Abstract
Background
There are no data on the impact of transcatheter aortic valve implantation (TAVI) on carotid and vertebral arterial blood flow. Our aim was to assess the effects of the orthostatic stress test on carotid and vertebral artery blood flow in patients with severe aortic stenosis (AS) undergoing TAVI.
Hypothesis
TAVI may have beneficial effect on carotid and vertebral artery flow in patients with severe aortic stenosis.
Methods
Thirty carefully selected patients with severe AS undergoing TAVI were enrolled. Peak systolic blood‐flow velocity and end‐diastolic velocity in the common carotid artery, internal carotid artery, and vertebral artery, as well as spectral analysis of flow pattern with time‐averaged maximum velocity (centimeters per second), time‐averaged mean velocity (centimeters per second), and flow volume (milliliters per minute) on both sides were measured by duplex ultrasound. Measurements were performed in the supine position and at 1 to 2 minutes after the assumption of the standing position at baseline and 3 months after TAVI.
Results
All duplex ultrasound parameters assessed in the supine position have significantly improved in patients after TAVI as compared to baseline (P < 0.001 for all). The orthostatic stress test induced decrease of carotid and vertebral arterial flow velocities in AS patients before and after TAVI. However, the drop in velocities and flow volume was numerically lower after TAVI.
Conclusions
TAVI may have some beneficial effect on extracranial artery blood flow by minimalization of its decrease as a response to orthostatic stress.
Keywords: aortic stenosis, transcatheter aortic valve implantation, orthostatic stress, Doppler ultrasound, cerebral blood flow
1. INTRODUCTION
Aortic stenosis (AS) is the most common type of valvular heart disease and mostly affects adults of advanced age (2%–7% of the population >65 years) with its primarily calcific form.1, 2 Surgical aortic valve replacement (AVR) was for decades the standard treatment for patients with symptomatic severe AS. Transcatheter aortic valve implantation (TAVI) is a less invasive treatment option for elderly, high‐risk patients with symptomatic severe AS than AVR. More importantly, TAVI improves survival3, 4 and quality of life5, 6 compared to medical treatment in inoperable patients. A successful TAVI procedure requires a complex selection process of patients, including detailed imaging information of the aortic valve anatomy and the peripheral arteries, and also critical clinical assessment by an interdisciplinary Heart Team.7, 8 There are limited data on responses of cerebral blood flow to the postural maneuver in AS.9 Passive orthostatic tests (without exercise) are helpful in diagnostic evaluation in other diseases predisposing to left ventricular outflow tract gradients.10 Also, no data on the possible impact of TAVI on carotid and vertebral arterial blood flow are available. Thus, our aim was to assess the effects of the orthostatic stress test on carotid and vertebral artery blood flow in patients with severe AS undergoing TAVI.
Figure 1.
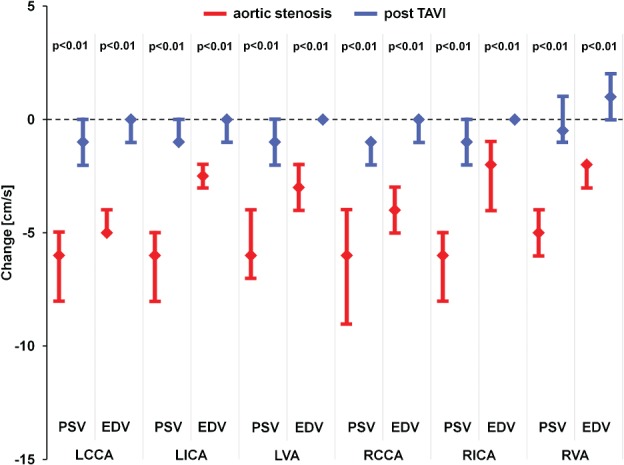
Change in PSV and EDV between the supine and upright position for patients with aortic stenosis (red) and TAVI patients (blue). Data are median with upper and lower quartiles. Abbreviations: EDV, end diastolic velocity; LCCA, left common carotid artery; LICA, left internal carotid artery; LVA, left vertebral artery; PSV, peak systolic velocity; RCCA, right common carotid artery; RICA, right internal carotid artery; RVA, right vertebral artery; TAVI, transcatheter aortic valve implantation.
