers with increased operator experience, the incidence of AKI is lower. Given that the most commonly employed approach is transfemoral, operator experience is certainly higher with this approach, and thus this further confounds the picture.5, 10, 14
Use of nephrotoxic contrast media has been associated with renal injury in a dose‐dependent manner (>100 mL) following TAVR in 1 study, though others have not confirmed these results.14, 19, 27 Widespread use of low‐osmolar contrast agents and minimization of contrast use in patients with CKD might explain a lack of association between contrast volume and AKI.19, 27 Inflammatory markers such as neutrophil‐to‐lymphocyte ratio and high‐sensitivity C‐reactive protein were found to be predictive of contrast‐induced AKI in patients undergoing emergency percutaneous coronary interventions.29 An interesting area of further investigation would be to attempt to replicate these results in patients undergoing TAVR.
Intraoperative hypotension, commonly associated with rapid pacing during deployment of the balloon‐expandable Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA), is thought to be associated with AKI, given the high sensitivity of renal tissue to hemodynamic perturbations.14 The self‐expandable CoreValve system (Medtronic, Minneapolis, MN) does not require rapid pacing. Whether the incidence of AKI with the use of the self‐expandable system is lower is an area where further analysis is needed. Reflective of intraoperative hypotension and ischemia‐related injuries, 1 study showed that use of an intra‐aortic balloon pump post‐TAVR was associated with increased incidence of AKI.4, 5
A large single‐center US study16 showed that, among patients with AKI post‐TAVR, the transapical approach was more commonly used and a larger valve more commonly deployed, as compared with patients without AKI. It is not clear whether the larger valve size is independently associated with AKI, and this is an area for further study.
3.3. Postoperative risk factors
Postoperative factors influencing the development of AKI post‐TAVR are related to the intraoperative course and postoperative complications. Postoperative anemia, which can be associated with intraoperative hemodynamic instability, was shown to be significantly associated with AKI, which can be attributed to aforementioned reasons.16 However, the optimal hemoglobin target for transfusion becomes tricky, as some patients undergoing TAVR could have a reduced left ventricular ejection fraction, and thus there is sometimes hesitation to liberally transfuse these patients in an attempt to avoid pulmonary edema.
Longer postoperative intensive care unit (ICU) stays also were associated with worse outcomes.16 Given that in patients with AKI following TAVR the length of ICU stay was noted to be longer, this forms a vicious cycle where association and causality can be attributed to a complicated procedure requiring ICU stay and also predisposing to increased AKI development.30
Further, continued smoking and decreased left ventricular ejection fraction were associated with worsening GFR in a recent study conducted by Beohar et al.26
It is interesting to note that TAVR does not increase mortality in patients with ESRD and is therefore an attractive option in the dialysis patient.31 One can argue that in subjects with ESRD, where all the functions of the kidney are met by renal replacement therapy (RRT), contrast‐mediated and athero‐embolic effects have minimal adverse impact.
4. IMPACT
4.1. Mortality
All of the aforementioned factors are associated with development of AKI, which is itself independently associated with increased 30‐day and 1‐year mortality.4, 5, 9, 16 Mortality rates at 30 days post‐TAVR among patients experiencing AKI have ranged from 10% to 30%, compared with 2% to 15% for those without AKI.2, 3, 5, 9, 25 One‐year mortality ranged from 10% to 70% for AKI patients, compared with 3% to 40% for patients without AKI.14 Severity of post‐TAVR AKI was also important, with need for RRT being associated with the highest mortality. Further, AKI was found to be a predictor of sepsis, which is independently associated with increased mortality and length of stay (LOS).32
4.2. Hospital stay and need for RRT
Development of post‐TAVR AKI was independently associated with increased LOS, although it was less than that for patients with post‐SAVR AKI.33, 34 Need for hemodialysis, on the other hand, was higher in TAVR patients with AKI when compared with SAVR, with the highest reported incidence at 21%, compared with <3%. This can be explained by increased baseline CKD and overall risk in patients undergoing TAVR.
The mean LOS for patients who developed complications following TAVR increased by 2 days, whereas the cost of their stay, depending on the complication, increased up to $40 000. Given these LOS issues and increased ICU use, it can be inferred that hospital cost is greater for patients with AKI vs without.33, 34 Additionally, all cause 30‐day readmission rates post‐TAVR are >15%, and AKI was found to be an independent predictor of readmission.33
5. PREVENTION
No standard protocol for prevention of AKI is currently available. Optimization of volume status prior to TAVR remains the standard of care. Hydration with resultant plasma expansion results in suppression of the renin‐angiotensin‐aldosterone system and can thereby prevent renal vasoconstriction. However, overhydration can result in the development of congestive heart failure, which is why maintenance of euvolemic status is of paramount importance.35 Apart from optimal hydration, techniques such as the use of sodium bicarbonate in dextrose coupled with N‐acetylcysteine in water have also been tried, but without conclusive results.36, 37 Promising techniques such as the RenalGuard System (RenalGuard Solutions, Inc., Milford, MA), which is a device for precisely controlling fluid administration according to diuretic‐induced urine output, have been reported in the literature, but have yet to gain widespread acceptance.38 Devices for cerebrovascular protection from emboli following TAVR have been tried in small samples, and the applicability of these devices for renal emboli prevention would be an interesting topic for further study.39 Avoidance and discontinuation of concomitantly used nephrotoxic agents, such as nonsteroidal anti‐inflammatory drugs and aminoglycosides, remains important.
