Abstract
Background
Data on treatment results of lipid‐lowering therapy (LLT) in familial hypercholesterolemia (FH) are limited, particularly in Asian patients.
Hypothesis
We sought to evaluate the target achievement rate and associated variables in Korean patients with FH after maximal statin‐based LLT.
Methods
We enrolled 146 patients with heterozygous FH, and 90 patients were finally analyzed. Patients were initially prescribed rosuvastatin 10 mg or atorvastatin 20 mg, and the regimen was adjusted to achieve the low‐density lipoprotein cholesterol (LDL‐C) target of 100 mg/dL. The primary evaluation point was the achievement rate of the LDL‐C targets at 12 months: LDL‐C < 100 mg/dL and ≥50% LDL‐C reduction. The associations between clinical variables and target achievement were also analyzed.
Results
At 12 months, 58% of patients were receiving high‐intensity regimens, whereas 46% were receiving combination therapy. The mean pre‐ and post‐treatment LDL‐C levels were 229 and 118 mg/dL, respectively. Twenty‐eight percent of patients achieved LDL‐C < 100 mg/dL, and 47% achieved ≥50% LDL‐C reduction. Pretreatment LDL‐C and high‐intensity regimens indicated a negative tendency toward the attainment of LDL‐C < 100 mg/dL. Conversely, pretreatment LDL‐C and diabetes mellitus were positively associated with a higher rate of ≥50% LDL‐C reduction.
Conclusions
The target achievement of LDL‐C < 100 mg/dL was low, and 50% LDL‐C reduction was moderately achieved in Korean patients with FH receiving maximal statin‐based LLT. Pretreatment LDL‐C levels and diabetes mellitus were associated with target achievement. Our results provide rare and informative data on FH treatment in Asian patients.
Keywords: Asian Continental Ancestry Group, Ezetimibe, Hydroxymethylglutaryl‐CoA Reductase Inhibitors, Hyperlipoproteinemia Type II, Therapeutics
1. INTRODUCTION
Lipid‐lowering therapy (LLT) with statins has been established as the mainstay of cardiovascular protection.1, 2 Particularly, it is an indispensable preventive measure in patients with familial hypercholesterolemia (FH), in whom cholesterol lowering has clearly shown a reduction of cardiovascular (CV) risk.3, 4, 5
Despite the increased CV risk in patients with FH, it has been pointed out that affected patients are often undertreated.6 In addition, in a recent study performed in the United States, only two‐thirds of patients with FH are receiving statin treatment, and only half are taking high‐intensity statins.7 Therefore, further attention is needed to diagnose these patients and appropriately provide intensive LLT in a timely manner.
There have been several limitations regarding data on the treatment results of FH. On one hand, although a few studies with FH registries were conducted with large cohorts, the LLT was not based on predefined protocols.8, 9, 10, 11, 12 On the other hand, most studies with treatment protocols in FH are clinical trials comparing efficacy of standard treatment and addition of novel agents, for instance proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.13, 14 In this regard, data on maximal statin‐based LLT are limited and the numbers of patients who participated in those kinds of studies are small.15 In particular, such data in Asian patients with FH have been scarce.
The aim of our study was to evaluate the rate of target achievement in Korean patients with FH after maximal statin‐based LLT. In addition, we analyzed the clinical variables associated with achieving the targets.
2. METHODS
2.1. Study population
The Korean Society of Lipid and Atherosclerosis supported this study, and 9 university hospitals in Korea participated.16, 17, 18 The study protocol was approved by the institutional review board of each hospital, and all study subjects provided written informed consent. One hundred forty‐six consecutive unrelated men and women older than 19 years who met the Simon‐Broome diagnostic criteria for heterozygous FH were enrolled.19 Briefly, diagnosis of FH was based upon (1) a total cholesterol level > 290 mg/dL or a low‐density lipoprotein‐cholesterol (LDL‐C) level > 190 mg/dL plus tendon xanthomas in the patient or relatives; (2) DNA‐based evidence of a mutation in LDLR, APOB, or PCSK9; or (3) LDL‐C > 190 mg/dL plus family history of premature coronary artery disease (CAD) or plus family history of elevated total cholesterol >290 mg/dL in adult relatives. Among them, 90 patients who accepted the treatment protocol, followed up for ≥12 months (a period for 6 follow‐up visits and 5 regimen adjustments), and had data on pre‐ and post‐treatment LDL‐C levels were finally analyzed.
