Abstract
Histologic examination of the myocardium, valves, and cardiac blood vessels is often as important as the gross examination. The diagnostic features and categories of heart disease are many and varied, possibly more than any other organ. We present a review of the histologic features of forensically important heart disease.
Keywords: Forensic pathology, Cardiovascular pathology, Autopsy, Heart, Sudden death
Introduction
Heart histology is not always required in a thorough forensic autopsy. For example, gross diagnosis of severe coronary artery atherosclerosis, myocardial infarcts, or aortic dissection is generally adequate for determining cause and manner of death and many pathologists will not routinely perform histologic examination in those cases. The diagnosis of many other forms of heart disease, however, requires histologic examination. Cardiomyopathies, myocarditis, endocarditis, nonatherosclerotic coronary arterial disease, and conduction system abnormalities are all diagnosed microscopically. Our recommendation for adequate histologic sampling of the heart is presented in “Adult Heart Dissection Technique” (1). We present a brief atlas of forensically important cardiovascular histopathology.
Discussion
Hypertensive and Atherosclerotic Disease
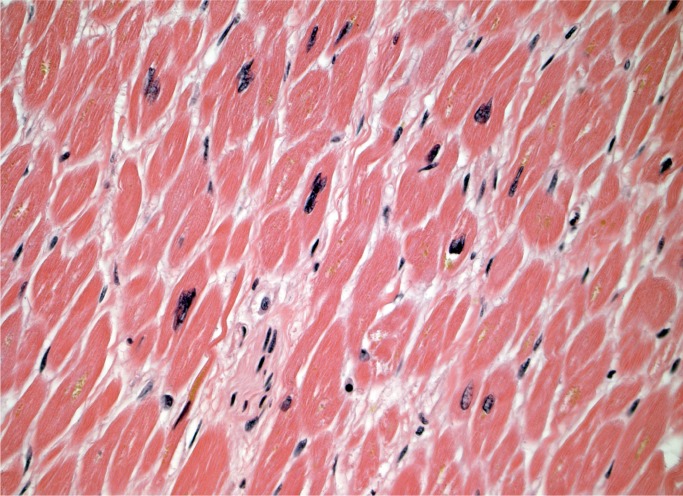
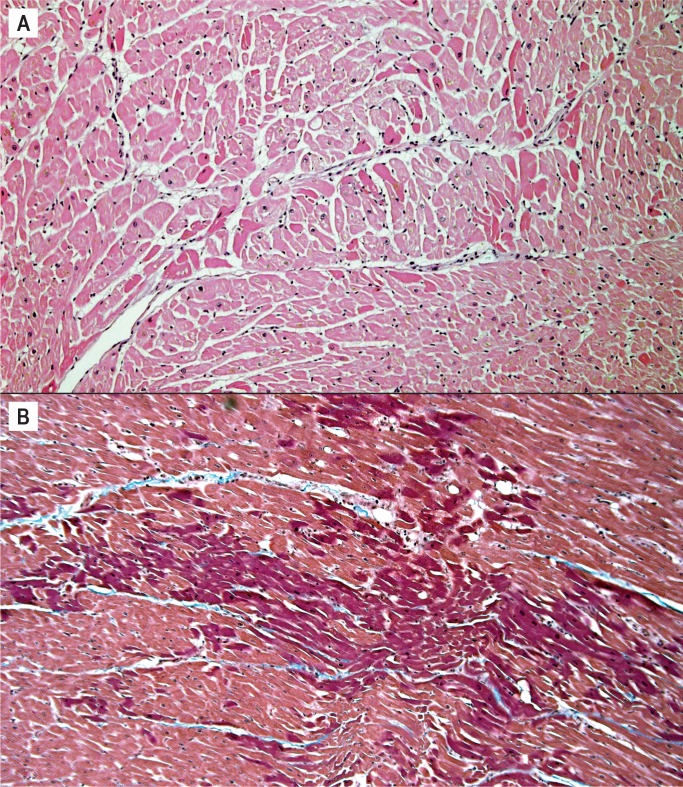
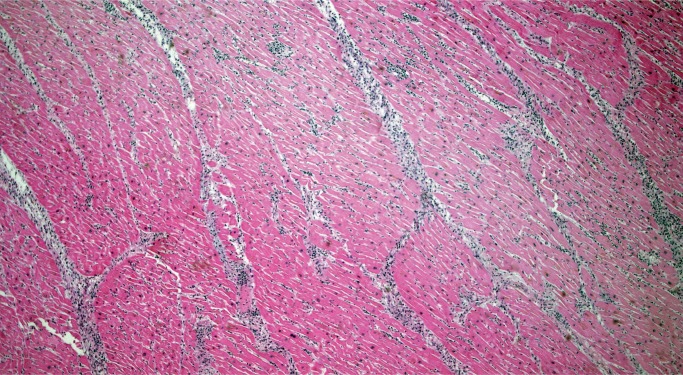
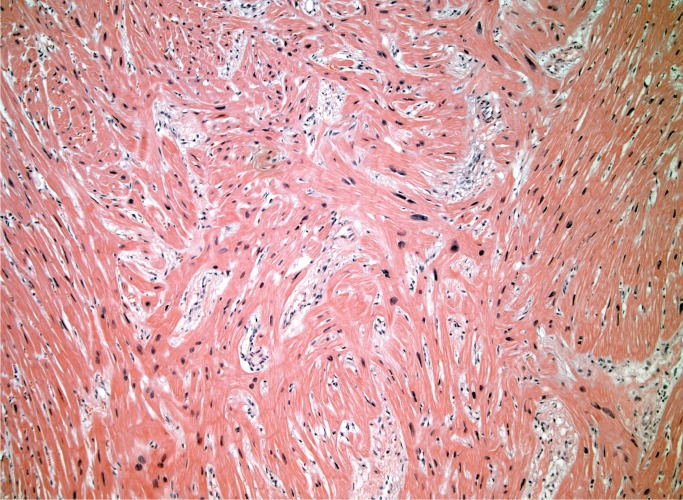
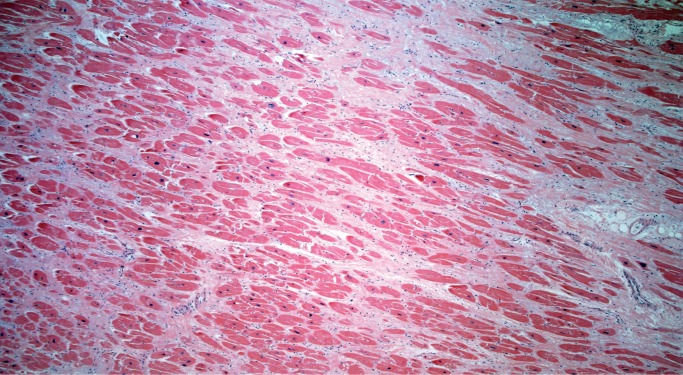
Myocyte hypertrophy and fibrosis are nonspecific histologic findings, but also some of the most commonly encountered in forensic cases. Myocyte hypertrophy is best evaluated in correlation with heart size. The classic histologic description is rectangular, hyperchromatic nuclei, often called “box-car” nuclei (Image 1). Myocytes may be obviously enlarged or barely noticeably enlarged, which is why correlation with heart size in crucial.
Image 1:
Hypertrophic myocytes with “boxcar” nuclei (H&E, x400).
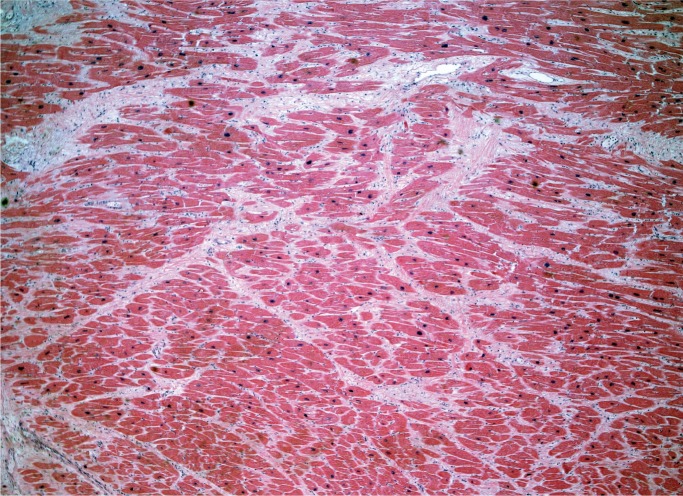
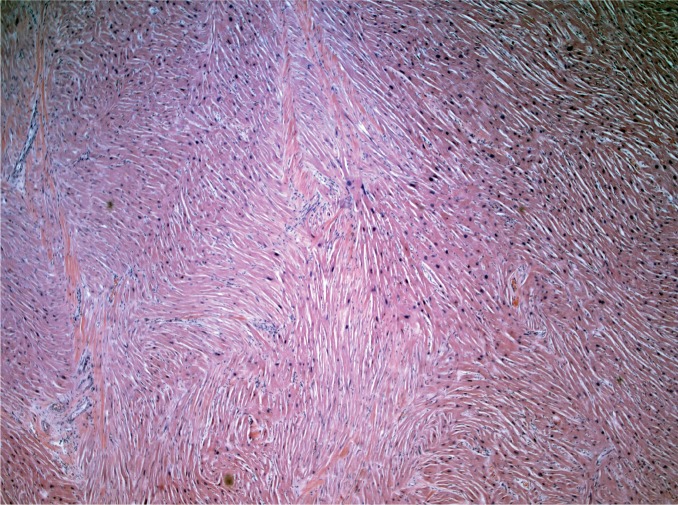
Myocardial fibrosis can be divided into three main categories: interstitial, perivascular, and replacement-type fibrosis. Interstitial and perivascular fibrosis often go together and increase with age. It is a nonspecific finding and presents in a variety of cardiac conditions such as hypertensive heart disease, cardiomyopathies, and small vessel disease (Image 2). Replacement fibrosis is characterized as areas of fibrosis not large enough to be considered an infarct, typically less than 3 mm in size. It too is nonspecific and can be seen in a number of conditions including ischemia, healed myocarditis, hypertensive heart disease, and cardiomyopathies (2).
Image 2:
Interstitial fibrosis (H&E, x40).
Coronary Artery Disease and Myocardial Infarct
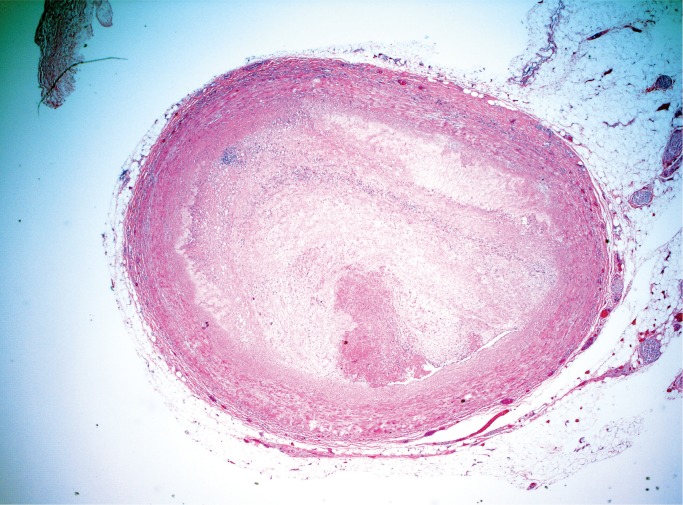
Atherosclerotic coronary artery disease is a regular part of any forensic practice and frequently does not require histologic examination. It is recommended, however, to at least retain the most severely narrowed arterial segments in a stock jar in case questions arise later. It is good practice to submit a section of a suspected coronary thrombus to differentiate postmortem clot or plaque hemorrhage grossly appearing as luminal thrombus (Image 3) (3).
Image 3:
Coronary artery thrombus (H&E, x20).
Aging Infarcts
Aging of myocardial infarcts is best done histologically. Myocardial infarcts heal from the borders inward and larger infarcts take longer to heal, accordingly it is important that sections are submitted from the margins of the infarct. Sections from the central portion of the infarct will incorrectly place the infarct at a younger age.
