Abstract
While reportedly a relatively common finding at the autopsy of decedents with metastatic neoplasms, dural metastases are infrequently described in the medical literature and only 55 cases of subdural hemorrhage associated with dural metastases have been described, with only one of these cases associated with head trauma. We report a 50-year-old incarcerated male who died as the result of acute and chronic subdural hemorrhage associated with recent minor head trauma and dural metastases, which were most likely of pancreatic origin. He had sustained a fall, possibly due to a seizure in his jail cell, developed an acute subdural hemorrhage, and died, necessitating an autopsy. Metastatic tumor in the dura and other organs was identified upon histologic examination and found to be CK7 and CK20 positive and TTF-1 and CDX2 negative, consistent with a pancreatic adenocarcinoma. In addition, marantic endocarditis was identified, which can occur in individuals with mucinous tumors, such as a pancreatic neoplasm. This case report offers the second description of a subdural hemorrhage occurring in association with both dural metastases and recent head trauma and confirms the importance of histologic examination of the subdural hemorrhage and adjacent dura at autopsy for reasons other than just timing of the event.
Keywords: Forensic pathology, Forensic, Autopsy, Subdural hemorrhage, Dura, Metastases
Introduction
Up to 8-9% of individuals who have died from or with metastatic neoplasms are reported to have dural metastases at autopsy; however, only 198 cases of dural metastases were described in the medical literature between 1904 and 2003 (1). The mechanism of dural metastasis is most often through hematogenous spread or direct invasion from cranial metastases although direct extension to the dura from a cerebral parenchymal metastasis may rarely occur (1). Occasionally, dural metastases are associated with a chronic subdural hemorrhage, with 55 such cases reported in the literature (2). Head trauma is also a well-known cause of a subdural hemorrhage; however, the combination of well-documented head trauma, dural metastases, and a subdural hemorrhage has been reported apparently only once (PubMed search [www.ncbi.nlm.nih.gov/pubmed/] using the key words, “subdural”, “trauma”, and “metastasis”). We report a similar case of a dural metastatic adenocarcinoma in an individual with recent head trauma and subdural hemorrhage.