2.2. Clinical and genetic data collection
Each patient underwent history‐taking, physical examination, and laboratory assessment. CAD was defined as any prior diagnosis of CAD such as myocardial infarction, coronary artery revascularization, or positive results of exercise or pharmacologic stress tests. In patients under ongoing LLT, any lipid‐lowering agent was washed out for 4 weeks unless the patient had a history of atherosclerotic CV or cerebrovascular diseases. The patients fasted for ≥12 hours before blood sampling, and samples were analyzed in 4 hours by a laboratory certified by the Korean Society of Laboratory Medicine.
Pathogenic mutation analysis was performed only in 79 patients, as described previously.18 Briefly, after genomic DNA was extracted, we obtained DNA sequences of 3 FH genes (LDLR, APOB, and PCSK9) by whole‐exome sequencing for 62 subjects or targeted‐exome sequencing for the other 17 subjects. For whole‐exome sequencing, the Agilent SureSelect Enrichment System (SureSelect All Exon 50 Mb or SureSelect All Exon V4 + UTRs kit; Agilent, Santa Clara, CA) was used, whereas for targeted sequencing, DNA fragments were enriched through solution‐based hybridization capture and sequenced with an Illumina HiSeq2500 platform (Illumina, San Diego, CA).
2.3. Treatment protocol
When a patient with FH agreed to follow the treatment protocol (see Supporting Information, Figure, in the online version of this article), rosuvastatin 10 mg or atorvastatin 20 mg daily was first recommended at the physician's discretion. If a patient preferred other statins, he or she was allowed to take them. Then, if the statin was tolerable, it was up‐titrated every 2 months to reach the LDL‐C target of 100 mg/dL.20 The final regimen was maintained when the target was achieved, whereas ezetimibe was added when it was not achieved. In patients whose LDL‐C was not sufficiently reduced thereafter, resin or niacin was combined appropriately. If the statin was not tolerable during this process, we decreased the statin dose, or switched to other statins and/or added ezetimibe. All participants received standard of care treatment for medical conditions.
2.4. Statistical analysis
Continuous variables are presented as mean ± SD and analyzed by using an independent t test. Categorical variables are reported as counts and proportions and analyzed by using Pearson χ2 tests or Fisher exact tests. We defined pretreatment LDL‐C as the documented values before drug therapy. Post‐treatment LDL‐C was defined as the values obtained at 12 months after drug treatment. The primary evaluation point was the achievement rate of the LDL‐C targets at 12 months: LDL‐C < 100 mg/dL and ≥50% LDL‐C reduction. The target achievement rate was further analyzed according to the lipid‐lowering intensities of the treatment regimens. In addition, the associations between clinical variables and LDL‐C target achievement were analyzed. To identify the relationship between clinical variables and target achievement, we performed logistic regression analysis. A P value of <0.05 was considered statistically significant. The SPSS statistical package version 23.0 (IBM Corp., Armonk, NY) was used for all analyses.
3. RESULTS
3.1. Clinical characteristics of the study population
The clinical and laboratory characteristics of the study patients are listed in Table 1. The mean patient age was 54 years. Of the patients, 39% were female and 27% had CAD. Definite type of FH was diagnosed in 20% of patients; 37% had mutations in 3 FH‐related genes. The mean pretreatment LDL‐C level was 229 mg/dL.
Table 1.
Clinical and laboratory parameters of the study population
| Variables | Total Population, N = 90 |
|---|---|
| Age, y | 54 ± 11 |
| Female sex | 35 (38.5) |
| Medical history | |
| DM | 6 (6.7) |
| HTN | 35 (38.9) |
| Current smoking | 12 (13.5) |
| CAD | 24 (26.7) |
| Family history | |
| Hypercholesterolemia | 51 (56.7) |
| Premature CADa | 44 (48.9) |
| BMI, kg/m2 | 25.0 ± 3.2 |
| Tendon xanthoma | 19 (21.1) |
| Type of clinical diagnosis | |
| Definite | 18 (20.0) |
| Possible | 72 (80.0) |
| Mutation positivity (n = 79) | 29 (36.7) |
| Lipids, mg/dL | |
| TC | 317 ± 49 |
| TG | 178 ± 97 |
| HDL‐C | 49.5 ± 15.0 |
| LDL‐C | 229 ± 44 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; SD, standard deviation; TC, total cholesterol; TG, triglycerides.