2. METHODS
We included 30 carefully selected patients awaiting TAVI who underwent carotid duplex ultrasound in our department and who fulfilled the following inclusion criteria: severe (aortic valve area [AVA] <0.8 cm2), isolated AS; preserved (>50%) left ventricle ejection fraction; no significant atherosclerosis in carotid and vertebral arteries; sinus rhythm. A total of 130 patients with AS were screened. The study protocol was approved by the institutional ethical board and complied with the Declaration of Helsinki. Informed consent was obtained from the subjects. To omit potentially confounding factors, 16 patients were excluded from the study due to detected atherosclerosis in carotid/vertebral arteries. Additional exclusion criteria were atrial flutter/fibrillation (25 patients excluded), more than mild concomitant mitral valve dysfunction, and/or more than mild aortic insufficiency (14 patients excluded). In regard to hypotensive therapy in this highly selected group of patients, only β‐blockers were administered, and the therapy remained unchanged after TAVI (45 patients excluded). The ultrasound transducer (4–10 MHz linear‐array transducer, Vivid 7; General Electric, Fairfield, CT) was used to perform carotid duplex ultrasound routinely in the supine position with peak systolic velocity and end‐diastolic velocity assessment in common carotid, internal carotid, and vertebral arteries. In the second part of examination during orthostatic test, the patient stood for 1 to 2 minutes, and carotid duplex ultrasound with velocity measurements was repeated again. Heart rate was assessed at baseline and after 1 to 2 minutes of orthostatic stress. Additionally, we assessed spectral analysis of flow pattern with time‐averaged maximum velocity (centimeters per second), time‐averaged mean velocity (centimeters per second), and flow volume (milliliters per minute) at baseline and in an upright position. Doppler ultrasound assessment was repeated 3 months after TAVI in the same way. Each duplex ultrasound parameter was assessed repeatedly 3 times, and a mean value was used for analysis.8 Standard echocardiography was performed using the Vivid 7 (General Electric). We obtained the M‐mode and 2‐dimensional echocardiograms for each patient, which was followed by a pulsed‐ and continuous‐wave Doppler ultrasound. We used conventional techniques to measure the echocardiographic parameters. Patient selection for candidates for TAVI was performed by a multidisciplinary heart team supported by clinical and imaging resources. TAVI procedures were performed using Edwards Sapien XT and Edwards Sapien 3 (Edwards Lifesciences, Irvine, CA), Medtronic Corevalve (Medtronic Inc., Minneapolis, MN), JenaValve (JenaValve Technology, Munich, Germany), and Lotus (Boston Scientific, Marlborough, MA). Access routes were transfemoral and transapical. Procedures were performed under general anesthesia or analgosedation.11
2.1. Statistical analysis
Continuous variables were presented as median (interquartile range [IQR]). Categorical variables were expressed as numbers (percentages). Differences between duplex ultrasound parameters assessed in the supine and upright positions as well as at baseline and follow‐up were assessed using the Wilcoxon signed rank test. All tests were 2‐tailed, and a P value of <0.05 was considered statistically significant. All statistical analysis was performed using SPSS software version 15.0 (IBM, Armonk, NY).
3. RESULTS
The median of age of the patients was 83.0 years (IQR, 79.0–85.3 years), and 26.7% of patients were males. The median AVA was 0.6 cm2 (IQR, 0.6–0.9 cm2) and medians of maximal/mean transaortic gradient were 80.0 mm Hg (IQR, 72.0–102.0 mm Hg)/46.0 (42.0–53.0 mm Hg). All patients were considered high risk according to the logistic EuroScore I 13.1% (IQR, 9.5%–23.2%) and Society of Thoracic Surgeons score 5.6% (IQR, 4.0%–13.7)%. Details of baseline characteristics, procedural, and postprocedural data are shown in Table 1. All duplex ultrasound parameters assessed in the supine position were higher after TAVI when compared to preprocedural assessment (P < 0.001 for all). The orthostatic stress test induced decrease of carotid and vertebral arterial flow velocities in AS patients before and after TAVI. However, the drop in velocities and flow volume was numerically lower after TAVI. Detailed analysis is shown in Tables 2 and 3. The median heart rate in AS patients before TAVI was 76.0 bpm (IQR, 65.5–79 bpm) at baseline and 76.0 bpm (IQR, 66.5–78.5 bpm) in the upright position (P = not significant), and 76.0 bpm (IQR, 65.5–79.0 bpm) and 76.0 bpm (65.5–78.5 bpm), respectively, after TAVI (P = not significant). Diameter of the left and right common carotid artery was 0.65 cm (IQR, 0.59–0.74 cm). Additionally, stroke volume and stroke volume index before and after TAVI were significantly different: 66.2 mL/beat/m2 (IQR, 54.6–83.50 mL/beat/m2) vs 70.8 mL/beat/m2 (IQR, 59.0–88.7 mL/beat/m2) (P < 0.001) and 37.0 mL/beat/m2 (IQR, 28.7–40.9 mL/beat/m2) vs 39.1 mL/beat/m2 (30.9–45.5 mL/beat/m2) (P < 0.001), respectively.