Case Report
The decedent was a 50-year-old incarcerated male who had reportedly struck his head as the result of a fall; however, following the autopsy, viewing of video recordings related to the reported fall indicated that he may instead have had a seizure causing a subsequent fall of less than one foot in height. Although he was active in the interim, three days after the traumatic event he was found unresponsive in his jail cell and then transported to the local hospital where an acute subdural hemorrhage was diagnosed by computed tomography (CT) scan. He was not considered a neurosurgical candidate and subsequently died. Scene investigation revealed scattered smears of blood on the floor and jail bed and blood-soaked towels in the cell, with medical personnel estimating a blood loss of 500 mL. His past medical history included diabetes mellitus, drug and alcohol abuse, hepatitis C, and a remote below the knee amputation. An autopsy was performed.
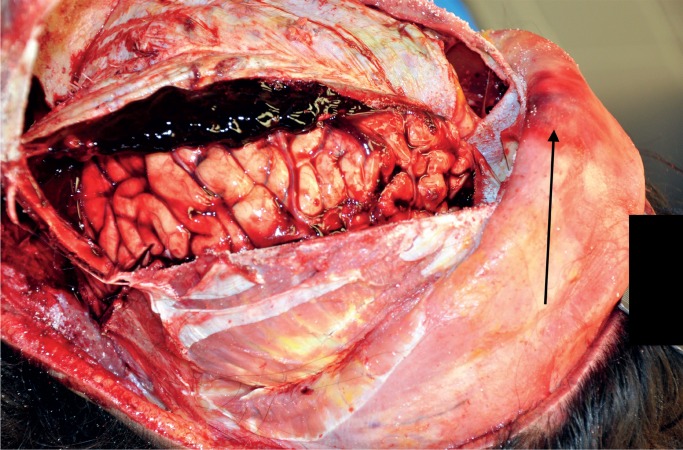
External examination revealed a 3.5 cm crusted abrasion on the bridge of his irregularly angled nose (comment: possible remote non-healed nasal fracture) and a few scattered contusions of the trunk and extremities. Internal examination revealed a 3.5 cm subgaleal hemorrhage in the midline of the frontal region of the scalp and that the cranium was intact; however, loosely adherent subdural hemorrhage was at the cerebral convexities and floors of the middle and posterior cranial fossae, with the largest collection at the right cerebral convexity, which was estimated at 20 mL in volume, consistent with an acute subdural hemorrhage (Image 1). In addition, the dura was thickened, suggestive of a remote subdural hemorrhage. No obvious mass lesions were identified in the thickened dura mater; representative sections were obtained for the purpose of microscopic aging of the subdural hemorrhage. Enlarged peri-aortic and peri-pancreatic nodes were identified at autopsy and initially felt to represent either a reactive process or potentially a lymphoma based upon their homogenous, smooth, tan glossy texture, but were confirmed to have metastatic adenocarcinoma by microscopic