The Prospective Randomized Trial of Prevention Measures in Patients at High Risk for Contrast Nephropathy (PRINCE) study showed that increasing urine output was found to be protective against contrast‐induced renal injury.40 More recently, methods such as the RenalGuard System, which administers intravenous fluids to exactly match the volume of urine produced by using furosemide, showed promising outcomes in a single‐center study.9 Large studies are needed to validate the benefits of these single‐center trials before such methods are incorporated into the standard of care. The landmark Prevention of Contrast Renal Injury With Different Hydration Strategies (POSEIDON) trial highlighted the value of using left ventricular end‐diastolic pressure–guided fluid management in the relative risk reduction of contrast nephropathy. When feasible, measurement of this parameter invasively or noninvasively may offer valuable information on optimizing renal perfusion in these high‐risk patients.35
6. CONCLUSION
AKI is frequent following TAVR. One of the problems in measuring the scope of the problem and efficacy of prevention has been lack of standard definitions. With standardization of definitions, we can hope to see greater accuracy in the characterization of post‐TAVR AKI. Further, with more advanced tools and renoprotective systems, we may see a reduction in the development of AKI following TAVR. For now, it is important to identify those patients who are at greatest risk of developing AKI after TAVR. These are patients with multiple underlying comorbidities, including CKD, and possibly those undergoing the procedure at low‐volume centers. At this time, hydration remains the mainstay of preventive therapy.
Conflicts of interest
The authors declare no potential conflicts of interest.
Ram P, Mezue K, Pressman G, Rangaswami J. Acute kidney injury post–transcatheter aortic valve replacement. Clin Cardiol. 2017;40:1357–1362. 10.1002/clc.22820
REFERENCES
- 1. Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. [DOI] [PubMed] [Google Scholar]
- 2. Vidi VD, Rab Z, Sraow DI, et al. Incidence, predictors and outcomes of acute kidney injury following transcatheter aortic valve replacement. J Am Coll Cardiol. 2014;63(12 suppl):A1747 10.1016/S0735-1097(14)61750-2. [DOI] [Google Scholar]
- 3. Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 4. Thongprayoon C, Cheungpasitporn W, Srivali N, et al. Incidence and risk factors of acute kidney injury following transcatheter aortic valve replacement. Nephrology. 2015;21:1041–1046. [DOI] [PubMed] [Google Scholar]
- 5. Thongprayoon C, Cheungpasitporn W, Srivali N, et al. AKI after transcatheter or surgical aortic valve replacement. J Am Soc Nephrol. 2016;27:1854–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagata M, Ninomiya T, Doi Y, et al. Trends in the prevalence of chronic kidney disease and its risk factors in a general Japanese population: the Hisayama Study [published correction appears in Nephrol Dial Transplant. 2010;25:4123–4124]. Nephrol Dial Transplant. 2010;25:2557–2564. [DOI] [PubMed] [Google Scholar]
- 7. Thakar CV, Christianson A, Himmelfarb J, et al. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6:2567–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 9. Barbanti M, Gulino S, Capranzano P, et al. Acute kidney injury with the RenalGuard system in patients undergoing transcatheter aortic valve replacement: the PROTECT‐TAVI Trial (Prophylactic Effect of Furosemide‐Induced Diuresis with Matched Isotonic Intravenous Hydration in Transcatheter Aortic Valve Implantation). JACC Cardiovasc Interv. 2015;8:1595–1604. [DOI] [PubMed] [Google Scholar]
- 10. Villablanca PA, Mathew V, Thourani VH, et al. A meta‐analysis and meta‐regression of long‐term outcomes of transcatheter versus surgical aortic valve replacement for severe aortic stenosis. Int J Cardiol. 2016;225:234–243. [DOI] [PubMed] [Google Scholar]
- 11. Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–269. [DOI] [PubMed] [Google Scholar]
- 12. Najjar M, Yerebakan H, Sorabella RA, et al. Acute kidney injury following surgical aortic valve replacement. J Card Surg. 2015;30:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helgason D, Helgadottir S, Viktorsson SA, et al. Acute kidney injury and outcome following aortic valve replacement for aortic stenosis. Interact Cardiovasc Thorac Surg. 2016;23:266–272. [DOI] [PubMed] [Google Scholar]
- 14. Najjar M, Salna M, George I. Acute kidney injury after aortic valve replacement: incidence, risk factors and outcomes. Expert Rev Cardiovasc Ther. 2015;13:301–316. [DOI] [PubMed] [Google Scholar]
- 15. Strauch JT, Scherner MP, Haldenwang PL, et al. Minimally invasive transapical aortic valve implantation and the risk of acute kidney injury. Ann Thorac Surg. 2010;89:465–470. [DOI] [PubMed] [Google Scholar]
- 16. Barbash IM, Ben‐Dor I, Dvir D, et al. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J. 2012;163:1031–1036. [DOI] [PubMed] [Google Scholar]