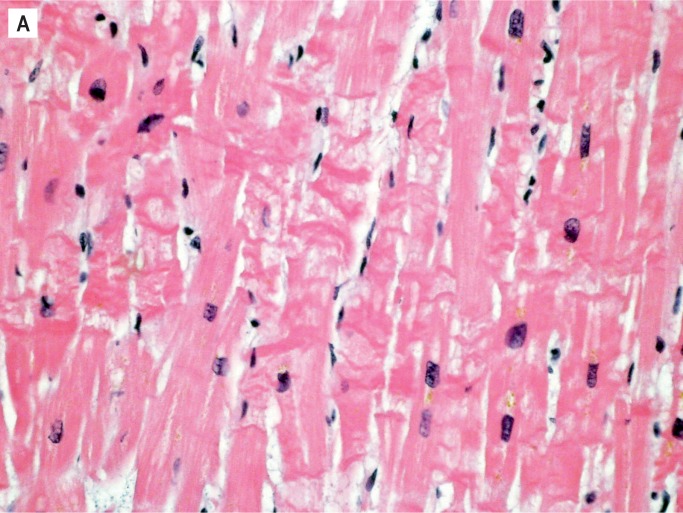
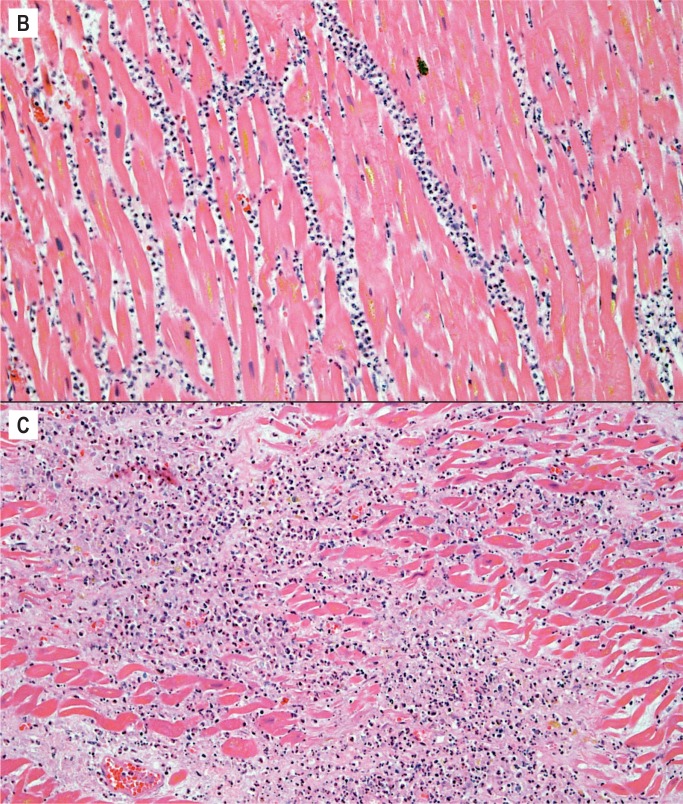
The histologic aging of infarcts requires evaluating the presence and extent of several criteria and their relationship to one another. These criteria include the appearance of the myocyte, type and extent of interstitial inflammatory cell infiltrate, and presence and extent of collagen deposition. Over the course of the healing process, the predominant histologic feature changes. Early in an infarct, it is hypereosinophilia of myocytes, usually accompanied by contraction bands and loss of myocyte nuclei (Image 4A). Myocytes may have a wavy appearance and there may be interstitial edema; these histologic changes can be appreciated by 4-12 hours. The next to appear is the interstitial inflammatory cell infiltrate. Neutrophils arrive first and are the hallmark of early ischemia, defined as the first 72 hours. They are initially seen in perivascular spaces as they marginate through blood vessels. In the first 24-48 hours, neutrophils increase within the interstitium (Image 4B), and by 48-72 hours they start to degenerate. Essentially, the interstitial neutrophils are mainly intact in the first 48 hours, there is an approximately equal mix of intact and degenerating neutrophils from 24-72 hours, and degenerating neutrophils and abundant cellular debris predominate closer to 72 hours (Image 4C). If blood flow is re-established in the early ischemic period, there may be abundant interstitial hemorrhage within the infarcted area, caused by the reperfusion.
Image 4:
Healing progression of a myocardial infarct. A) Four to 12 hours (H&E, x400).
Image 4:
Healing progression of a myocardial infarct. B) 24 to 48 hours (H&E, x200), C) Less than 72 hours (H&E, x200).
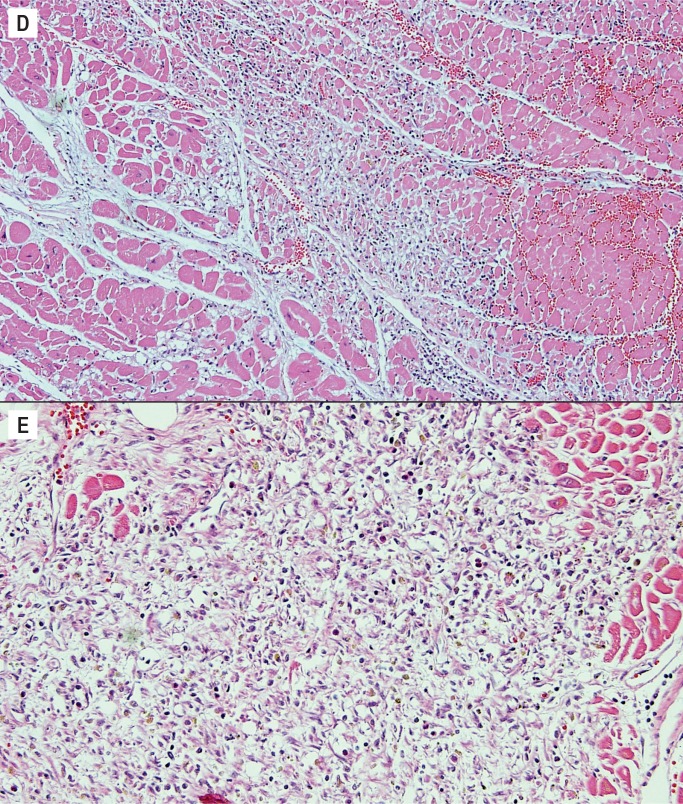
Around day three, the inflammatory cell infiltrate transitions to mononuclear cells. The neutrophils begin the phagocytic process of removing the ischemic myocytes, and this continues in the next stage of three to five days. At this stage, there is early removal of myocytes and the interstitial cells consist of lymphocytes, pigment laden histiocytes, and myofibroblasts. Collagen is not yet present (Image 4D). The interstitial cells continue to increase in the next stage, five to seven days. This is the most cellular stage of the healing process with large numbers of interstitial inflammatory cells (Image 4E). This stage also is the peak time period when ischemic myocytes have been removed and collagen has not yet been deposited. Therefore, this is a time of greatest weakness of the wall and is often the time period of acute myocardial rupture.
Image 4:
Healing progression of a myocardial infarct. D) Three to five days (H&E, x100), E) Five to seven days (H&E, x200).
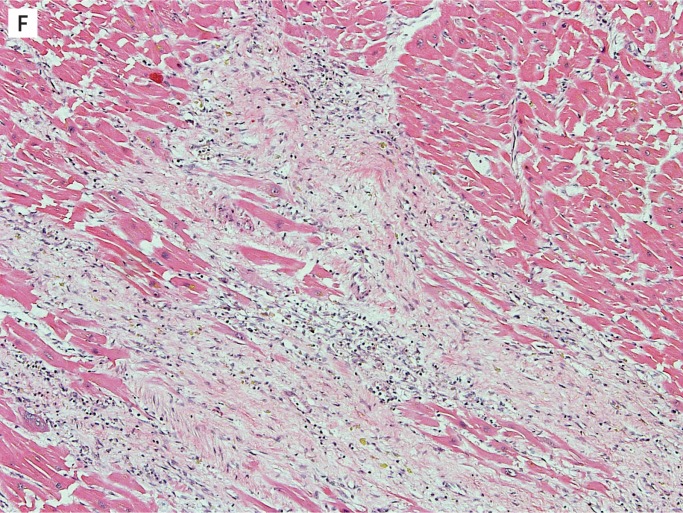
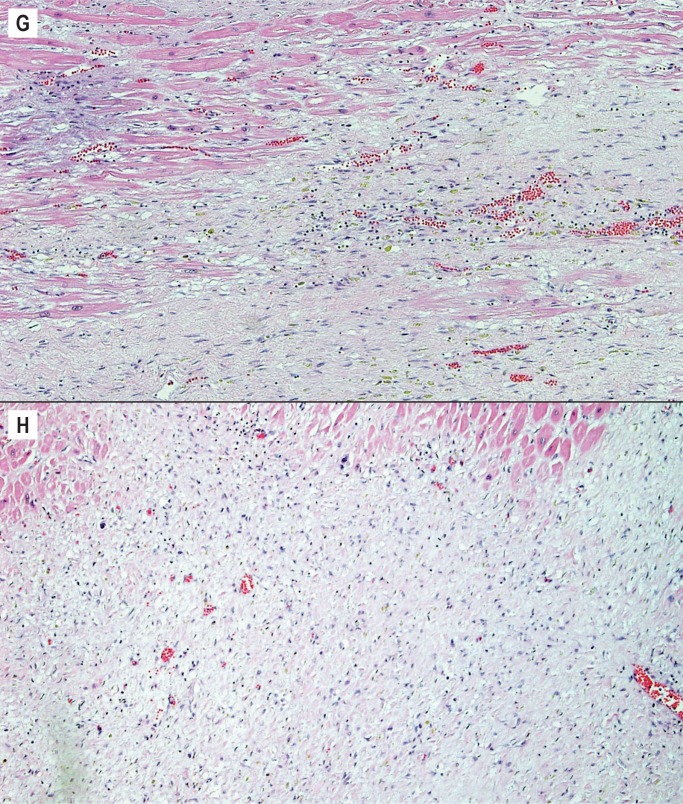
After one week, collagen deposition begins and the inflammatory cells begins to dissipate. From this point on, the age depends on the extent of collagen deposition and the extent of remaining inflammatory cells. By one to two weeks, there is only early collagen deposition and inflammatory cells are still numerous (Image 4F). By two to four weeks, the collagen deposition increases and there are fewer inflammatory cells (Image 4G). By one to two months, the collagen is well established and there are only a few residual inflammatory cells (Image 4H). After the two month period, the scar is formed and there is dense collagen with essentially no inflammation.
Image 4F:
Healing progression of a myocardial infarct, one to two weeks (H&E, x100),
Image 4:
Healing progression of a myocardial infarct. G) Two to four weeks (H&E, x100), H) One to two months (H&E, x100).
The well healed scar does undergo additional changes such as fatty infiltration and neovascularization, however, these are more variable features and an infarct is not further aged after two months (Image 4I). Remaining myocytes entrapped within areas of scar are common in a healed infarct and are a potential focus of arrhythmias (Image 4J) (4).
Image 4:
Healing progression of a myocardial infarct. I) Healed, greater than two months (H&E, x12.5), J) Greater than two months with entrapped myocytes within scar (H&E, x40).
Neurocardiogenic Injury
Neurocardiogenic injury, also known as Takotsubo cardiomyopathy, neurogenic stunned myocardium, or stress cardiomyopathy, is occasionally seen in forensic cases and may be mistaken for myocarditis. This condition is typically a transient myocardial dysfunction in the absence of coronary disease. It occurs as the result of a sudden surge of catecholamines delivered directly to the myocardium, or a period of global myocardial hypoperfusion, leading to diffuse myocardial injury. The sudden sympathetic stimulation resulting in a catecholamine surge may be due to any number of underlying causes. Intracranial processes (traumatic or nontraumatic) are the most common cause of neurocardiogenic injury, but it is also seen in asphyxial deaths such as hangings or drownings, overdoses, prolonged resuscitation in which catecholaminergic medications are administered, and is frequently seen confined to the right ventricle in cases of pulmonary embolus. It is also occasionally seen as the result of an extremely emotionally distressing life event with subsequent sudden collapse, the so-called “death by a broken heart.” Clinically, there may be electrocardiogram (EKG) changes and elevated troponin levels suggestive of ischemia, wall motion abnormalities on echocardiogram, and ventricular failure/ low ejection fraction, often in a patient with no heart disease but with other severe conditions as described above or in someone found unresponsive within an unknown down time interval (5). A previously hospitalized patient may come to autopsy with concerns of a cardiomyopathy or heart disease above and beyond the underlying cause of death.
On gross examination, the heart may be normal or there may be a mottled appearance to the myocardium. There is often an area of endocardial hemorrhage in the left ventricular aspect of the ventricular septum, which is part of this overall process and does not represent a contusion or other type of myocardial injury.