examination. The mitral valve had a small (0.2-0.3 cm) sterile vegetation (i.e., nonbacterial thrombotic endocarditis or marantic endocarditis). The liver did not appear cirrhotic grossly; however, microscopic examination revealed increased fibrosis of the portal tracts with some septae and occasional ill-defined nodules. Other autopsy findings included cerebral edema with associated secondary brainstem hemorrhage (i.e., Duret hemorrhage), consistent with the clinical diagnosis of herniation (Image 2), mild coronary artery atherosclerosis, a heart weight of 560 g, and features suggestive of anemia (e.g., pale conjunctivae, pale renal cortices, and essentially no lividity, even given the greater than 18 hours elapsed between death and autopsy).
Image 1:
Acute subdural hemorrhage. The black arrow indicates the frontal subgaleal hemorrhage.
Image 2:
Secondary brainstem hemorrhage (i.e., Duret hemorrhage). The patient had a history of herniation.
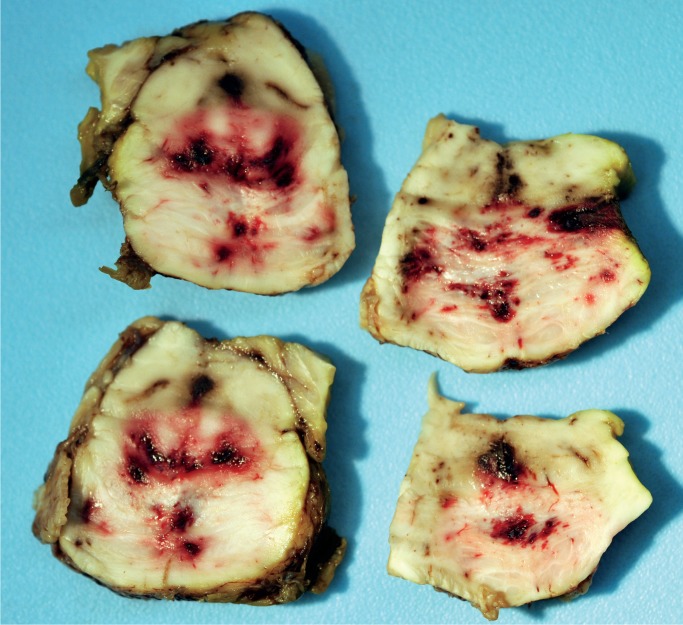
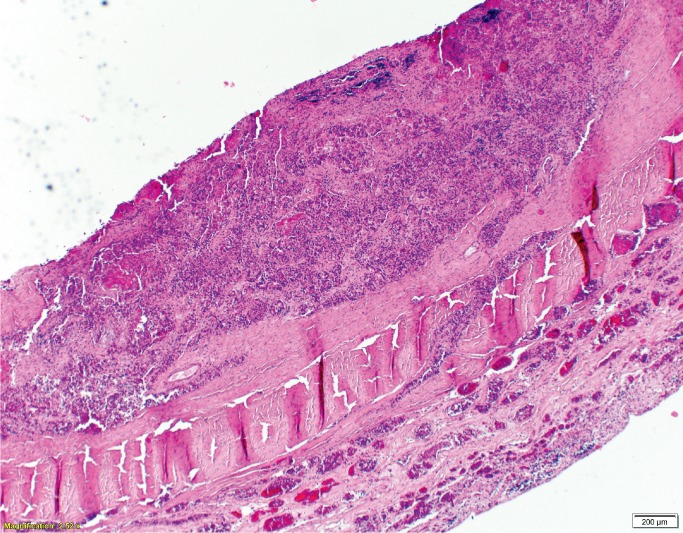
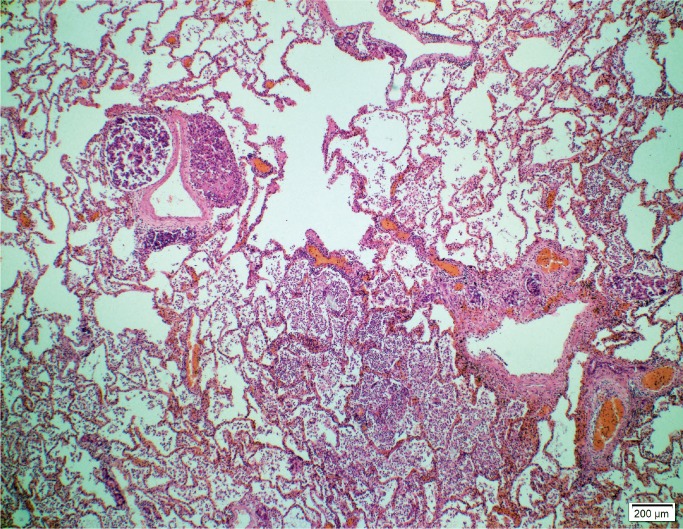
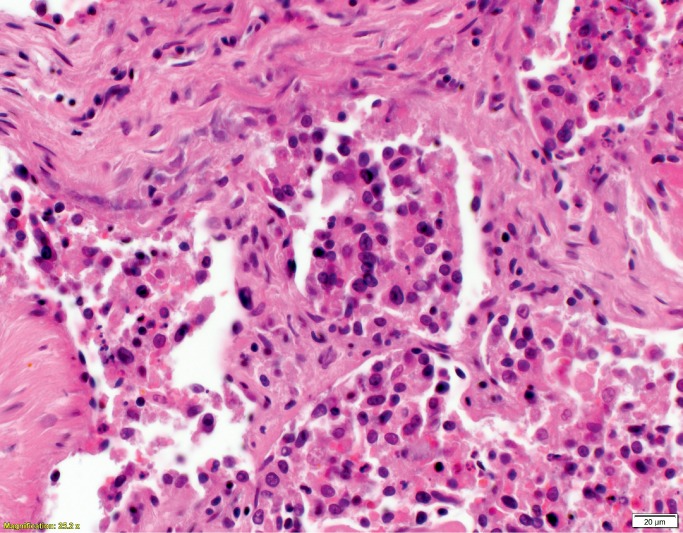
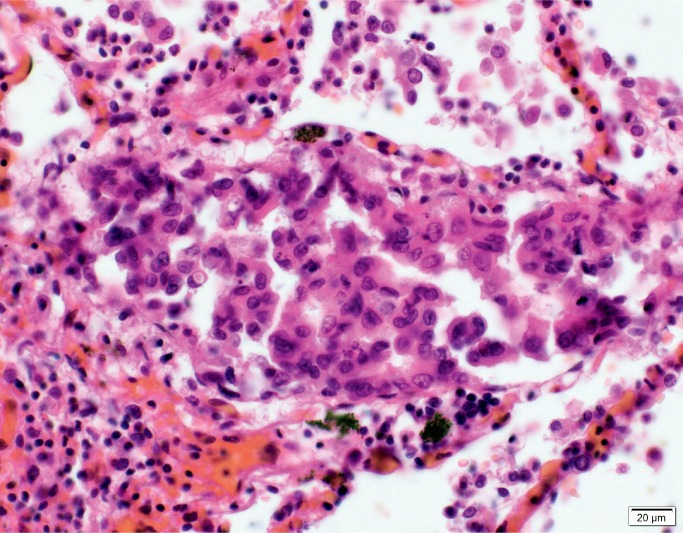
Histologic examination of the subdural hemorrhage and dura mater revealed areas with aggregates of intact red blood cells and a focal thin layer of inflammatory cells and fibroblast-like cells between the red blood cells and the dura mater and other areas of a thick layer of fibrous tissue with pockets of fibrin, red blood cells, and neoplastic cells (Image 3). In addition to the collections of neoplastic cells associated with the subdural hemorrhage, histologic examination identified metastatic neoplasm in the pituitary gland, lungs, left adrenal gland, and lymph nodes (Image 4). The tumor had focal glandular differentiation and evidence of mucus production, consistent with an adenocarcinoma. Immunohistochemical staining of the neoplasm revealed strong positivity for CK7 and CK20 but was negative for TTF-1 and CDX2. No primary source of a neoplasm was identified by gross examination. The small and large intestine were not opened; however, during evisceration, no abnormality of either was identified (e.g., no firm palpable mass, focal fibrosis, or other form of adherence). The testes were not examined. The pancreas had been serially sectioned.