Data are presented as n (%) or mean ± SD.
Premature CAD is defined as CAD at age < 50 years in a grandparent, aunt, or uncle or at age < 60 years in a parent, sibling, or child.
3.2. Treatment regimens
Among 90 patients who agreed to the treatment protocol, 84 patients (93%) were prescribed rosuvastatin 10 mg or atorvastatin 20 mg. The treatment regimens at 12 months after drug treatment were categorized according to the lipid‐lowering intensity (Table 2).21 Fifty‐eight percent and 42% of participants were taking high‐intensity and moderate‐intensity regimens, respectively, whereas no patients were taking low‐intensity regimens. Among the patients who were taking high‐intensity treatment, 6%, 40%, and 12% were receiving triple, dual, and single agents, respectively.
Table 2.
Lipid‐lowering regimens at 12 months
| Regimen | No. (%) |
|---|---|
| High intensity (triple combination) | 5 (5.6) |
| Atorvastatin 80 mg + ezetimibe 10 mg + resin and/or niacin | 3 (3.3) |
| Rosuvastatin 20 mg + ezetimibe 10 mg + resin and/or niacin | 2 (2.2) |
| High intensity (dual combination) | 36 (40.0) |
| Atorvastatin 80 mg + ezetimibe 10 mg | 6 (6.7) |
| Rosuvastatin 20 mg + ezetimibe 10 mg | 11 (12.2) |
| Atorvastatin 40 mg + ezetimibe 10 mg | 5 (5.6) |
| Rosuvastatin 10 mg + ezetimibe 10 mg | 7 (7.8) |
| Atorvastatin 20 mg + ezetimibe 10 mg | 2 (2.2) |
| Simvastatin 40 mg + ezetimibe 10 mg | 1 (1.1) |
| Pitavastatin 2 mg + ezetimibe 10 mg | 2 (2.2) |
| Pravastatin 40 mg + ezetimibe 10 mg | 1 (1.1) |
| Pravastatin 20 mg + ezetimibe 10 mg | 1 (1.1) |
| High intensity (single) | 11 (12.2) |
| Atorvastatin 80 mg | 1 (1.1) |
| Rosuvastatin 20 mg | 6 (6.7) |
| Atorvastatin 40 mg | 4 (4.4) |
| Moderate intensity | 38 (42.2) |
| Rosuvastatin 10 mg | 8 (8.9) |
| Atorvastatin 20 mg | 16 (17.8) |
| Pitavastatin 4 mg | 1 (1.1) |
| Rosuvastatin 5 mg | 4 (4.4) |
| Atorvastatin 10 mg | 9 (10.0) |
3.3. Target achievement
Data on target achievement are presented in the Figure 1 and Supporting Information, Table, in the online version of this article. The mean pretreatment and post‐treatment LDL‐C levels were 229 and 118 mg/dL, respectively, and the mean percent change was −46%. Twenty‐eight percent of patients achieved LDL‐C < 100 mg/dL, and 2% of participants further achieved <70 mg/dL. Meanwhile, the post‐treatment LDL‐C levels reached 100 to 129 and 130 to 159 mg/dL in 46% and 19% of patients, respectively. The proportion of patients who achieved ≥50% LDL‐C reduction was 47%. Both pretreatment and post‐treatment LDL‐C levels were higher in patients who were receiving stronger‐intensity regimens at 12 months.
Figure 1.