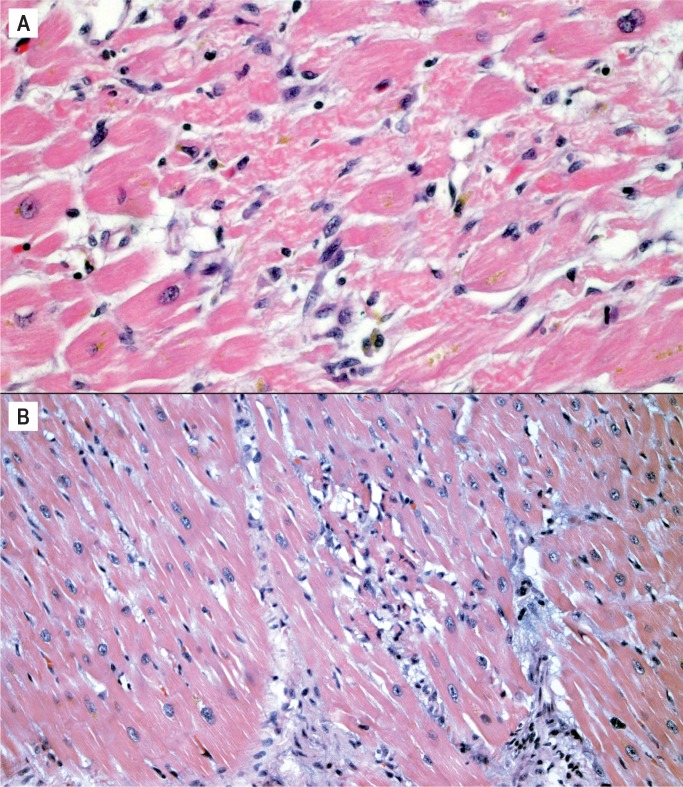
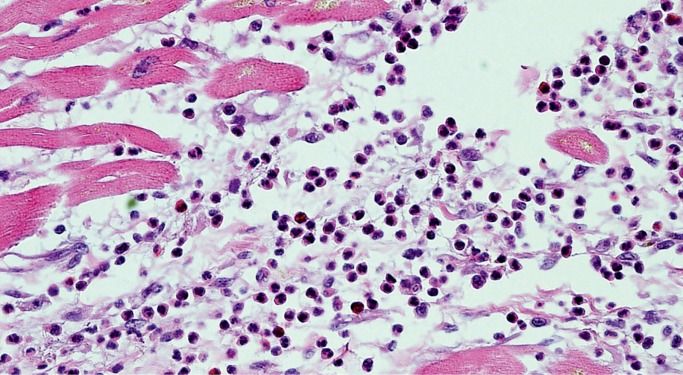
The histologic appearance of neurogenic cardiomyopathy is nonspecific, variable, and exists on a spectrum. It must be distinguished from both viral myocarditis and ischemic infarction. Although the myocyte injury resembles infarction, it does not progress in the same sequence and cannot be aged like infarcts. Diffuse hypereosinophilic myocytes, with or without contraction bands and cytoplasmic clumping (which are often seen together), may be seen in the earliest phase (Image 5). They are often surrounded by a mixed population of leukocytes and can be referred to as coagulative myocytolysis/contraction band necrosis as the process evolves (Image 6) (6 –8). An important aspect of this entity is its rapid appearance. The hypereosinophilic myocytes surrounded by leukocytes can happen within minutes. In the acute stage of this process, it may be confused with viral myocarditis; however, viral myocarditis does not have hypereosinophilic myocytes and contraction bands. In viral myocarditis, the predominant inflammatory cell is typically the lymphocyte. Lymphocytes surround myocytes and actively destroy them, but the myocytes may look normal histologically (Image 7A). This is in contrast to coagulative myocytolysis/contraction band necrosis, where leukocytes respond to an injured/hypereosinophilic myocyte (Image 7B).
Image 5:
Neurocardiogenic injury. A) Scattered hypereosinophilic myocytes (H&E, x100), B) Hypereosinophilic myocytes highlighted by a Masson trichrome stain (trichrome, x100).
Image 6:
Hypereosinophilic myocytes with contraction bands and cytoplasmic clumping surrounded by mixed inflammatory cells A) (H&E, x400), B) (H&E, x200).
Image 7:
Myocarditis vs. neurocardiogenic injury. A) Lymphocytic myocarditis with leukocytes surrounding and destroying structurally normal myocytes without associated hypereosinophilia or cytoplasmic clumping (H&E, x400); B) Coagulative myocytolysis/contraction band necrosis with hypereosinophilic myocytes and cytoplasmic clumping surrounded by a mixed population of leukocytes, differentiating it from lymphocytic myocarditis (H&E, x400).
Neurocardiogenic injury resolves if there is a survival period. Histologic features during this period include removal of the injured myocyte, collagen deposition, and the presence of pigment-laden histiocytes and myofibroblasts (Image 8). This process will also be patchy throughout the myocardium, which aids in distinguishing it from typical ischemia.
Image 8:
Resolving coagulative myocytolysis/contraction band necrosis with removal of injured myocytes, collage deposition, and remaining mononuclear inflammatory cells (H&E, x200).
Infiltrative Diseases
Lymphocytic and Mixed Cellular Myocarditis
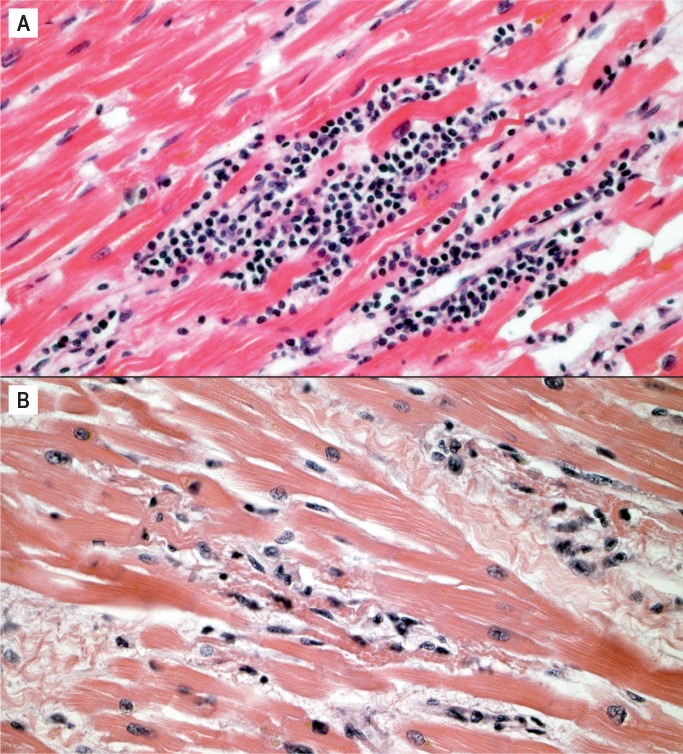
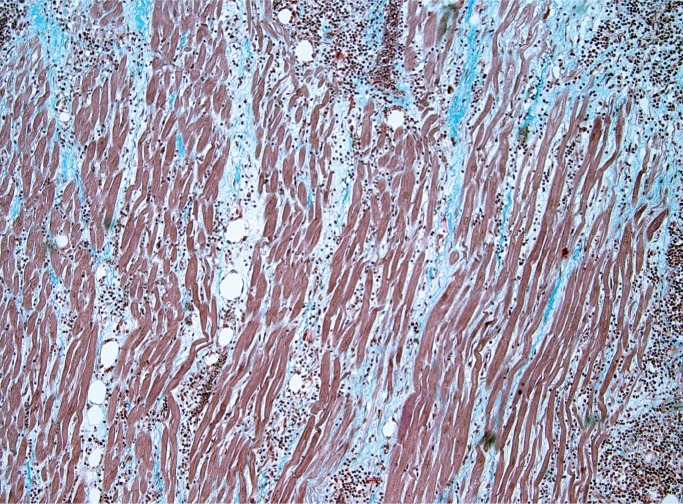
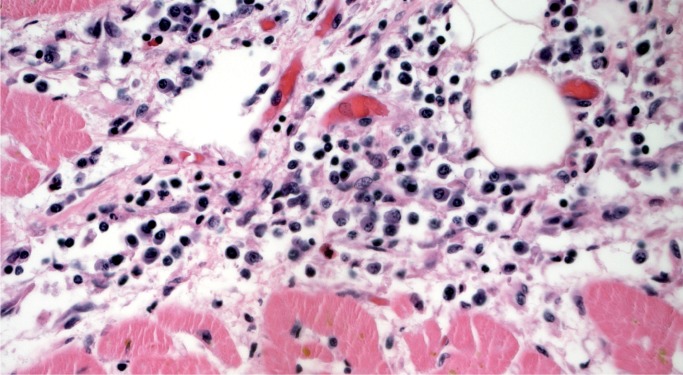
Myocarditis is a potential cause of sudden cardiac death at any age and is an important consideration in the forensic practice. As mentioned, it may be challenging to distinguish myocarditis from neurogenic cardiac injury (stress cardiomyopathy) or ischemic injury (Image 9). It is a microscopic diagnosis consisting of myocardial inflammation with associated myocyte injury. The cellular composition may be lymphocyte predominant (Image 10), lymphohistiocytic, or mixed cellular (with some eosinophils and/or neutrophils) (Image 11). If neutrophils are the dominant cell type, bacterial and fungal etiologies must be considered, possibly resulting from conditions such as endocarditis with abscesses and/or septic emboli, sepsis, or direct extension from pneumonia. The Dallas criteria are frequently cited as diagnostic requirements for myocarditis, which requires myocyte necrosis or degenerative changes to be seen, but these criteria were developed for endomyocardial biopsies and were never intended for postmortem or post-transplant cardiac examination (9). Myocarditis is not associated with myocyte injury away from areas of inflammation, which is part of what distinguishes it from ischemia and neurocardiogenic injury (Image 12). The myocyte injury pattern seen in postmortem cardiac sections consists of destruction of myocytes by inflammation, or patchy fibrosis associated with residual inflammation. Other authors have proposed criteria involving counting the number of lymphocytes or requiring immunohistochemical (IHC) stains for the diagnosis of myocarditis; this is of questionable practical utility in a forensic setting (9). Mixed cellular and lymphocytic myocarditis are presumably viral, although molecular and IHC testing for viruses on autopsy material is often low-yield.
Image 9:
Florid myocarditis with transmural lymphocytic inflammation with myocyte injury and removal (H&E, x20).
Image 10:
Lymphocyte predominant myocarditis (H&E, x200).
Image 11:
Mixed cellular myocarditis with eosinophils, lymphocytes, and some neutrophils (H&E, x400).
Image 12:
Masson trichrome stain of myocarditis is negative for hypereosinophilic myocytes, distinguishing it from neurocardiogenic injury or ischemia (trichrome, x100).