Image 3A:
Dural metastases (H&E, x40).
Image 4A:
Pulmonary metastases (H&E, x40)
Image 3B:
Dural metastases (H&E, x400).
Image 4B:
Pulmonary metastases (H&E, x400).
The cause of death was certified as complications of acute on chronic subdural hemorrhage associated with dural metastatic adenocarcinoma and recent minor head trauma. The decedent’s diabetes mellitus and chronic alcoholism were listed as other significant conditions. The manner of death was certified as accident.
Discussion
The number of cases in the medical literature describing subdural hemorrhage associated with dural metastases is small, and only one of these previously reported cases was associated with head trauma (3). The mechanisms proposed by other authors for the development of a subdural hemorrhage in the background of dural metastases are rupture of blood vessels formed by neo-vascularization of the hemorrhage, obstruction of dural venous vessels leading to dilation and rupture, or a preexisting subdural hemorrhage serving as a medium for the deposition of blood-borne metastases (1). The tumor type associated with this process is not specific. In their review, which included their own two cases and eleven other cases of metastatic tumor to the dura associated with subdural hemorrhage previously described in the literature, Ambiavagar and Sher listed the source tumor as prostate (three), pancreas (two), stomach (two), and one each for penis, breast, lung, cervix, Ewing’s sarcoma, and anaplastic carcinoma (no site specified) (4). Laigle-Donadey et al. added to this list of causative neoplasms as they indicated that the most common sources of metastases to the dura are prostate, breast, lung, and stomach neoplasms (1); however, 45% of dural metastases are due to a wide variety of tumor types other than those just listed, with pancreatic neoplasms representing only three reported cases. In this current case, the neoplasm was an adenocarcinoma; however, the definitive primary site was not identified at autopsy. Immunohistochemical staining of the tumor was CK7 and CK20 positive and TTF-1 and CDX2 negative; tumors that are often TTF-1 negative and CK7 and CK20 positive include ampullary adenocarcinoma, bronchioalveolar adenocarcinoma (comment: 21% were positive for TTF-1), signet ring cell carcinoma of the stomach, transitional cell carcinoma, and ovarian mucinous cystadenocarcinoma (5), none of which were identified at autopsy, and lung adenocarcinomas are most often TTF-1 positive (5). As the intestine was not examined at the time of autopsy, metastatic colonic adenocarcinoma was a consideration; however, of 12 cases of metastatic colonic adenocarcinoma, Roy et al. found that 100% stained positive for CK20 and CDX2, whereas only one stained positive for CK7 (6). Therefore, the source of the metastases to the dura was not likely from the colon. Two distinctly possible sources for the metastatic tumor to the dura based upon the immunohistochemical staining pattern are the bladder and the pancreas. Of five cases of urothelial carcinoma, 100% stained positive for CK7, 40% stained positive for CK20, and none stained positive for CDX2 (5). Of tumors that are most often considered in a TTF-1 negative, CK7 positive, and CK20 negative group, gastric adenocarcinoma, pancreatic adenocarcinoma, cholangiocarcinoma, and neuroendocrine carcinoma of the ampulla of Vater most commonly have CK20 positivity (around 40+% of cases) (5). No lesion of the stomach, gallbladder, or ampulla of Vater was identified and the bladder and renal pelves were inspected at autopsy and no tumor was identified; however, a pancreatic neoplasm could have been missed during sectioning of that organ.
As described above, the mitral valve had nonbacterial thrombotic endocarditis, a condition that is associated with metastatic neoplasms (7). In their review of 16 individuals with nonbacterial thrombotic endocarditis, Rohner et al. found that seven had mucinous malignancies, three of the stomach, two of the lung, one of the colon, and one of the pancreas (8). As the bladder and stomach were examined and no tumor was identified nor was thickening of the stomach wall identified, one likely possibility, if the marantic endocarditis identified at autopsy was the result of a neoplasm, is a pancreatic adenocarcinoma. As stated previously, the main primary tumors that metastasize to the dura are those of the prostate, breast, lung, and stomach. While a definitive diagnosis of a primary pancreatic adenocarcinoma cannot be confirmed in the current case, immunohistochemical staining, combined with the autopsy findings, is most consistent with a metastatic pancreatic adenocarcinoma. Previously, only three cases of dural metastases from a pancreatic primary have apparently been recorded in the literature (1).