- 17. Sinning JM, Ghanem A, Steinhäuser H, et al. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:1141–1149. [DOI] [PubMed] [Google Scholar]
- 18. Khawaja MZ, Thomas M, Joshi A, et al. The effects of VARC‐defined acute kidney injury after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis. EuroIntervention. 2012;8:563–570. [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto M, Hayashida K, Mouillet G, et al. Renal function–based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2013;6:479–486. [DOI] [PubMed] [Google Scholar]
- 20. Hoste EAJ, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. Eur Heart J. 2012;33:2403–2418. [DOI] [PubMed] [Google Scholar]
- 22. Auffret V, Campalo‐Parada F, Regueiro A, et al. Serial changes in cognitive function following transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:2129–2141. [DOI] [PubMed] [Google Scholar]
- 23. Lai KS, Herrmann N, Saleem M, et al. Cognitive outcomes following transcatheter aortic valve implantation: a systematic review. Cardiovasc Psychiatry Neurol. 2015;2015:209569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldfarb S, McCullough PA, McDermott J, et al. Contrast‐induced acute kidney injury: specialty‐specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clin Proc. 2009;84:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neragi‐Miandoab S, Michler RE. A review of most relevant complications of transcatheter aortic valve implantation. ISRN Cardiol. 2013;2013:956252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beohar N, Doshi D, Thourani V, et al. Association of transcatheter aortic valve replacement with 30‐day renal function and 1‐year outcomes among patients presenting with compromised baseline renal function: experience from the PARTNER 1 Trial and Registry. JAMA Cardiol. 2017;2:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jhaveri KD, Saratzis AN, Wanchoo R, et al. Endovascular aneurysm repair (EVAR)‐ and transcatheter aortic valve replacement (TAVR)‐associated acute kidney injury. Kidney Int. 2017;91:1312–1323. [DOI] [PubMed] [Google Scholar]
- 28. Elmariah S, Farrell LA, Daher M, et al. Metabolite profiles predict acute kidney injury and mortality in patients undergoing transcatheter aortic valve replacement. J Am Heart Assoc. 2016;5:e002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yuan Y, Qiu H, Hu X, et al. Predictive value of inflammatory factors on contrast‐induced acute kidney injury in patients who underwent an emergency percutaneous coronary intervention. Clin Cardiol. 2017;40:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mack MJ, Brennan JM, Brindis R, et al; STS/ACC TVT Registry. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069–2077. [DOI] [PubMed] [Google Scholar]
- 31. Rau S, Wessely M, Lange P, et al. Transcatheter aortic valve implantation in dialysis patients. Nephron Clin Pract. 2012;120:c86–c90. [DOI] [PubMed] [Google Scholar]
- 32. Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kolte D, Khera S, Sardar MR, et al. Thirty‐day readmissions after transcatheter aortic valve replacement in the United States. Circ Cardiovasc Interv. 2017;10:e004472. [DOI] [PubMed] [Google Scholar]
- 34. Arnold SV, Lei Y, Reynolds MR, et al; for the PARTNER Investigators . Costs of periprocedural complications in patients treated with transcatheter aortic valve replacement: results from the Placement of Aortic Transcatheter Valve trial. Circ Cardiovasc Interv. 2014;7:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic‐guided fluid administration for the prevention of contrast‐induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383:1814–1823. [DOI] [PubMed] [Google Scholar]
- 36. Ashworth A, Webb ST. Does the prophylactic administration of N‐acetylcysteine prevent acute kidney injury following cardiac surgery? Interact Cardiovasc Thorac Surg. 2010;11:303–308. [DOI] [PubMed] [Google Scholar]
- 37. Scherner M, Wahlers T. Acute kidney injury after transcatheter aortic valve implantation. J Thorac Dis. 2015;7:1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Visconti G, Focaccio A, Donahue M, et al. RenalGuard System for the prevention of acute kidney injury in patients undergoing transcatheter aortic valve implantation. EuroIntervention. 2016;11:e1658–e1661. [DOI] [PubMed] [Google Scholar]
- 39. Rodés‐Cabau J, Kahlert P, Neumann FJ, et al. Feasibility and exploratory efficacy evaluation of the Embrella Embolic Deflector system for the prevention of cerebral emboli in patients undergoing transcatheter aortic valve replacement: the PROTAVI‐C pilot study. JACC Cardiovasc Interv. 2014;7:1146–1155. [DOI] [PubMed] [Google Scholar]
- 40. Stevens MA, McCullough PA, Tobin KJ, et al. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy: results of the P.R.I.N.C.E. study. Prevention of Radiocontrast‐Induced Nephropathy Clinical Evaluation. J Am Coll Cardiol. 1999;33:403–411. [DOI] [PubMed] [Google Scholar]