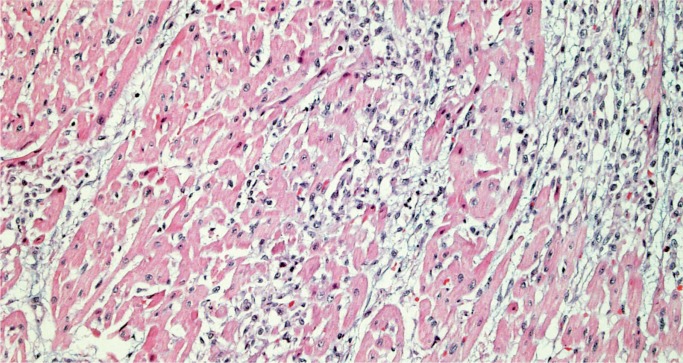
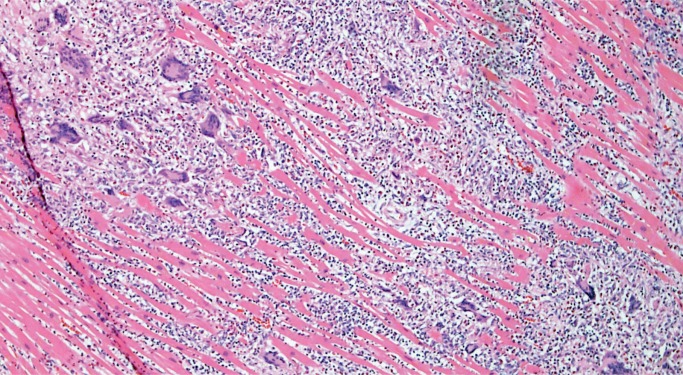
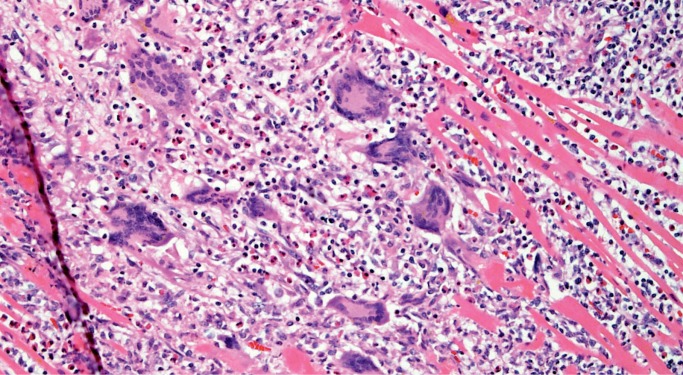
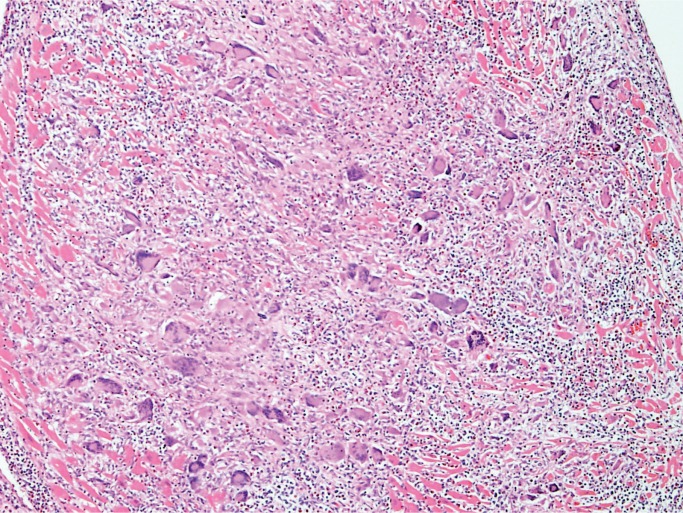
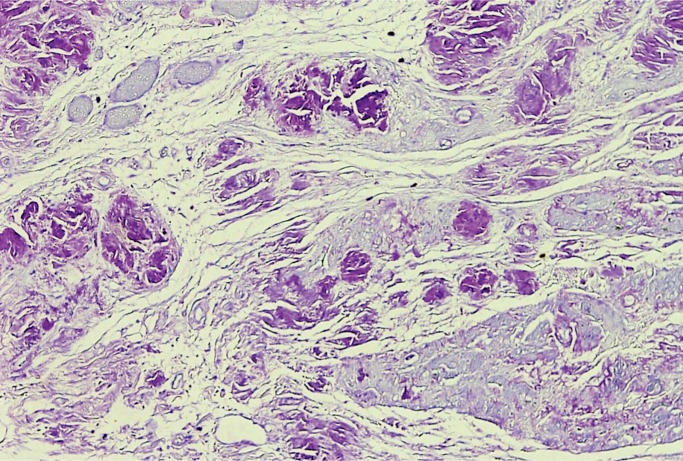
Giant Cell Myocarditis
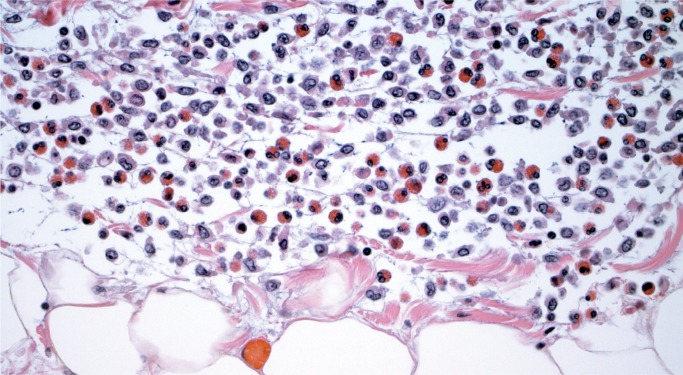
Giant cell myocarditis (GCM) is a rare, rapidly progressive and frequently fatal disease typically affecting young and middle-aged adults. It is considered idiopathic, but it has a strong association with autoimmune disorders, which are present in up to 20% of cases (10, 11). Histologically, there is florid myocarditis with lymphocytes, histiocytes, and multinucleated giant cells (CD68 positive macrophages) (Image 13). Numerous eosinophils are a hallmark of this process and a distinguishing feature from sarcoidosis, which is the most common differential diagnosis (Image 14). Significant fibrosis may be present, though not as extensive as that typically seen with sarcoidosis (Image 15). Noncaseating granulomas are not seen in GCM, also distinguishing it from sarcoidosis (12).
Image 13:
Florid myocarditis with numerous multinucleated giant cells, without granuloma formation (H&E, x100).
Image 14:
Numerous eosinophils are a distinguishing feature of giant cell myocarditis (H&E, x400).
Image 15:
Giant cell myocarditis with fibrosis (H&E, x200).
Lyme Carditis
Carditis is a rare complication of systemic infection by the tick-borne spirochete Borrelia burgdorferi, which causes Lyme disease. Patients may be acutely ill with Lyme disease or have minimal symptoms; heart block may be a presenting sign. The histologic appearance of Lyme carditis is characterized by florid inflammation involving the myocardium, epicardium, and endocardium. Often, there is a perivascular pattern of inflammation, which is said to resemble a “road map” on low power (Image 16). The cellular composition is lymphohistiocytic with frequent plasma cells (Image 17). This constellation of cardiac findings should prompt serologic testing for Lyme in the decedent. A Warthin-Starry stain or IHC may show spirochetes (13).
Image 16:
Lyme myocarditis with perivascular “road map” pattern on low power (H&E, x40).
Image 17:
Plasma cells are a prominent inflammatory component of Lyme carditis (H&E, x400).
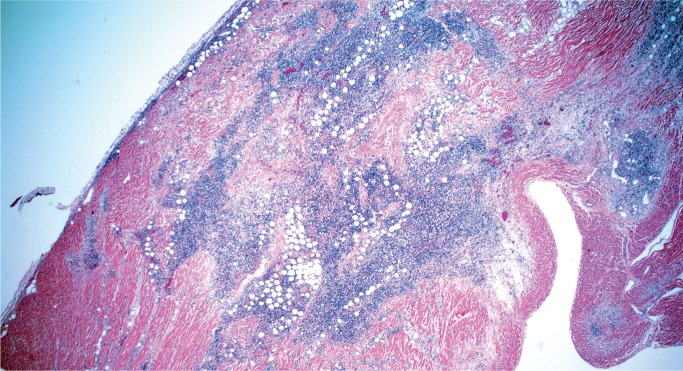
Sarcoidosis
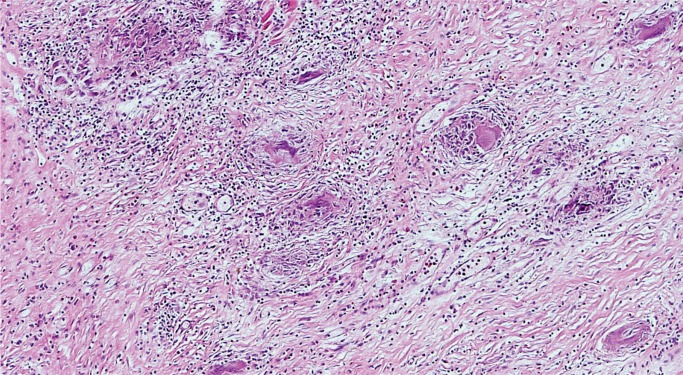
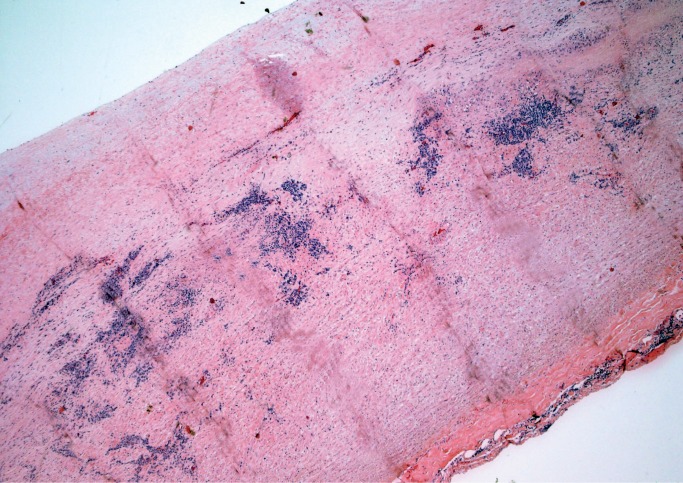
Sarcoidosis is an idiopathic systemic disease that may affect any organ and the heart is no exception. As in other tissues, sarcoidosis is characterized by patchy mononuclear inflammatory infiltrate with prominent noncaseating granulomas containing multinucleated giant cells (Image 18), usually in a background of prominent myocardial fibrosis, which often can be recognized on gross exam (Image 19). Typical asteroid bodies may be seen but are not a diagnostic requirement. Cardiac sarcoidosis may infiltrate the atrioventricular node. It is often referred to as “granulomatous myocarditis” if definite extracardiac involvement is not identified or unknown. Grocott methenamine silver (GMS), Gram, and acid-fast stains for organisms are negative (12).
Image 18:
Granulomatous myocarditis (sarcoidosis) features poorly formed, noncaseating granulomas (H&E, x200).
Image 19:
Cardiac sarcoidosis. A background of dense myocardial fibrosis can usually be seen on gross examination (H&E, x20).
Cardiomyopathy
An exhaustive list of cardiomyopathies and variants is beyond the scope of this paper, and only the more commonly occurring cardiomyopathies will be covered.
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant genetic disorder with hundreds of identified culprit mutations; extreme care is required in making the diagnosis (14). Diagnostic features include cardiomegaly with left ventricular hypertrophy, hypertrophic myocytes, and myocyte disarray and/or myofiber bundle disorder. Classically, myocyte disarray occupying at least 20% of the overall area of any one section is needed to make the diagnosis of HCM. Myocytes or myofiber bundles have a haphazard arrangement and there is increased interstitial fibrosis (Images 20 and 21). Intramyocardial arteries are often atypical with thickened media and narrowed lumina, which may cause small areas of ischemia leading to areas of replacement fibrosis (15).
Image 20:
Myocyte disarray and myocyte hypertrophy in a case of hypertrophic cardiomyopathy (H&E, x200).
Image 21:
Myofiber bundle disorder, where bundles of myocytes are haphazardly arranged, may be a feature of hypertrophic cardiomyopathy (H&E, x40).
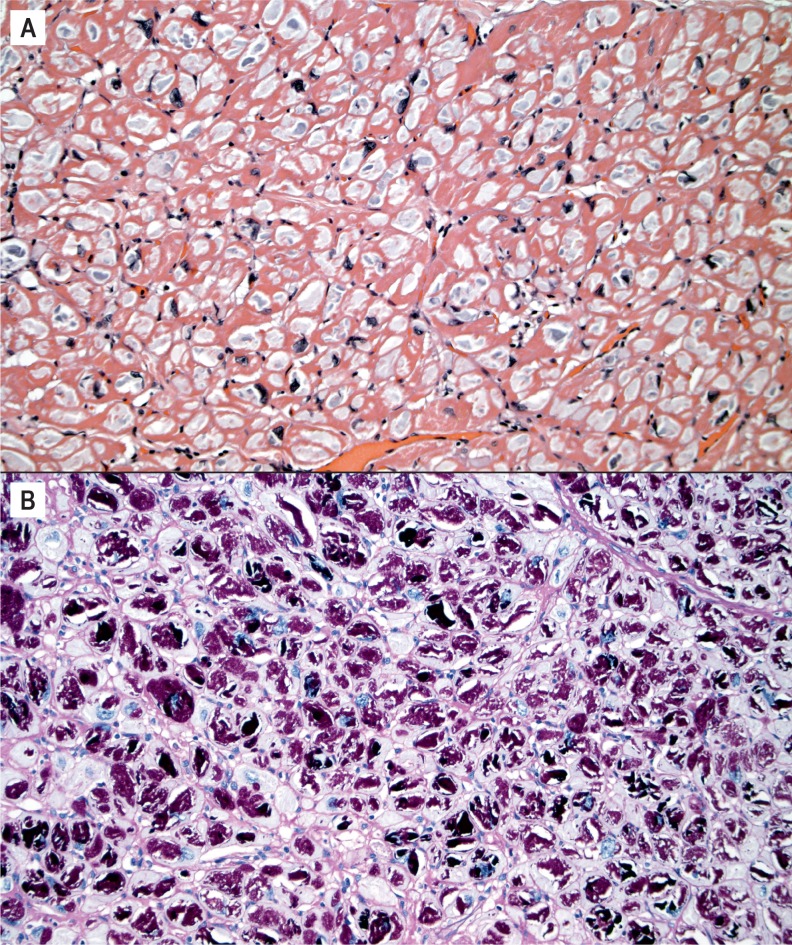
Certain glycogen storage diseases can clinically and grossly mimic HCM, though the distinction can be made histologically. There is no myocyte disarray, but instead enlarged myocytes with cytoplasmic vacuoles containing glycogen, which can be highlighted with periodic acid–Schiff (PAS) stain (Image 22) (16).
Image 22:
Periodic acid–Schiff positive vacuolated myocytes in a glycogen storage disease A) (H&E, x200), B) (Periodic acid-Schiff without diastase, x200).