Conclusion
In addition to 1) the presentation of a condition that is rarely reported in the medical literature (i.e., dural metastases associated with a subdural hemorrhage) and 2) the second apparent report of a subdural hemorrhage occurring in an individual with both dural metastases and recent head trauma, this report also illustrates that while histologic examination of the dura mater and a subdural hemorrhage can be used to help determine the age, examination may also help identify an underlying causative or contributory etiology. In this current case, the subdural hemorrhage had at least two ages (with both a more recent and a more remote component identified upon histologic examination). The timing of the events combined with what was previously described in the medical literature by Tomlin et al. suggest that the dural metastases may have most contributed to the chronic component of the subdural hemorrhage, whereas the head trauma contributed most to the acute component (3). However, given the relatively minor nature of the head trauma, it is our opinion that the dural metastases may have contributed to a greater propensity to bleed as dural metastases by themselves have been shown to increase the risk for the development of a spontaneous subdural hemorrhage through the mechanisms of neo-vascularization or dural sinus obstruction.
Authors
Janet R. Julson BS, University of North Dakota School of Medicine and Health Sciences - Pathology
Roles: Project conception and/or design, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work, principal investigator of the current study.
Timothy Weiland MD, Altru Hospital - Pathology
Roles: Data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work, writing assistance and/or technical editing.
Walter L. Kemp MD PhD, University of North Dakota School of Medicine and Health Sciences - Pathology
Roles: Project conception and/or design, data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work, general supervision, writing assistance and/or technical editing.
Footnotes
Ethical Approval: As per Journal Policies, ethical approval was not required for this manuscript
Statement of Human and Animal Rights: This article does not contain any studies conducted with animals or on living human subjects
Statement of Informed Consent: No identifiable personal data were presented in this manuscript
Disclosures & Declaration of Conflicts of Interest: The authors, reviewers, editors, and publication staff do not report any relevant conflicts of interest
Financial Disclosure: The authors have indicated that they do not have financial relationships to disclose that are relevant to this manuscript
References
- 1). Laigle-Donadey F, Taillibert S, Mokhtari K, et al. Dural metastases. J Neurooncol. 2005. October; 75(1):57–61. PMID: 16215816 10.1007/s11060-004-8098-1. [DOI] [PubMed] [Google Scholar]
- 2). George KJ, Lau A, Ellis M, et al. Metastatic coagulopathic subdural hematoma: a dismal prognosis. Surg Neurol Int. 2012; 3:60 PMID: 22754725. PMCID: PMC3385075 10.4103/2152-7806.97004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Tomlin JM, Alleyne CH. Transdural metastasis from adenocarcinoma of the prostate mimicking subdural hematoma: case report. Surg Neurol. 2002. November; 58(5):329–31; discussion 331. PMID: 12504300 10.1016/s0090-3019(02)00835-2. [DOI] [PubMed] [Google Scholar]
- 4). Ambiavagar PC, Sher J. Subdural hematoma secondary to metastatic neoplasm: report of two cases and a review of the literature. Cancer. 1978. October; 42(4):2015–8. PMID: 361219 . [DOI] [PubMed] [Google Scholar]
- 5). Jagirdar J. Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma of the lung. Arch Pathol Lab Med. 2008. March; 132(3):384–96. PMID: 18318581 10.1043/1543-2165(2008)132[;384:AOITTD];2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6). Roy S, Smith MA, Cieply KM, et al. Primary bladder adenocarcinoma versus metastatic colorectal adenocarcinoma: a persisting diagnostic challenge. Diagn Pathol. 2012. November 2; 7:151 PMID: 23121893. PMCID: PMC3502416 10.1186/1746-1596-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Liu J, Frishman WH. Nonbacterial thrombotic endocarditis: pathogenesis, diagnosis, and management. Cardiol Rev. 2016. Sep-Oct; 24(5):244–7. PMID: 27501336 10.1097/CRD.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 8). Rohner RF, Prior JT, Sipple JH. Mucinous malignancies, venous thrombosis and terminal endocarditis with emboli. A syndrome. Cancer. 1966. December; 19(12):1805–12. PMID: 5927940 . [DOI] [PubMed] [Google Scholar]