Table 1.
Baseline characteristics of patients with severe aortic stenosis: procedural and postprocedural data (N = 30)
| Variable | Value |
|---|---|
| Age, y, median (IQR) | 83.0 (79.0–85.3) |
| Male, no. (%) | 8 (26.7%) |
| Body mass index, kg/m2, median (IQR) | 27.1 (25.4–30.0) |
| Arterial hypertension (only β‐blocker), no. (%) | 24 (80.0%) |
| Diabetes mellitus, no. (%) | 14 (46.7%) |
| Hyperlipidemia, no. (%) | 308 (100.0%) |
| Previous pacemaker, no. (%) | 0 (0.0%) |
| Atrial fibrillation, no. (%) | 0 (0.0%) |
| Aortic valve parameters | |
| TG max, mm Hg, median (IQR) | 80.0 (72.0–102.0) |
| TG mean, mm Hg, median (IQR) | 46.0 (42.0–53.0) |
| Aortic valve area, cm2, median (IQR) | 0.6 (0.6–0.9) |
| LVEF, %, median (IQR) | 61.5 (50.0–65.0) |
| Risk of surgery | |
| Logistic EuroScore I [%], median (IQR) | 13.1 (9.5–23.2) |
| Society of Thoracic Surgeons Score [%], median (IQR) | 5.6 (4.0–13.7) |
| Device implanted, no. (%) | |
| Edwards Sapien, Sapien XT, Sapien 3 | 13 (46.7%) |
| Medtronic CoreValve, Evolut | 8 (26.6%) |
| Jena Valve | 3 (10.0%) |
| Lotus | 5 (16.7%) |
| Aortic regurgitation after TAVI, no. (%) | |
| None | 24 (80.0%) |
| Grade 1 | 6 (20.0%) |
| Grade 2 | 0 (0.0%) |
| Grade 3 | 0 (0.0%) |
| TG max after TAVI, mm Hg, median (IQR) | 12.0 (10.0–20.3) |
| TG mean after TAVI, mm Hg, median (IQR) | 7.0 (5.3–11.8) |
Abbreviations: IQR, interquartile range; LVEF, left ventricle ejection fraction; TAVI, transcatheter aortic valve implantation; TG, transaortic gradient.
Table 2.