Dilated Cardiomyopathy
Dilated cardiomyopathy is the most common form of cardiomyopathy. The diagnostic criteria are nonspecific and depend heavily on clinical history. Dilated cardiomyopathy can be divided into primary and secondary forms, but most commonly are classified as idiopathic. Numerous inheritable forms of dilated cardiomyopathy exist, some of which can be identified with genetic testing. Secondary forms are caused by a variety of conditions, some of which include post-viral, cardiotoxic medications/agents, stimulant use, alcohol, and peripartum cardiomyopathy. Evaluation of family members is recommended as a genetic cause can often not be ruled out.
The diagnosis of dilated cardiomyopathy is not made histologically. The microscopic findings are very nonspecific and consist of myocyte hypertrophy (Image 23) and myocardial fibrosis (Image 24). Occasional foci of myocarditis and/or myocardial remodeling may be seen (15).
Image 23:
Myocyte hypertrophy with irregular nucleli in dilated cardiomyopathy (H&E, x200).
Image 24:
Nonspecific interstitial fibrosis in a case of dilated cardiomyopathy (H&E, x40).
Arrhythmogenic Cardiomyopathy
Arrhythmogenic cardiomyopathy (AC) is a distinct cardiomyopathy, which clinically is often lumped under dilated cardiomyopathy. Arrhythmogenic cardiomyopathy is a potentially inheritable disease. There are dominant and recessive mutations and variable penetrance of the disease. The underlying molecular mutations involve demosomes. Initially described as a right ventricular process, it is now known to be more complex. Isolated right ventricular involvement, isolated left ventricular involvement, or biventricular involvement occur even within the same family, with the same mutation (17).
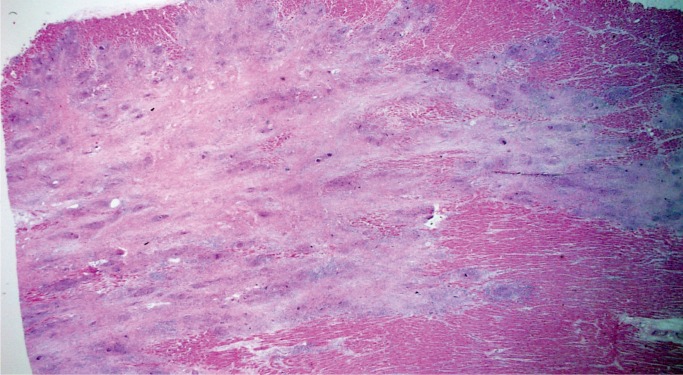
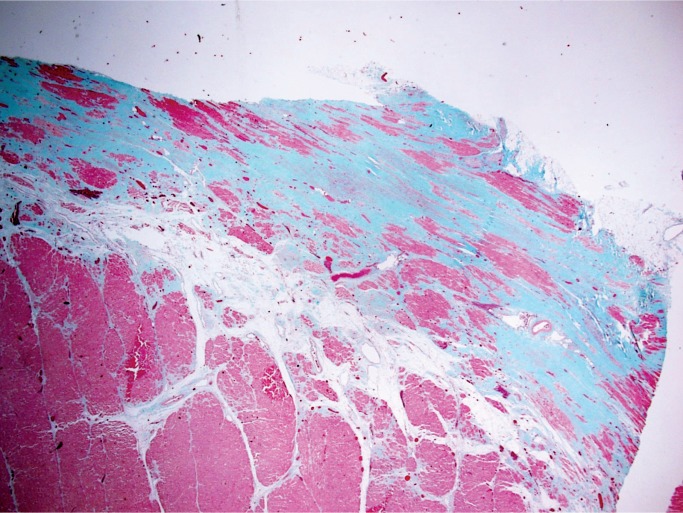
Histologically, there is fibrofatty replacement of the subepicardium in a band-like distribution (Image 25). With left ventricular involvement, the posterior and lateral walls are the most commonly involved; however, circumferential involvement of the subepicardium of the free walls and compact myocardium of the septum is common. Myocyte degeneration with vacuolated, pale staining cells is seen in myocytes entrapped within the areas of fibrofatty replacement (Image 26). Scattered foci of myocarditis are common as remodeling of dying/apoptotic cells continues; this is not a separate infectious process (18).
Image 25:
Subepicardial fibrofatty replacement of the left ventricular myocardium in the classic band-like distribution of arrhythmogenic cardiomyopathy (trichrome, x12.5).
Image 26:
Arrhythmogenic cardiomyopathy: Degenerating myocytes with vacolated, pale-staining cytoplasm entrapped in areas of fibrofatty replacement (H&E, x400).
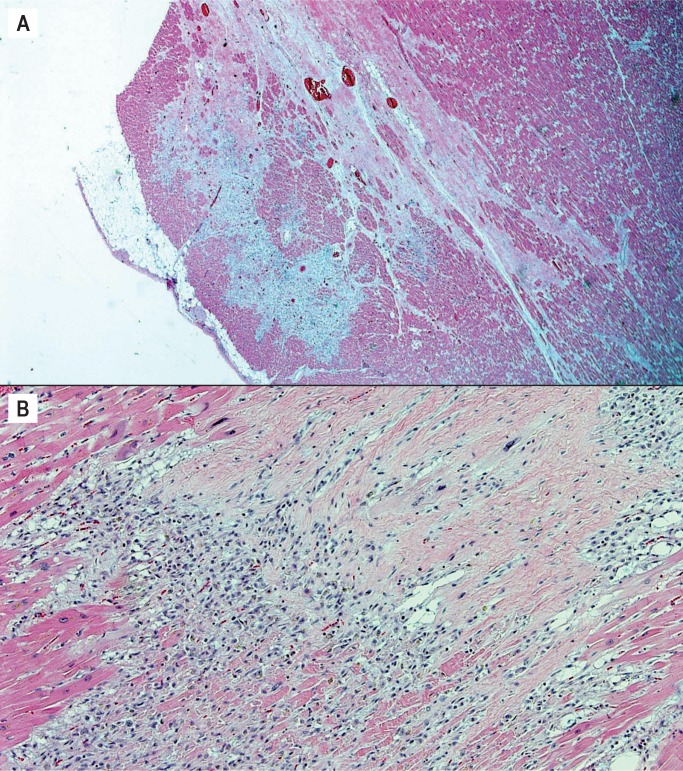
There is an active phase of the disease before the fibrosis is complete in which the predominant finding is myocarditis/remodeling. The key to establishing the diagnosis is recognizing the pattern of subepicardial band-like involvement (Image 27). In some cases, the fibrofatty replacement may be only recognized histologically and the heart may appear grossly unremarkable. This stresses the importance of adequate full thickness sampling of the myocardium of both ventricles (19).
Image 27:
Arrhythmogenic cardiomyopathy variant. A) Active phase of arrhythmogenic cardiomyopathy with active remodeling in the classic subepicardial band-like distribution (H&E, x25); B) Active remodeling in active phase of arrhythmogenic cardiomyopathy (H&E, x200).
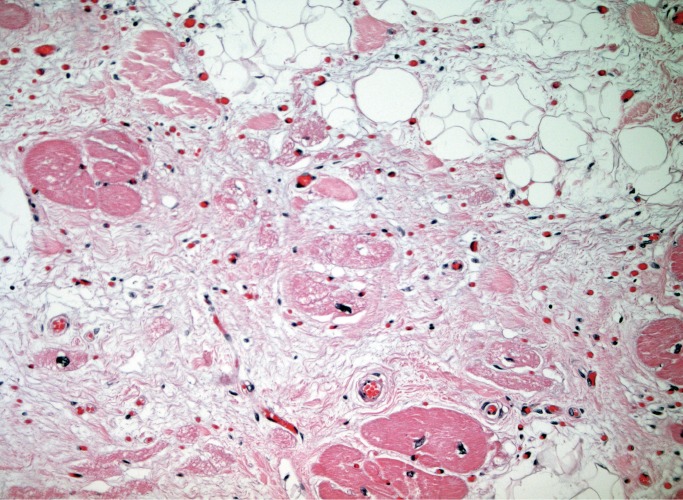
In AC involving the right ventricle, the main differential diagnosis is fatty infiltration, which is a benign condition and can be seen in as many as 50% of normal hearts in elderly patients. Arrhythmogenic cardiomyopathy on the right side will show fibrofatty replacement of the subepicardial myocardium, meaning myocytes have been destroyed and replaced by fibrosis and adipose tissue, sometimes to the extent that only a single layer of subendocardial myocytes remains (Image 28). Myocyte degeneration can be seen in the areas of fibrofatty replacement and there may be detached islands of remaining myocytes that appear to be disconnected from one another and from remaining subendocardial myocytes. This is in contrast to fatty infiltration, which will have little to no fibrosis and an infiltrative pattern to the fat deposition rather than a replacement pattern (Image 29). Myocyte degeneration is not a feature and detached islands of myocytes will not be present in fatty infiltration. Typically in fatty infiltration the right ventricle has normal or increased myocardial thickness, whereas the myocardium is thinned in right-sided AC (20).
Image 28:
Fibrofatty replacement of right ventricular myocardium with remaining detached islands of myocytes in arrhythmogenic cardiomyopathy (H&E, x12.5).
Image 29:
In contrast to right-sided arrhythmogentic cardiomyopathy, fatty infiltration of the right ventricle shows expansion of the free wall myocardium with infiltrating fat in a lace-like pattern. Detached groups of myocytes, fibrofatty replacement, and myocyte degeneration are not present (H&E, x12.5).
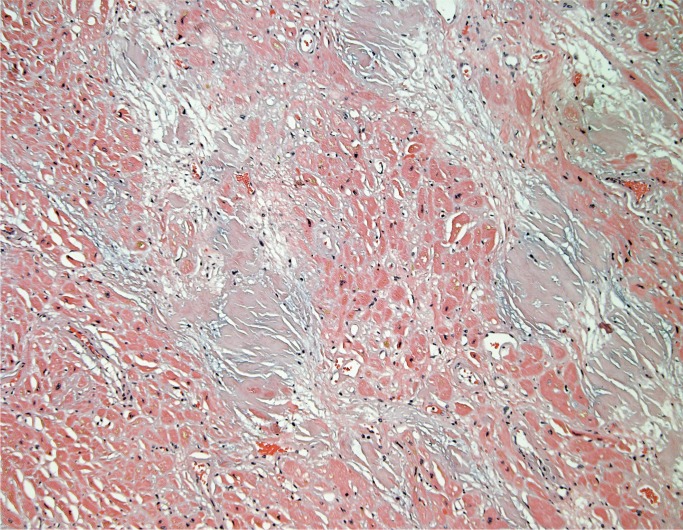
Amyloidosis
Amyloidosis may affect any organ and may be seen anywhere in the heart including valves, endocardium, myocardial interstitium and vessels (Image 30). Congo red staining has traditionally been used for identifying amyloid; however, it requires a polarizer and staining quality is variable depending on the experience of the lab. Crystal violet is an alternative stain that is more consistent and easier to see on conventional light microscopy (Image 31) (21).
Image 30:
Interstitial amyloid deposits have a lavender appearance on H&E (H&E, x100).
Image 31:
Crystal violet stains amyloid dark purple (Crystal violet, x100).
Nonatherosclerotic Arterial Disease
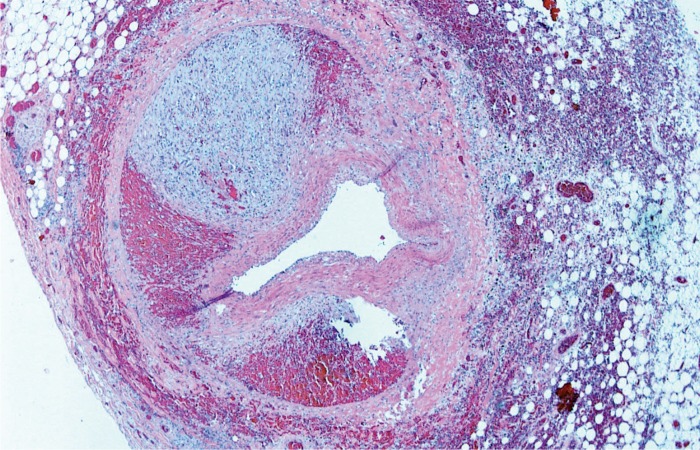
Coronary Artery Dissection
Spontaneous coronary artery dissection (SCAD) is gaining attention as a cause of acute coronary artery syndrome in women (22). This process has a female predominance, however it can occur in either sex. The most common associated risks are pregnancy (including the postpartum period) and there is a suggested association with fibromuscular dysplasia in other organs. Hormones are thought to play a role but this association is poorly understood (22 –25).
On gross examination, SCAD can be easily missed and overlooked as postmortem blood clot. Involvement of multiple arteries, often small branches, is not uncommon.