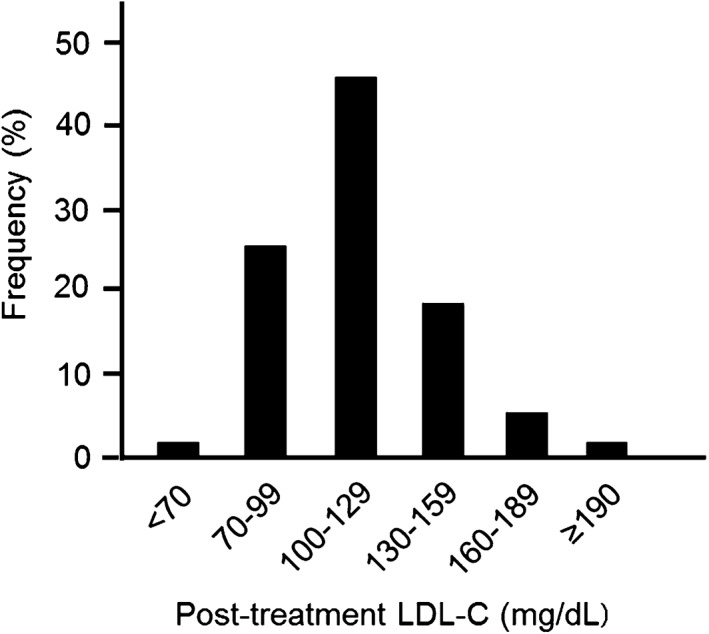
Distribution of post‐treatment LDL‐C levels in the study population (n = 90). Abbreviations: LDL‐C, low‐density lipoprotein cholesterol
3.4. Clinical variables associated with the target achievement
The results of the analyses of associations between variables and the target achievement are listed in Table 3. Pretreatment LDL‐C (odds ratio [OR]: 0.99, P = 0.07) and high‐intensity regimens (OR: 0.39, P = 0.051) indicated a negative tendency toward the attainment of LDL‐C < 100 mg/dL. However, no variables showed statistically significant associations with the achievement. Although patients with pathogenic mutations reached the target less frequently, the difference was not statistically significant. Conversely, pretreatment LDL‐C (OR: 1.02, P = 0.01) and diabetes mellitus (DM; OR: 6.35, P = 0.01) were found to be positively associated with a higher rate of ≥50% LDL‐C reduction (OR: 1.02, P = 0.01).
Table 3.
Association of variables with LDL‐C target achievement
| Variables | LDL‐C < 100 mg/dL, n = 25 | LDL‐C ≥ 100 mg/dL, n = 65 | OR (95% CI) | P Value | ≥50% LDL‐C reduction, n = 42 | <50% LDL‐C reduction, n = 48 | OR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|
| Age | 56 ± 9 | 53 ± 12 | 1.03 (0.99‐1.08) | 0.19 | 55 ± 11 | 52 ± 11 | 1.03 (0.99‐1.07) | 0.20 |
| Female sex | 7 (28.0) | 28 (43.1) | 0.51 (0.18‐1.36) | 0.19 | 14 (33.3) | 21 (43.8) | 0.64 (0.27‐1.51) | 0.31 |
| DM | 3 (12.0) | 3 (4.6) | 2.82 (0.49‐16.24) | 0.23 | 5 (11.9) | 1 (2.1) | 6.35 (0.97‐124.50) | 0.01 |
| HTN | 11 (44.0) | 24 (36.9) | 1.34 (0.52‐3.43) | 0.54 | 17 (40.5) | 18 (37.5) | 1.13 (0.48‐2.66) | 0.77 |
| Current smoking | 3 (12.5) | 9 (13.8) | 0.89 (0.18‐3.32) | 0.87 | 6 (14.6) | 6 (12.5) | 1.20 (0.35‐4.16) | 0.77 |
| CAD | 4 (16.0) | 20 (30.8) | 0.43 (0.11‐1.31) | 0.16 | 10 (23.8) | 14 (29.2) | 0.76 (0.29‐1.94) | 0.57 |
| BMI | 24.6 ± 2.9 | 25.1 ± 3.4 | 0.95 (0.80‐1.11) | 0.52 | 24.4 ± 3.2 | 25.4 ± 3.2 | 0.90 (0.78‐1.03) | 0.14 |
| Tendon xanthoma | 4 (16.0) | 15 (23.1) | 0.63 (0.17‐2.00) | 0.46 | 8 (19.0) | 11 (22.9) | 0.79 (0.28‐2.19) | 0.65 |
| Mutation positivity (n = 79) | 4 (21.1) | 25 (41.7) | 0.37 (0.10‐1.17) | 0.11 | 13 (37.1) | 16 (36.4) | 1.03 (0.41‐2.60) | 0.94 |
| Definite type | 4 (16.0) | 14 (21.5) | 0.69 (0.18‐2.20) | 0.56 | 8 (19.0) | 10 (20.8) | 0.89 (0.31‐2.52) | 0.83 |
| Pretreatment LDL‐C | 215 ± 28 | 235 ± 48 | 0.99 (0.97‐1.00) | 0.07 | 243 ± 51 | 217 ± 34 | 1.02 (1.00‐1.03) | 0.01 |
| High‐intensity regimen | 10 (40.0) | 41 (63.1) | 0.39 (0.15‐0.99) | 0.051 | 25 (59.5) | 26 (54.2) | 1.24 (0.54‐2.90) | 0.61 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; DM, diabetes mellitus; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; OR, odds ratio; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
4. DISCUSSION
In the present study, we found that 28% of the study patients achieved LDL‐C < 100 mg/dL, whereas 47% of patients attained ≥50% LDL‐C reduction at 12 months after maximal statin‐based LLT. These results were obtained with high‐intensity regimens in 58% and combination regimens in 46%. Pretreatment LDL‐C and DM were positively associated with a higher rate of 50% LDL‐C reduction. Although several studies reported the results of real‐world practice in patients with FH, studies on treatment with a predefined protocol, like ours, have not been available. In this regard, our results provide rare and informative data on FH treatment, particularly in Asian patients. The target achievement rate was similar to or modestly higher than that in Western reports, although the intensity of our regimen was slightly weaker than that in those studies. Nevertheless, the achievement rate remains far from satisfactory.