Carotid duplex ultrasound data
| Variable | Supine | Upright | P Value |
|---|---|---|---|
| Before TAVI, n = 30 | |||
| PSV LCCA, cm/s | 95.5 (93.0–100.0) | 90.0 (89.0–93.0) | <0.001 |
| EDV LCCA, cm/s | 25.0 (24.0–26.0) | 20.0 (20.0–21.0) | <0.001 |
| Left TAMAX, cm/s | 46.6 (43.2–48.9) | 42.2 (41.2–45.1) | <0.001 |
| Left TAMEAN, cm/s | 22.6 (22.1–24.3) | 20.1 (20.0–21.0) | <0.001 |
| LCCA flow volume, mL/min | 699.6 (631.2–703.9) | 671.3 (610.2–689.4) | <0.001 |
| PSV LICA, cm/s | 93.5 (91.0–97.0) | 86.5 (84.0–91.0) | <0.001 |
| EDV LICA, cm/s | 23.0 (22.0–24.0) | 20.0 (20.0–21.0) | <0.001 |
| PSV LVA, cm/s | 48.0 (45.0–49.0) | 42.0 (39.0–45.0) | <0.001 |
| EDV LVA, cm/s | 15.0 (15.0–17.0) | 12.0 (12.0–13.0) | <0.001 |
| PSV RCCA, cm/s | 96.0 (94.0–100.0) | 90.0 (86.0–94.0) | <0.001 |
| EDV RCCA, cm/s | 25.0 (23.0–27.0) | 20.0 (20.0–21.0) | <0.001 |
| TAMAX, cm/s | 46.3 (43.2–48.2) | 42.2 (41.0–45.0) | <0.001 |
| TAMEAN, cm/s | 22.7 (22.0–24.2) | 20.0 (19.9–21.2) | <0.001 |
| Flow volume, mL/min | 699.1 (629.1–704.2) | 674.7 (609.2–690.2) | <0.001 |
| PSV RICA, cm/s | 93.0 (91.0–98.0) | 87.0 (84.0–92.0) | <0.001 |
| EDV RICA, cm/s | 22.0 (22.0–25.0) | 20.0 (20.0–21.0) | <0.001 |
| PSV RVA, cm/s | 46.5 (45.0–49.0) | 41.5 (40.0–43.0) | <0.001 |
| EDV RVA, cm/s | 14.5 (13.0–15.0) | 12.0 (12.0–13.0) | <0.001 |
| After TAVI, n = 30 | |||
| PSV LCCA, cm/s | 98.5 (96.0–103.0) | 97.5 (95.0–100.0) | <0.001 |
| EDV LCCA, cm/s | 26.0 (25.0–27.0) | 25.0 (24.0–27.0) | <0.001 |
| TAMAX, cm/s | 49.0 (45.5–51.0) | 48.2 (43.6–50.0) | <0.001 |
| TAMEAN, cm/s | 24.0 (23.5–25.9) | 24.0 (22.5–25.0) | <0.001 |
| Flow volume, mL/min | 722.3 (654.7–732.0) | 718.6 (649.2–724.0) | <0.001 |
| PSV LICA, cm/s | 96.0 (94.0–100.0) | 96.0 (93.0–100.0) | <0.001 |
| EDV LICA, cm/s | 24.5 (23.0–26.0) | 24.0 (22.0–26.0) | 0.004 |
| PSV LVA, cm/s | 50.0 (45.0–52.0) | 50.0 (45.0–51.0) | <0.001 |
| EDV LVA, cm/s | 15.0 (15.0–17.0) | 15.5 (15.0–17.0) | 0.023 |
| PSV RCCA, cm/s | 98.5 (96.0–102.0) | 97.5 (95.0–99.0) | <0.001 |
| EDV RCCA, cm/s | 26.0 (25.0–27.0) | 25.0 (24.0–27.0) | 0.001 |
| TAMAX, cm/s | 49.0 (45.5–52.0) | 47.7 (43.6–50.0) | <0.001 |
| TAMEAN, cm/s | 24.0 (23.5–25.9) | 23.7 (22.5–25.0) | <0.001 |
| Flow volume, mL/min | 723.3 (654.7–732.0) | 718.2 (649.2–724.0) | <0.001 |
| PSV RICA, cm/s | 96.0 (94.0–100.0) | 96.0 (93.0–99.0) | <0.001 |
| EDV RICA, cm/s | 24.0 (23.0–26.0) | 24.0 (23.0–26.0) | 0.27 |
| PSV RVA, cm/s | 49.0 (46.0–51.0) | 50.0 (45.0–50.8) | 0.006 |
| EDV RVA, cm/s | 14.5 (13.3–15.0) | 15.0 (15.0–16.0) | 0.001 |
Abbreviations: EDV, end‐diastolic velocity; LCCA, left common carotid artery; LICA, left internal carotid artery; LVA, left vertebral artery; PSV, peak systolic velocity; RCCA, right common carotid artery; RICA, right internal carotid artery; RVA, right vertebral artery; TAMAX, time‐averaged maximum velocity; TAMEAN, time‐averaged mean velocity; TAVI, transcatheter aortic valve implantation.
Table 3.