Histologically, there is separation of the layers of the media with thrombus accumulation in the false channel and compression of the true lumen (Image 32). The dissection heals by organization of the thrombus, eventually turning to collagen (Image 33). The unique and, as yet unexplained, histologic feature of SCAD is abundant adventitial eosinophils (Image 34) (26).
Image 32:
Spontaneous coronary artery dissection with separation of the layers of the media and blood in the false channel (H&E, x40).
Image 33:
Healing spontaneous coronary artery dissection (H&E, x40).
Image 34:
Adventitial eosinophils, an unexplained histologic feature often seen in spontaneous coronary artery dissection (H&E, x200).
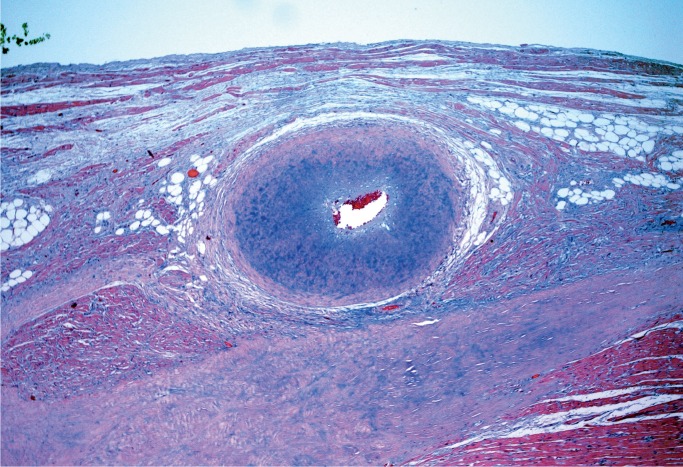
Kawasaki Disease
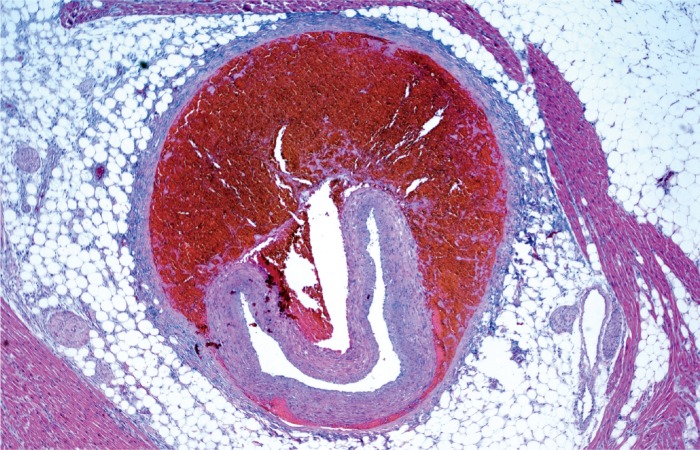
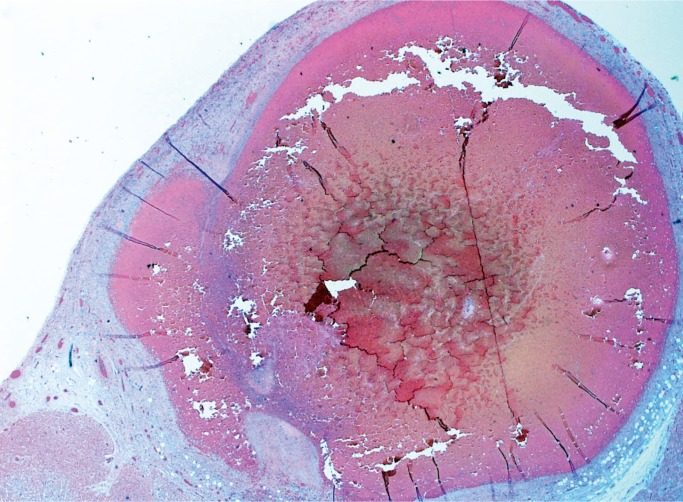
Mucocutaneous lymph node syndrome, also called Kawasaki disease, is a form of vasculitis with an unknown cause. It has an acute phase in infancy and early childhood in which patients present with high fever, rash, conjunctivitis, palmar and plantar erythema, tongue swelling and erythema, and enlarged lymph nodes. The histologic manifestation of the acute phase is an acute necrotizing vasculitis (Image 35). The disease usually is self limited with complete resolution, but some patients develop coronary artery aneurysms which can become several centimeters in diameter. These aneurysms rarely rupture, but instead become occluded by thrombus (Image 36), a possible cause of sudden death (26, 27).
Image 35:
Kawasaki disease in an infant. Necrotizing vasculitis with intimal thickening in an epicardial artery (H&E, x40).
Image 36:
Healed Kawasaki disease with formation of a coronary artery aneurysm, which is occluded by thrombus (H&E, x12.5).
Takayasu Arteritis
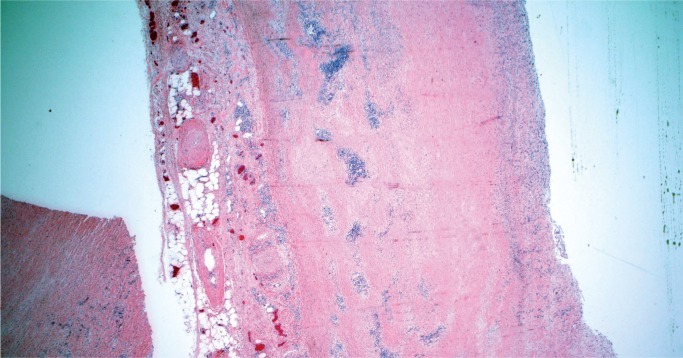
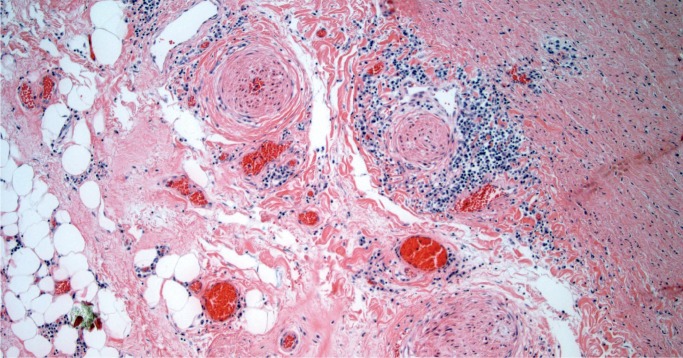
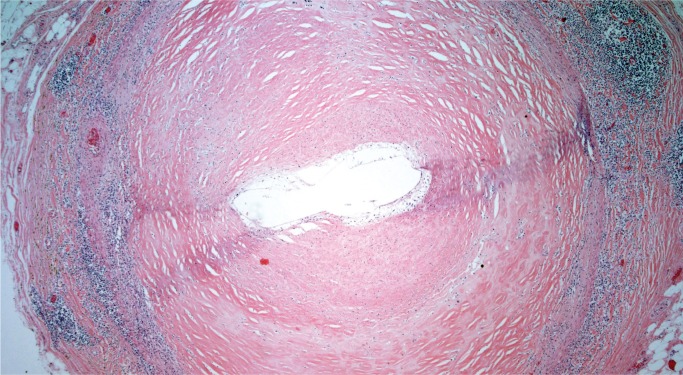
Takayasu arteritis involves large arteries and major branches and is of unknown etiology. This disease is more common in young women and in people of Asian or African descent. Gross findings include marked thickening of the aortic wall and its branches with a “tree bark” texture to the aortic intima. In the acute phase, inflammation is seen in the outer two-thirds of the media and in the adventitia, with occasional giant cells. In the late phase, there is still remaining patchy mononuclear inflammation with medial fibrosis and destruction of the elastic lamina. There is marked fibrous thickening of the intima (Image 37). A characteristic feature is marked intimal proliferation in the vasa vasorum with obliteration of the lumen (Image 38). There may be intervening unaffected segments of aorta. Longitudinal sections of aorta are preferable to document arteritis, while cross-sections of the coronary arteries are adequate. Coronary arteries may be involved, sometimes with critical (nonatherosclerotic) narrowing by marked medial fibrosis and intimal thickening (Image 39). Scattered giant cells may be seen (26, 27).
Image 37:
Low power Image of healing phase of Takayasu arteritis showing patchy lymphoplasmacytic inflammation in the outer media and adventitia with medial thickening (H&E, x20).
Image 38:
Typical appearance of vaso vasorum in the healed phase of Takayasu arteritis with intimal thickening and obliteration of the lumens with residual inflammation (H&E, x100).
Image 39:
Medial fibrosis and intimal thickening in a coronary artery involved by Takayasu arteritis (H&E, x40).
Giant Cell Arteritis
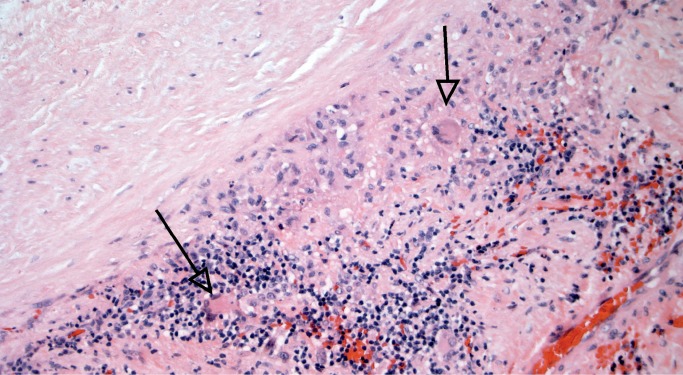
Giant cell arteritis, also called temporal arteritis, is another vasculitis of uncertain etiology. It is a panarteritis involving large and medium-sized arteries, predominantly affecting elderly patients. Clinical manifestations are varied and depend upon the distribution of affected arteries. Grossly, there is often an aortic aneurysm and a “tree bark” appearance to the intima, as seen in other forms of aortitis. Histologically, the inflammation is mononuclear with giant cells (Image 40). Healed lesions have intimal thickening with few remaining areas of inflammation (Image 41). Giant cells may also be present in Takayasu arteritis; the distinguishing features are patient age and the distinctive involvement of the vasa vasorum in Takayasu is not seen in giant cell arteritis. As mentioned previously, longitudinal sections of aorta and cross-sections of coronary arteries are preferred (26, 27).
Image 40:
Mononuclear inflammatory cells with occasional giant cells (arrows) in giant cell aortitis (H&E, x200).
Image 41:
Healing giant cell aortitis with medial inflammation and intimal thickening (H&E, x40).
Valve Disease
Infective Endocarditis
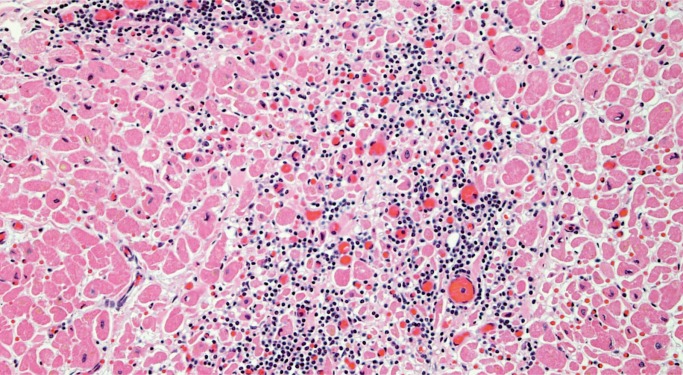
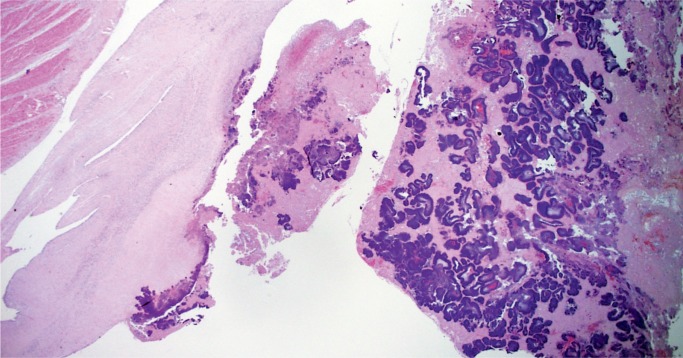
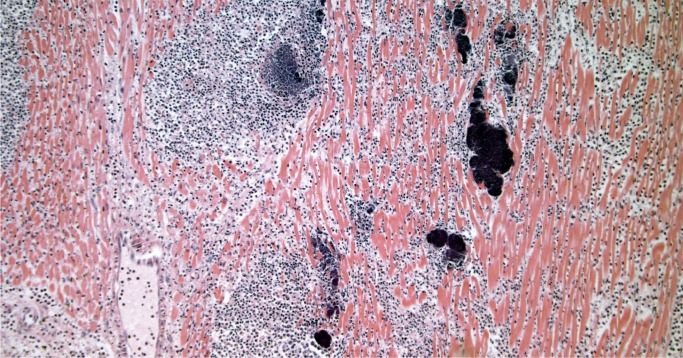
Infective endocarditis is often seen in forensic autopsies in association with drug use. Grossly, infective endocarditis should be suspected when perforations or tears in valve leaflets/cusps associated with vegetations are identified. Sections of the vegetations and valve should be taken to confirm the presence of active inflammation and to distinguish from nonbacterial thrombotic endocarditis (Image 42). It is also important to take histologic sections of the myocardium as left-sided vegetations may result in septic emboli to the myocardium (Image 43), which may be an important part of the mechanism of death. Gram stain may reveal microorganisms (Image 44), and a GMS stain may be helpful in addition as it not only shows fungal hyphae but may reveal degenerating bacteria that do not react to Gram stain in patients who have received antibiotics (28). All thrombi or suspected vegetations on a prosthetic valve should be examined histologically with stains for organisms as the gross appearance of infective vegetations often differs from those affecting native valves and may be mistaken for a benign thrombus.