In previous Western studies, the target achievement rates for LDL‐C < 100 mg ranged from 11% to 25%, whereas the rates for ≥50% LDL‐C reduction ranged from 41% to 47%. In a study performed in the United States, the Study of Awareness and Detection of Familial Hypercholesterolemia (CASCADE‐FH) registry, LDL‐C < 100 mg was reached in 25%, and ≥50% LDL‐C reduction was attained in 41% of patients.11 Although these treatment results are similar to ours, these were accomplished by more frequent use of combination regimens (56%) than in our study. In a study in the Netherlands, LDL‐C < 2.5 mmol/L (97 mg/dL) was achieved in 21% of patients, whereas a considerable proportion of patients received submaximal treatment. In this study, ≥50% LDL‐C reduction was achieved in 47% of patients.8 Likewise, in a French study, 19% of the study patients reached LDL‐C < 100 mg/dL, in cases treated with maximal LLT with statins plus other agents.10 On the other hand, the achievement of the same target was only 11% in the Spanish Familial Hypercholesterolaemia Cohort Study (SAFEHEART) registry in Spain, although 72% of patients were receiving maximal LLT that can lower LDL‐C ≥ 50%.12 In a study performed in Norway, LDL‐C < 2.6 mmol/L (100 mg/dL) was reached in 12% of patients; however, adequate LLT was given in only 47% of patients.9
Although Asian studies on the treatment results of FH are extremely limited, several small Japanese data on fixed regimens are available. In a study with triple combination therapy of rosuvastatin 20 mg, ezetimibe 10 mg, and colestimide 3.62 g, the achievement rate of LDL‐C < 100 mg/dL was 44%.15 In Japanese studies, the mean LDL‐C reductions were 57%, 61%, and 66% after treatment with rosuvastatin 40 mg alone,22 atorvastatin 20 to 40 mg plus colestimide 3 g,23 and the triple combination mentioned above,15 respectively.
In our study, a few variables were associated with the target achievement. The CASCADE‐FH registry demonstrated that achieving LDL‐C < 100 mg/dL was associated with old age and high‐intensity regimens, whereas ≥50% LDL‐C reduction was correlated with old age, high pretreatment LDL‐C, and high‐intensity regimens.11 The relationship between pretreatment LDL‐C and ≥50% LDL‐C reduction in this study is in accordance with our result. Conversely, the SAFEHEART registry demonstrated that achievement of LDL‐C < 100 mg/dL was associated with type 2 DM, absence of cardiovascular disease, defective LDLR mutation, and ezetimibe use.10 The association of DM with the target achievement rate was similar to our study. However, the underlying cause of this finding is uncertain, and further study is needed for clarification. The negative correlation between LDLR mutation and attaining the target is concordant with a Brazilian study. This study showed that the attainment rates of LDL‐C < 3.4 mmol/L (131 mg/dL) were 23%, 27%, and 47% in groups with null‐, defective‐, and no mutations, respectively.24 Although the achievement of LDL‐C < 100 mg/dL was less frequent in mutation carriers in our study, the difference was not statistically significant. This finding in our study might have been affected by the smaller sample size or ethnic characteristics.