Comparison of carotid doppler ultrasound parameters of AS patients before and after transcatheter aortic valve implantation in terms of orthostatic stress
| Variable | Before TAVI, n = 30 | After TAVI n = 30 | P Value | |
|---|---|---|---|---|
| PSV LCCA, cm/s | Supine | 95.5 (93.0 to 100.0) | 98.5 (96.0 to 102.8) | <0.001 |
| Upright | 90.0 (89.0 to 93.8) | 97.5 (95.0 to 100.0) | <0.001 | |
| Delta | −6.0 (−7.8 to −4.3) | −1.0 (−2.0 to 1.0) | <0.001 | |
| EDV LCCA, cm/s | Supine | 25.0 (24.0 to 26.0) | 26.0 (25.0 to 27.0) | <0.001 |
| Upright | 20.0 (20.0 to 21.0) | 25.0 (24.0 to 27.0) | <0.001 | |
| Delta | −4.5 (−5.0 to −4.0) | 0.0 (−1.0 to −0.0) | <0.001 | |
| LCCA TAMAX, cm/s | Supine | 46.6 (43.2 to 48.9) | 48.5 (44.5 to 51.8) | <0.001 |
| Upright | 42.7 (41.2 to 45.2) | 47.9 (43.5 to 50.2) | <0.001 | |
| Delta | −2.8 (−3.9 to −1.8) | −1.0 (−1.7 to 0.0) | <0.001 | |
| LCCA TAMEAN, cm/s | Supine | 22.5 (22.0 to 24.3) | 24.0 (23.5 to 25.1) | <0.001 |
| Upright | 20.1 (19.5 to 21.2) | 23.7 (22.5 to 24.9) | <0.001 | |
| Delta | −2.3 (−3.0 to −1.8) | −0.8 (−1.0 to −0.3) | <0.001 | |
| LCCA flow volume, mL/min | Supine | 699.2 (627.7 to 703.3) | 721.1 (652.4 to 731.1) | <0.001 |
| Upright | 659.7 (610.1 to 690.7) | 717.6 (646.7 to 721.2) | <0.001 | |
| Delta | −20.0 (−28.4 to −14.4) | −4.8 (−8.8 to −3.3) | <0.001 | |
| PSV LICA, cm/s | Supine | 93.5 (91.0 to 97.0) | 96.0 (92.5 to 100.0) | <0.001 |
| Upright | 87.0 (84.3 to 91.0) | 96.0 (91.5 to 99.8) | <0.001 | |
| Delta | −6.0 (−7.0 to −4.3) | −1.0 ( to −1.8 to 0.0) | <0.001 | |
| EDV LICA, cm/s | Supine | 23.0 (22.0 to 24.0) | 24.0 (23.0 to 26.0) | 0.001 |
| Upright | 20.0 (20.0 to 21.0) | 24.0 (22.0 to 25.8) | <0.001 | |
| Delta | −2.0 (−3.0 to −2.0) | 0.0 (−1.0 to 0.0) | <0.001 | |
| PSV LVA, cm/s | Supine | 48.5 (45.0 to 49.8) | 50.0 (46.0 to 52.0) | <0.001 |
| Upright | 42.0 (39.3 to 45.0) | 50.0 (45.3 to 51.0) | <0.001 | |
| Delta | −6.0 (−7.0 to −4.0) | −1.0 (−2.0 to 0.0) | <0.001 | |
| EDV LVA, cm/s | Supine | 15.0 (15.0 to 16.8) | 15.0 (15.0 to 16.8) | <0.001 |
| Upright | 12.0 (12.0 to 13.0) | 15.0 (15.0 to 16.0) | <0.001 | |
| Delta | −3.0 (−4.0 to −2.0) | 0.0 (0.0 to 0.0) | <0.001 | |
| PSV RCCA, cm/s | Supine | 96.0 (94.0 to 100.0) | 98.5 (96.0 to 102.0) | <0.001 |