Image 42:
Vegetation on a mitral valve in bacterial endocarditis with bacterial colonies discernable on H&E stain and areas of acute inflammation (H&E, x12.5).
Image 43:
Microabscesses in the myocardium due to septic emboli from left-sided bacterial endocarditis. Bacterial colonies are discernable on H&E (H&E, x100).
Image 44:
Gram stain of vegetation confirming the presence of Gram-positive cocci (Gram stain, x40).
Nonbacterial Thrombotic Endocarditis
The vegetations in nonbacterial thrombotic endocarditis consist of thrombi without microorganisms in variable phases of organization (Images 45 –47). Nonbacterial thrombotic endocarditis may develop on valves without underlying pathology and are associated with systemic diseases or disorders such as acquired immunodeficiency syndrome (AIDS), systemic lupus erythematosus, hypercoagulable states, or disseminated intravascular coagulation, and may also be associated with medical intervention such as prior surgery or intracardiac catheters (28). Grossly, there is usually no visible damage to the valve; sections must be taken to rule out infective vegetations.
Image 45:
Acute nonbacterial thrombotic endocarditis on a mitral valve consisting of fibrin without inflammation or organisms (H&E, x20).
Image 46:
Organizing nonbacterial thrombotic endocarditis with fibrin being converted to fibrosis without inflammation or organisms (H&E, x20).
Image 47:
Old nonbacterial thrombotic endocarditis vegetation that is calcified and hyalinized (H&E, x20).
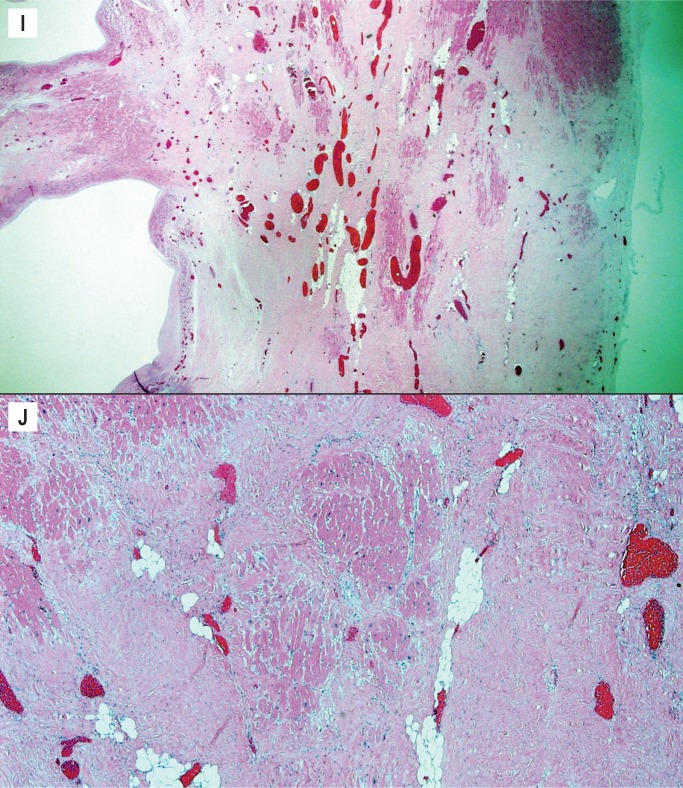
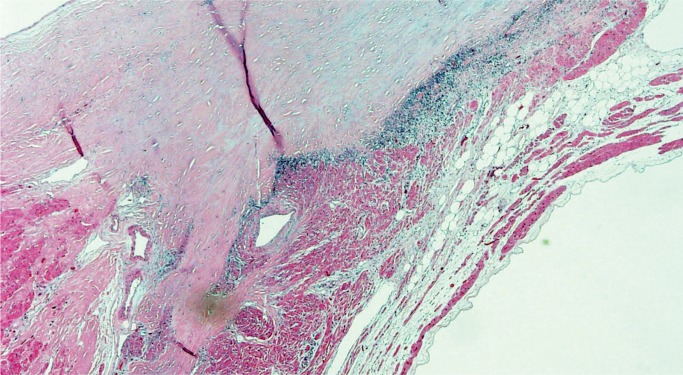
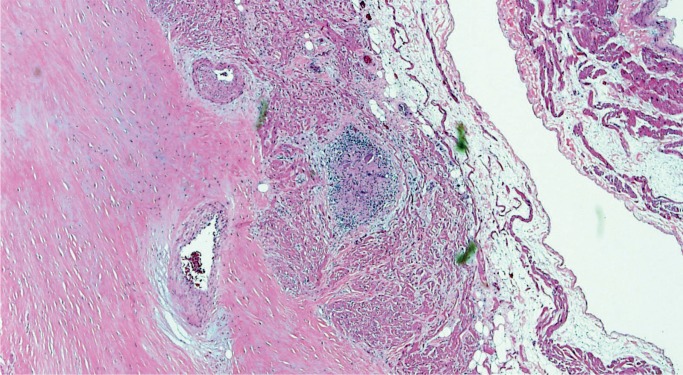
Mitral Valve Prolapse
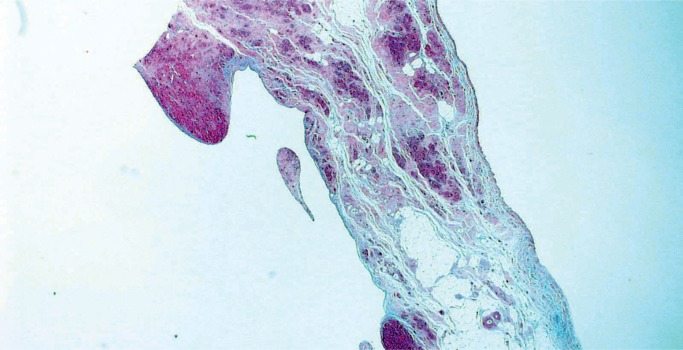
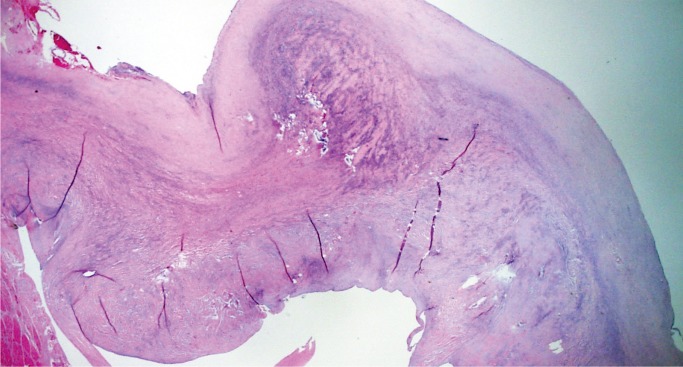
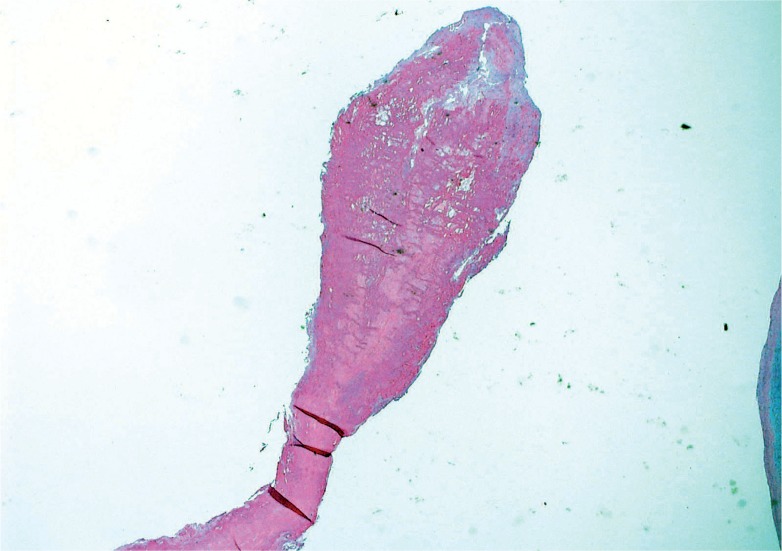
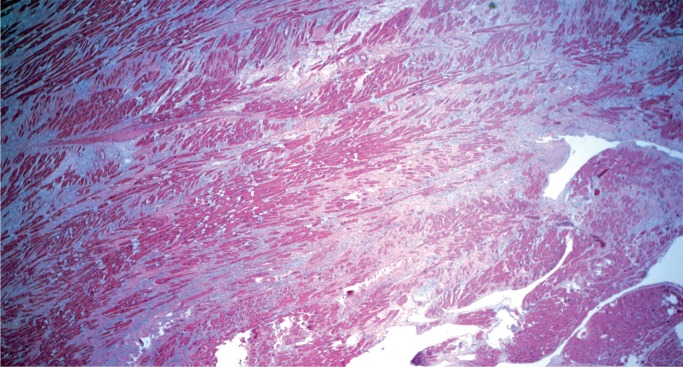
Mitral valve prolapse (MVP) is a common and benign condition in most people (Image 48). A known risk factor in severe MVP is chordae tendineae rupture, which results in flail leaflet with sudden onset heart failure (Image 49). In a small subset of severe or “malignant” MVP, there is a risk of malignant arrhythmia and sudden cardiac death. The proposed mechanism is ventricular remodeling, especially in the posterolateral wall of the left ventricle, thought to be the result of chronic myocardial strain from the prolapsing leaflet (Image 50) (29, 30). The histologic appearance of the mitral leaflet does not always correlate to disease severity. It is more important to rule out thrombi on the valve and evaluate for the presence of myocardial fibrosis.
Image 48:
Mitral valve prolapse with marked expansion of the spongiosa layer, disruption of the fibrosa, and fibrous thickening of the atrial and ventricular surfaces (H&E, x12.5).
Image 49:
Ruptured chord in mitral valve prolapse; note the ragged appearance and thin layer of fibrin over the site of rupture (H&E, x40).
Image 50:
Marked interstitial fibrosis/ventricular remodeling in the posterior wall of the left ventricle in mitral valve prolapse. This finding is often confined to the midventricular level (H&E, x40).
Conduction System Abnormalities
Atrioventricular Nodal Tumor
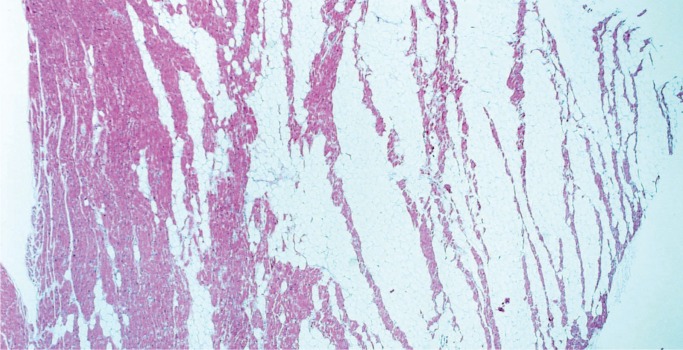
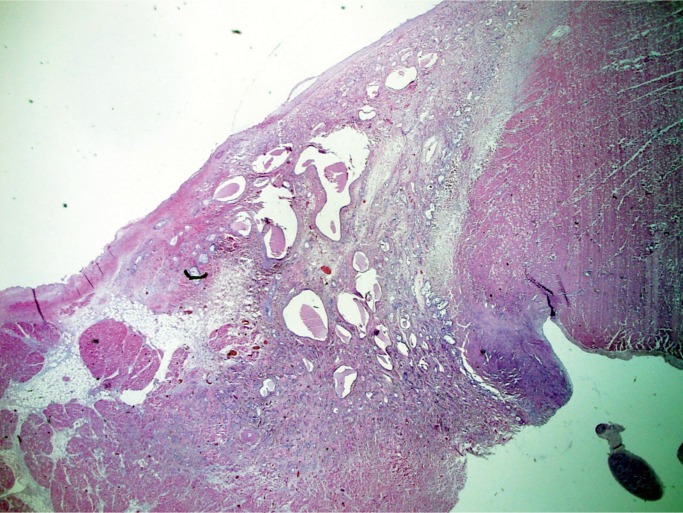
Atrioventricular (AV) nodal tumor, or cystic tumor of the AV node, is a microscopic, benign, congenital neoplasm located in the region of the AV node that is a potential cause of sudden death. There is a female predilection with a wide age range at presentation, although it is usually in childhood. Patients usually present with complete heart block or may be asymptomatic until the patient dies suddenly. Any case of congenital heart block should be a suspected AV nodal tumor. Most are diagnosed at autopsy. Once thought to be mesothelial in origin, they are now considered to be endodermal rests and the term “mesothelioma” is no longer used. Histologically, these tumors consist of variable-sized cysts lined with cuboidal, transitional, or squamous cells in a background of dense fibrosis (Image 51) (31, 32).