We discovered that the post‐treatment LDL‐C was higher in patients treated with stronger‐intensity regimens (see Table 3 and Supporting Information, Table, in the online version of this article). Because the pretreatment LDL‐C was higher in these patients, it can be assumed that post‐treatment LDL‐C was higher despite more reduction of the absolute amount of LDL‐C. Thus, it is the severity of the disease and not the treatment intensity which explains this observation. In addition, in the current study, the target of ≥50% LDL‐C reduction was more frequently achieved in patients with higher pretreatment LDL‐C. However, it seems inappropriate to be satisfied with attainment of ≥50% LDL‐C reduction in this population. The CV risk may be greater when patients with FH have higher baseline LDL‐C. Thus, reducing LDL‐C levels more aggressively in these patients, rather than being content with 50% reduction, would induce more clinical benefit. A PCSK9 inhibitor recently has shown CV risk reduction,25 and it is expected to play a certain role in further LLT in patients with FH in the near future.
4.1. Study limitations
To date, data on the treatment results of Asian patients with FH are only partially reported. Although we tried to enroll the most available number of patients, it might not be sufficient to analyze the variables determining the results. Larger and longer‐term studies may be helpful to clarify the effect of LLT, including appropriate agents and doses, and desirable targets, on the clinical outcomes in this population. In 24 patients who took moderate‐intensity regimens in our study, the post‐treatment LDL‐C levels were 100 to 129 mg/dL (see Supporting Information, Table, in the online version of this article). Thus, it is possible that higher‐intensity regimens in these patients might have raised the target achievement rate to some extent. Data on statin intolerance are not well reported. However, based on the best available data in these cases, we found that the final LLT regimens were determined by clinician‐patient discussion rather than strict up‐titration. We cannot rule out cases in which a clinician or patient preferred not using a higher‐dose statin after considering the patient's condition or history regarding pharmacotherapy.
5. CONCLUSION
The goal attainment of LDL‐C < 100 mg/dL was low and ≥50% LDL‐C reduction was moderately achieved in Korean patients with FH receiving maximal statin‐based LLT. Our results were not inferior to those of foreign studies. Additionally, a few variables, including LDL‐C, were identified to be associated with the target achievement. Our results provide rare and informative data on FH treatment, particularly in Asian patients.
Supporting information
Supplementary Figure 1 Flow‐chart of the treatment protocol. LDL‐C: low‐density lipoprotein‐cholesterol
Table S1 Post‐treatment LDL‐C levels and the proportion of patients achieving 50% LDL‐C reduction with each regimen category
ACKNOWLEDGMENTS
The authors are grateful to Jiyeong Jeong, RN, for her excellent assistance with clinical data collection and patient care.
Conflicts of interest
The authors declare no potential conflicts of interest.
Oh J, Lee CJ, Kim DI, et al. Target achievement with maximal statin‐based lipid‐lowering therapy in Korean patients with familial hypercholesterolemia: A study supported by the Korean Society of Lipid and Atherosclerosis. Clin Cardiol. 2017;40:1291–1296. 10.1002/clc.22826
Author contributions: Jaewon Oh and Chan Joo Lee contributed equally to this work.
Funding information This research was financially supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2012R1A4A1029061 and 2014R1A1A2056104); the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (2015M3A9B6029138); and the National Research Council of Science & Technology grant by the Korean government, MSIP (No. CAP‐12‐2‐KBSI).