| Upright | 90.0 (86.5 to 94.0) | 97.5 (95.0 to 99.0) | <0.001 | |
| Delta | −5.5 (−8.0 to −4.0) | −1.0 (−2.0 to to 1.0) | <0.001 | |
| EDV RCCA, cm/s | Supine | 24.5 (23.0 to 25.0) | 26.0 (25.0 to 27.0) | <0.001 |
| Upright | 20.0 (20.0 to 21.0) | 25.0 (24.0 to 26.8) | <0.001 | |
| Delta | −4.0 (−5.0 to −3.0) | 0.0 (−1.0 to 0.0) | <0.001 | |
| RCCA TAMAX, cm/s | Supine | 46.3 (43.1 to 48.9) | 48.5 (44.5 to 51.8) | <0.001 |
| Upright | 42.7 (41.1 to 45.1) | 47.9 (43.5 to 50.2) | <0.001 | |
| Delta | −2.8 (−3.6 to −1.9) | −1.0 (−1.7 to −0.5) | <0.001 | |
| RCCA TAMEAN, cm/s | Supine | 22.5 (21.7 to 24.2) | 24.0 (23.5 to 25.1) | <0.001 |
| Upright | 20.0 (19.7 to 21.2) | 23.7 (22.5 to 24.9) | <0.001 | |
| Delta | −2.3 (−3.1 to −2.0) | −0.7 (−1.1 to 0.0) | <0.001 | |
| RCCA Flow volume, mL/min | Supine | 698.6 (626.3 to 703.7) | 722.0 (653.2 to 731.1) | <0.001 |
| Upright | 659.3 (609.2 to 690.0) | 716.6 (646.7 to 721.2) | <0.001 | |
| Delta | −21.0 (−28.5 to −14.3) | −6.9 (−8.8 to −3.1) | <0.001 | |
| PSV RICA, cm/s | Supine | 93.0 (91.0 to 98.0) | 96.0 (93.3 to 99.8) | <0.001 |
| Upright | 87.0 (84.0 to 92.0) | 96.0 (92.3 to 99.0) | <0.001 | |
| Delta | −6.0 (−7.8 to −5.0) | −1.0 (−2.0 to 0.0) | <0.001 | |
| EDV RICA, cm/s | Supine | 22.0 (21.3 to 24.5) | 24.0 (23.0 to 26.0) | 0.002 |
| Upright | 20.0 (20.0 to 20.8) | 24.0 (23.0 to 26.0) | <0.001 | |
| Delta | −2.0 (−3.8 to −1.0) | 0.0 (0.0 to 0.0) | <0.001 | |
| PSV RVA, cm/s | Supine | 47.0 (45.0 to 49.0) | 49.0 (46.0 to 51.0) | <0.001 |
| Upright | 42.0 (41.0 to 43.0) | 50.0 (45.0 to 50.8) | <0.001 | |
| Delta | −5.0 (−6.0 to −4.0) | −0.5 (−1.8 to 1.0) | <0.001 | |
| EDV RVA, cm/s | Supine | 14.5 (13.3 to 15.0) | 14.5 (13.3 to 15.0) | <0.001 |
| Upright | 12.0 (12.0 to 13.0) | 15.0 (15.0 to 16.0) | <0.001 | |
| Delta | −2.0 (−3.0 to −2.0) | 1.0 (0.0 to 2.0) | <0.001 |
Abbreviations: EDV, end diastolic velocity; LCCA, left common carotid artery; LICA, left internal carotid artery; LVA, left vertebral artery; PSV, peak systolic velocity; RCCA, right common carotid artery; RICA, right internal carotid artery; RVA, right vertebral artery; TAMAX, time‐averaged maximum velocity; TAMEAN, time‐averaged mean velocity; TAVI, transcatheter aortic valve implantation.