Image 51:
Atrioventricular nodal tumor with variably sized cysts lined with cuboidal, transitional or squamous cells on a background of dense fibrosis (H&E, x12.5).
Myocarditis
Myocarditis (Image 52) and sarcoidosis (Image 53) may be found within the AV node and could be considered a cause of sudden death. In at least one case in our laboratory, sarcoid lesions were restricted to the region of the AV node.
Image 52:
Lymphocytic myocarditis within atrioventricular node (H&E, x40).
Image 53:
Granuloma with giant cells in the atrioventricular node in a case of sarcoidosis (H&E, x40).
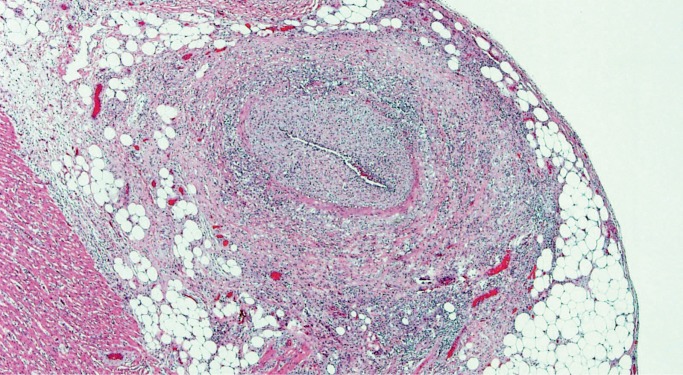
Dysplastic AV Nodal Artery
Narrowing of the AV nodal artery has a variety of names; dysplastic AV nodal artery, fibromuscular hyperplasia, and fibromuscular dysplasia have all been used. It is typified by nonatherosclerotic luminal narrowing of this small artery by intimal and medial thickening (Image 54). Several studies have concluded that severe (>75%) narrowing of the AV nodal artery is significant enough to explain sudden cardiac death, with a proposed mechanism of AV nodal ischemia (32, 33).
Image 54:
Atrioventricular nodal artery with greater than 75% luminal narrowing my medial and intimal thickening (H&E, x100).
Conclusion
Histologic examination of the heart is often an important part of a complete forensic autopsy, especially in cases of sudden death with no anatomic cause or suspected cardiomyopathy. Many cardiac diseases have diagnostic features that require histologic examination, or are enhanced by it, as presented here. Cardiac disease is vast and varied, many diagnoses are challenging, and the atlas we have presented here is not exhaustive. It behooves any forensic pathologist to have a cardiac pathology consultant available for equivocal or time consuming cases.
Authors
Emily R. Duncanson MD, Jesse E. Edwards Registry of Cardiovascular Disease
Roles: Project conception and/or design, data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work.
Shannon M. Mackey-Bojack MD, Jesse E. Edwards Registry of Cardiovascular Disease
Roles: Project conception and/or design, data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work.
Footnotes
Ethical Approval: As per Journal Policies, ethical approval was not required for this manuscript
Statement of Human and Animal Rights: This article does not contain any studies conducted with animals or on living human subjects
Statement of Informed Consent: No identifiable personal data were presented in this manuscript
Disclosures & Declaration of Conflicts of Interest: The authors, reviewers, editors, and publication staff do not report any relevant conflicts of interest
Financial Disclosure: The authors have indicated that they do not have financial relationships to disclose that are relevant to this manuscript
References
- 1). Duncanson ER, Mackey-Bojack SM. Adult heart dissection technique. Acad Forensic Pathol. 2011. September; 1(2):148–55. 10.23907/2011.020. [DOI] [Google Scholar]
- 2). Schoen FJ, Edwards W. Valvular heart disease In: Cardiovascular pathology. 3rd ed. Philadelphia: Churchill Livingstone; 2001. [Google Scholar]
- 3). Duncanson ER, Mackey-Bojack SM. Sudden death due to atherosclerotic coronary artery disease: a review of commonly accepted practices. Acad Forensic Pathol. 2015. March; 5(1):2–9. 10.23907/2015.001. [DOI] [Google Scholar]
- 4). Schoen FJ, Mitchell RN. Robbins & Cotran pathologic basis of disease. 9th ed. Philadelphia: Elseiver; c2014. Chapter 12, The heart; p. 523–78. [Google Scholar]
- 5). Berman M, Ali A, Ashley E, et al. Is stress cardiomyopathy the underlying cause of ventricular dysfunction associated with brain death? J Heart Lung Transplant. 2010. September; 29(9):957–65. PMID: 20627624 10.1016/j.healun.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 6). Samuels MA. The Brain-Heart Connection. Circulation. 2007. July 3; 116(1):77–84. PMID: 17606855 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- 7). Wybraniec M, Mizia-Stec K, Krzych L. Stress cardiomyopathy: yet another type of neurocardiogenic injury: ‘stress cardiomyopathy’. Cardiovasc Pathol. 2014. May-Jun; 23(3):113–20. PMID: 24462197 10.1016/j.carpath.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 8). Krishnamoorthy V, Mackensen GB, Gibbons EF, Vavilala MS. Cardiac dysfunction after neurologic injury: what do we know and where are we going? Chest. 2016. May; 149(5):1325–31. PMID: 26836901. PMCID: PMC4944787 10.1016/j.chest.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Edgecombe A, Veinot J. Myocarditis at post-mortem examination: a forensic perspective. Acad Forensic Pathol. 2011. September; 1(2):216–23. 10.23907/2011.028. [DOI] [Google Scholar]
- 10). Kandolin R, Lehtonen J, Salmenkivi K, et al. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail. 2013. January; 6(1):15–22. PMID: 23149495 10.1161/CIRCHEARTFAILURE.112.969261. [DOI] [PubMed] [Google Scholar]
- 11). Rosenstein ED, Zucker MJ, Kramer N. Giant cell myocarditis: most fatal of autoimmune diseases. Semin Arthritis Rheum. 2000. August; 30(1):1–16. PMID: 10966208 10.1053/sarh.2000.8367. [DOI] [PubMed] [Google Scholar]
- 12). Xu J, Brooks EG. Giant cell myocarditis: a brief review. Arch Pathol Lab Med. 2016. December; 140(12):1429–34. PMID: 27922771 10.5858/arpa.2016-0068-RS. [DOI] [PubMed] [Google Scholar]
- 13). Centers for Disease Control and Prevention (CDC). Three sudden cardiac deaths associated with Lyme carditis - United States, November 2012-July 2013. MMWR Morb Mortal Wkly Rep. 2013. December 13; 62(49):993–6. PMID: 24336130. PMCID: PMC4585587. [PMC free article] [PubMed] [Google Scholar]
- 14). Pinckard JK. Hypertrophic cardiomyopathy. Acad Forensic Pathol. 2011. September; 1(2):232–3. https://doi.org/10.23907/2011.031. [Google Scholar]
- 15). Gallo P, d’Amati G. Cardiomyopathies In: Cardiovascular pathology. 3rd ed. Philadelphia: Churchill Livingstone; 2001. [Google Scholar]
- 16). Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention . Circulation. 2006. April 11; 113(14):1807–16. PMID: 16567565 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 17). Singh SM, Casey SA, Berg AA, et al. Autosomal-dominant biventricular arrhythmogenic cardiomyopathy in a large family with a novel in-frame DSP nonsense mutation. Am J Med Genet. 2018. July 16; 176(7):1622–6. 10.1002/ajmg.a.38719. [DOI] [PubMed] [Google Scholar]
- 18). Mackey-Bojack SM, Roe SJ, Titus JL. Sudden death with circumferential subepicardial fibrofatty replacement: left-sided arrhythmogenic ventricular cardiomyopathy. Am J Forensic Med Pathol. 2009. June; 30(2):209–14. PMID: 19465822 10.1097/PAF.0b013e3181873c7d. [DOI] [PubMed] [Google Scholar]
- 19). Martins D, Ovaert C, Khraiche D, et al. Myocardial inflammation detected by cardiac MRI in Arrhythmogenic right ventricular cardiomyopathy: A paediatric case series. Int J Cardiol. 2018. May 31 pii: S0167-5273(18)31054-4. PMID: 29885824 10.1016/j.ijcard.2018.05.116. [DOI] [PubMed]
- 20). Burke AP, Farb A, Tashko G, Virmani R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases? Circulation. 1998. April 28; 97(16):1571–80. PMID: 9593562 10.1161/01.cir.97.16.1571. [DOI] [PubMed] [Google Scholar]
- 21). Lester WM, Gotlieb A. Cardiovascular effects of systemic diseases and conditions In: Cardiovascular Pathology. 3rd ed. Philadelphia: Churchill Livingstone; 2001. [Google Scholar]
- 22). Tweet MS, Hayes SN, Pitta SR, et al. . Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012. July 31; 126(5):579–88. PMID: 22800851 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]
- 23). Terrovitis JV, Kanakakis J, Nanas JN. Spontaneous coronary artery dissection as a cause of acute myocardial infarction in the postpartum period. Cardiol Rev. 2005. Jul-Aug; 13(4):211–3. PMID: 15949057 10.1097/01.crd.0000134862.82534.b3. [DOI] [PubMed] [Google Scholar]
- 24). Mortensen KH, Thuesen L, Kristensen IB, Christiansen EH. Spontaneous coronary artery dissection: a western Denmark Heart Registry study. Catheter Cardiovasc Interv. 2009. November 1; 74(5):710–7. PMID: 19496145 10.1002/ccd.22115. [DOI] [PubMed] [Google Scholar]
- 25). Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014. October; 7(5):645–55. PMID: 25294399 10.1161/CIRCINTERVENTIONS.114.001760. [DOI] [PubMed] [Google Scholar]
- 26). Mackey-Bojack SM, Duncanson ER, Roe SJ. Coronary heart disease: clinical, pathological, imaging, and molecular profiles. New York: Springer; c2012. Chapter 16, Pathology of sudden death in coronary arterial diseases; p. 291–305. [Google Scholar]
- 27). Thiene G, Basso C, Corrado D. Cardiovascular causes of sudden death In: Cardiovascular Pathology. 3rd ed. Philadelphia: Churchill Livingstone; 2001. [Google Scholar]
- 28). Silver MD, Silver MM. Valvular heart disease - conditions causing regurgitation In: Cardiovascular Pathology. 3rd ed. Philadelphia: Churchill Livingstone; 2001. [Google Scholar]
- 29). Basso C, Perazzolo Marra M, Rizzo S, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015. August 18; 132(7):556–66. PMID: 26160859 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 30). Lancellotti P, Garbi M. Malignant mitral valve prolapse: substrates to ventricular remodeling and arrhythmias. Circ Cardiovasc Imaging. 2016. August; 9(8): e005248 PMID: 27516480 10.1161/CIRCIMAGING.116.005248. [DOI] [PubMed] [Google Scholar]
- 31). Burke A, Virmani R. Heterotopias and tumors originating from ectopic tissues In: Atlas of tumor pathology: tumors of the heart and great vessels. Washington: Armed Forces Institute of Pathology; 1995. [Google Scholar]
- 32). Cohle SD. Histopathology of the cardiac conduction system in the investigation of sudden unexpected death. Acad Forensic Pathol. 2011. September; 1(2):206–15. 10.23907/2011.027. [DOI] [Google Scholar]
- 33). Slemp SN, Hoover RL, Prahlow JA. Sudden death in 14 month old due to fibromuscular dysplasia of atrioventricular nodal artery. Acad Forensic Pathol. 2011. December; 1(4):372–7. 10.23907/2011.052. [DOI] [Google Scholar]