REFERENCES
- 1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2. LaRosa JC, Grundy SM, Waters DD, et al; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 3. Versmissen J, Oosterveer DM, Yazdanpanah M, et al. Efficacy of statins in familial hypercholesterolaemia: a long‐term cohort study. BMJ. 2008;337:a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harada‐Shiba M, Sugisawa T, Makino H, et al. Impact of statin treatment on the clinical fate of heterozygous familial hypercholesterolemia. J Atheroscler Thromb. 2010;17:667–674. [DOI] [PubMed] [Google Scholar]
- 5. Besseling J, Hovingh GK, Huijgen R, et al. Statins in familial hypercholesterolemia: consequences for coronary artery disease and all‐cause mortality. J Am Coll Cardiol. 2016;68:252–260. [DOI] [PubMed] [Google Scholar]
- 6. Nordestgaard BG, Chapman MJ, Humphries SE, et al; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478a–3490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knickelbine T, Lui M, Garberich R, et al. Familial hypercholesterolemia in a large ambulatory population: statin use, optimal treatment, and identification for advanced medical therapies. J Clin Lipidol. 2016;10:1182–1187. [DOI] [PubMed] [Google Scholar]
- 8. Pijlman AH, Huijgen R, Verhagen SN, et al. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross‐sectional study in The Netherlands. Atherosclerosis. 2010;209:189–194. [DOI] [PubMed] [Google Scholar]
- 9. Leren TP, Berge KE. Subjects with molecularly defined familial hypercholesterolemia or familial defective apoB‐100 are not being adequately treated. PLoS One. 2011;6:e16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Béliard S, Carreau V, Carrié A, et al. Improvement in LDL‐cholesterol levels of patients with familial hypercholesterolemia: can we do better? Analysis of results obtained during the past two decades in 1669 French subjects. Atherosclerosis. 2014;234:136–141. [DOI] [PubMed] [Google Scholar]
- 11. deGoma EM, Ahmad ZS, O'Brien EC, et al. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States: data from the CASCADE‐FH Registry. Circ Cardiovasc Genet. 2016;9:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perez de Isla L, Alonso R, Watts GF, et al; SAFEHEART Investigators. Attainment of LDL‐cholesterol treatment goals in patients with familial hypercholesterolemia: 5‐year SAFEHEART Registry follow‐up. J Am Coll Cardiol. 2016;67:1278–1285. [DOI] [PubMed] [Google Scholar]
- 13. Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78‐week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raal FJ, Stein EA, Dufour R, et al; RUTHERFORD‐2 Investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD‐2): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:331–340. [DOI] [PubMed] [Google Scholar]
- 15. Kawashiri MA, Nohara A, Noguchi T, et al. Efficacy and safety of coadministration of rosuvastatin, ezetimibe, and colestimide in heterozygous familial hypercholesterolemia. Am J Cardiol. 2012;109:364–369. [DOI] [PubMed] [Google Scholar]
- 16. Han SM, Hwang B, Park TG, et al. Genetic testing of Korean familial hypercholesterolemia using whole‐exome sequencing. PLoS One. 2015;10:e0126706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwon M, Han SM, Kim DI, et al. Evaluation of polygenic cause in Korean patients with familial hypercholesterolemia: a study supported by the Korean Society of Lipidology and Atherosclerosis. Atherosclerosis. 2015;242:8–12. [DOI] [PubMed] [Google Scholar]
- 18. Shin DG, Han SM, Kim DI, et al. Clinical features of familial hypercholesterolemia in Korea: predictors of pathogenic mutations and coronary artery disease: a study supported by the Korean Society of Lipidology and Atherosclerosis. Atherosclerosis. 2015;243:53–58. [DOI] [PubMed] [Google Scholar]
- 19. Marks D, Thorogood M, Neil HA, et al. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168:1–14. [DOI] [PubMed] [Google Scholar]
- 20. Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 21. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(25 suppl 2):S46–S48]. Circulation. 2014;129(25 suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 22. Mabuchi H, Nohara A, Higashikata T, et al. Clinical efficacy and safety of rosuvastatin in Japanese patients with heterozygous familial hypercholesterolemia. J Atheroscler Thromb. 2004;11:152–158. [DOI] [PubMed] [Google Scholar]
- 23. Kawashiri MA, Higashikata T, Nohara A, et al. Efficacy of colestimide coadministered with atorvastatin in Japanese patients with heterozygous familial hypercholesterolemia (FH). Circ J. 2005;69:515–520. [DOI] [PubMed] [Google Scholar]
- 24. Santos PC, Morgan AC, Jannes CE, et al. Presence and type of low‐density lipoprotein receptor (LDLR) mutation influences the lipid profile and response to lipid‐lowering therapy in Brazilian patients with heterozygous familial hypercholesterolemia. Atherosclerosis. 2014;233:206–210. [DOI] [PubMed] [Google Scholar]
- 25. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Flow‐chart of the treatment protocol. LDL‐C: low‐density lipoprotein‐cholesterol
Table S1 Post‐treatment LDL‐C levels and the proportion of patients achieving 50% LDL‐C reduction with each regimen category