4. DISCUSSION
In our study, we evaluated the blood flow in extracranial arteries before and after TAVI and found improvement of velocities and flow volume after TAVI in either the supine position or after orthostatic stress; therefore, it can be a very valuable finding, especially regarding everyday activities but also quality of life. Patients with severe AS should routinely undergo a duplex ultrasound examination of the carotid and vertebral arteries as a part of a comprehensive assessment before AVR or TAVI. In patients with severe AS and preserved left ventricle ejection fraction, stroke volume may be reduced as compared to healthy subjects, as well as patients with even mild AS. These may negatively impact peripheral and cerebral blood flow. More interestingly, changes in cerebral blood flow can be even more pronounced during orthostatic stress. The fall in stroke volume can lead to a reduction in peripheral arterial blood flow. Stroke volume falls even further if the ventricle begins to exhibit systolic and diastolic dysfunction. In our study we enrolled only patients with preserved left ventricle ejection fraction. Orthostatic‐induced changes in duplex echocardiographic measures of transaortic gradient in patients with AS have been reported recently.12 Changes in carotid and vertebral artery blood flow in patients with severe AS have also been reported.9 In a study by Kleczyński et al.,9 a significantly higher blood velocity drop in carotid and vertebral arteries during orthostatic stress was found in patients with AS compared to healthy subjects. Standing is a fundamental activity of daily life and may induce a fall in cardiac patients predisposed to syncope. Importantly, syncope is recognized as an important problem in patients with cardiac disease, especially with left ventricular preload dependence. The orthostatic test is a provocative maneuver that is definitely physiologically based. In our study, we found that after orthostatic stress, there is also a small drop in blood velocities and flow volume that is numerically lower in contrast to baseline values. This may be a result of an almost physiological gradient across the prosthesis. Nonetheless, the gradient remains somewhat higher compared to a native healthy aortic valve, resulting in limiting the peripheral blood flow. Another factor that may contribute to our findings is the age of the patients (median of 83 years) influencing the orthostatic response. On the other hand, it remains unclear whether orthostatic stress evokes regional differences in cerebral blood flow. Cerebral blood flow in humans is greater in the supine position compared to the seated or upright positions.13 Sato et al showed that blood flow in the internal carotid artery and medial cerebral artery were reduced during the head‐up tilt test, but vertebral artery blood flow was maintained by dilatation of territories of the vertebrobasilar system.14 Furthermore, Ogoh et al recently provided data showing that the effect of graded orthostatic stress on vertebral artery blood flow is different than observed in the internal carotid artery. This response suggests that orthostatic tolerance may be associated with hemodynamic changes in posterior rather than anterior cerebral blood flow.15 Another recent study by Ogoh et al was conducted to identify the effects of shift in venous drainage from the brain on the cerebral circulation. Importantly, this study addressed both arterial and venous flow responses in the anterior and posterior parts of the brain. In supine humans, the main drainage from the brain is through the internal jugular vein, but the vertebral veins become important during orthostatic stress, because the internal jugular vein is partially collapsed. Results of the study support that vertebral vein compensates for the reduction in internal jugular vein blood flow when seated, and that the vertebral vein may influence vertebral artery blood flow.16
AS is known to modify initial upstroke time of velocity in peripheral arteries and carotid velocities. Cardon et al conducted a prospective study in 30 patients scheduled for AVR for AS. The goal was to establish postoperative correction of carotid flow disorders. Postoperatively (after surgical AVR), the authors observed a large decrease of initial upstroke time and higher peak systolic velocities in all studied arteries.17
TAVI is a favorable treatment option for elderly patients with severe AS who are at a high risk for surgery. It not only improves survival in patients with AS treated conservatively but is also noninferior to surgical treatment regarding survival in selected patients.18 A biological prosthesis implanted in a native aortic position provides and almost physiological gradient across the valve, and thus may restore peripheral arterial blood by reducing the afterload. In our cohort of patients, no significant paravalvular leak was observed after the procedure, which may have an impact on peripheral blood flow and mortality.19 After TAVI, high outflow resistance caused by a reduction in the valve orifice area while opening is diminished. This causes an initially large pressure gradient to occur across the aortic valve during ejection, such that the peak systolic pressure within the ventricle is significantly reduced. This leads to a decrease in ventricular wall stress (afterload), an increase in stroke volume, and a decrease in end‐systolic volume. Stroke volume increases because the velocity of fiber shortening is increased by the decreased afterload. The rise in stroke volume may lead to increased velocities in peripheral arteries.
4.1. Study limitations
The exclusion criteria of the study significantly constrained patient recruitment and resulted in relatively small sample size.
5. CONCLUSION
We evaluated the blood flow in extracranial arteries before and after TAVI and found improvement of velocities and flow volume after TAVI. TAVI seems to have a beneficial effect on cerebral blood flow. However, larger clinical investigations are required to support our hypothesis.
Conflicts of interest
The authors declare no potential conflicts of interest.
Kleczyński P, Dimitrow PP, Dziewierz A, Surdacki A and Dudek D. Transcatheter aortic valve implantation improves carotid and vertebral arterial blood flow in patients with severe aortic stenosis: practical role of orthostatic stress test Clin Cardiol, 2017;40:492–497. 10.1002/clc.22684
REFERENCES
- 1. Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. [DOI] [PubMed] [Google Scholar]
- 2. Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med . 2010; 363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 4. Bagienski M, Kleczynski P, Dziewierz A, et al. Early and mid‐term outcomes after transcatheter aortic valve implantation. Data from single center registry. Postepy Kardiol Interwencyjnej . 2016;12:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kleczyński P, Bagieński M, Sorysz D, et al. Short‐ and intermediate‐term improvement of patient quality of life after transcatheter aortic valve implantation: a single‐center study. Kardiol Pol. 2014;72:612–616. [DOI] [PubMed] [Google Scholar]
- 6. Kleczyński P, Bagieński M, Dziewierz A, et al. Twelve‐month quality of life improvement and all‐cause mortality in elderly patients undergoing transcatheter aortic valve replacement. Int J Artif Organs. 2016;39:444–449. [DOI] [PubMed] [Google Scholar]
- 7. Vahanian A, Alfieri O. Guidelines on valvular heart disease in clinical practice. EuroIntervention. 2013;9: S11–S13. [DOI] [PubMed] [Google Scholar]
- 8. Landes U, Barsheshet A, Finkelstein A, et al. Temporal trends in transcatheter aortic valve implantation, 2008‐2014: patient characteristics, procedural issues, and clinical outcome [published online October 26, 2016]. Clin Cardiol . doi: 10.1002/clc.22632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kleczyński P, Dimitrow PP, Dziewierz A, et al. Decreased carotid and vertebral arterial blood‐flow velocity in response to orthostatic unload in patients with severe aortic stenosis. Cardiol J. 2016;23: 393–401. [DOI] [PubMed] [Google Scholar]
- 10. Dimitrow PP, Cheng TO. Standing position alone or in combination with exercise as a stress test to provoke left ventricular outflow tract gradient in hypertrophic cardiomyopathy and other conditions. Int J Cardiol. 2010;143:219–222. [DOI] [PubMed] [Google Scholar]
- 11. Kleczyński P, Sorysz D, Rzeszutko Ł, et al. Current approach to transfemoral aortic valve replacement. Kardiol Pol. 2013;71:203–204. [DOI] [PubMed] [Google Scholar]
- 12. Dimitrow PP, Sorysz D. Orthostatic stress echocardiography as a useful test to measure variability of transvalvular pressure gradients in aortic stenosis. Cardiovasc Ultrasound. 2013;24;11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eicke BM, von Schlichting J, Mohr‐Ahaly S, et al. Lack of association between carotid artery volume blood flow and cardiac output. J Ultrasound Med. 2001;20:1293–1298. [DOI] [PubMed] [Google Scholar]
- 14. Sato K, Fisher JP, Seifert T, et al. Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp Physiol. 2012; 97:1272–1280. [DOI] [PubMed] [Google Scholar]
- 15. Ogoh S, Sato K, Okazaki K, et al. Blood flow in internal carotid and vertebral arteries during graded lower body negative pressure in humans. Exp Physiol. 2015;100:259–266. [DOI] [PubMed] [Google Scholar]
- 16. Ogoh S, Washio T, Sasaki H, et al. Coupling between arterial and venous cerebral blood flow during postural change. Am J Physiol Regul Integr Comp Physiol. 2016;311:R1255–R1261. [DOI] [PubMed] [Google Scholar]
- 17. Cardon C, Chenon D, Meimoun P, et al. Effect of aortic valve replacement for aortic stenosis on cervical arterial blood flow [in French]. Arch Mal Coeur Vaiss. 2001;94:103–107. [PubMed] [Google Scholar]
- 18. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 19. Kleczyński P, Zasada W, Bagieński M, et al. Paravalvular leak after TAVI: short‐term results. Data from Polish national POL‐TAVI registry. Cardiol J 2016;23:163–168. [DOI] [PubMed] [Google Scholar]


