Abstract
Forensic pathologists encounter hypoxic-ischemic (HI) brain damage or traumatic brain injuries (TBI) on an almost daily basis. Evaluation of the findings guides decisions regarding cause and manner of death. When there are gross findings of brain trauma, the cause of death is often obvious. However, microscopic evaluation should be used to augment the macroscopic diagnoses. Histology can be used to seek evidence for TBI in the absence of gross findings, e.g., in the context of reported or suspected TBI. Estimating the survival interval after an insult is often of medicolegal interest; this requires targeted tissue sampling and careful histologic evaluation. Retained tissue blocks serve as forensic evidence and also provide invaluable teaching and research material. In certain contexts, histology can be used to demonstrate nontraumatic causes of seemingly traumatic lesions. Macroscopic and histologic findings of brain trauma can be confounded by concomitant HI brain injury when an individual survives temporarily after TBI. Here we review the histologic approaches for evaluating TBI, hemorrhage, and HI brain injury. Amyloid precursor protein (APP) immunohistochemistry is helpful for identifying damaged axons, but patterns of damage cannot unambiguously distinguish TBI from HI. The evolution of hemorrhagic lesions will be discussed in detail; however, timing of any lesion is at best approximate. It is important to recognize artifactual changes (e.g., dark neurons) that can resemble HI damage. Despite the shortcomings, histology is a critical adjunct to the gross examination of brains.
Keywords: Forensic pathology, Axon injury, Hemorrhage, Hypoxia, Ischemia, Traumatic brain injury, Neuropathology
Introduction
Traumatic brain injury (TBI) can be categorized in several ways; physical mechanism (e.g., penetrating versus nonpenetrating; direction of acceleration), magnitude of force, single versus multiple, and temporal spectrum from acute to chronic. Relatively mild injuries such as concussions, by definition, have no gross or histologic findings, but may have occurred in individuals who died as a consequence of trauma to another part of the body or drug/alcohol toxicity. More severe brain injuries, which can cause death, usually present with intracranial hemorrhages (e.g., epidural, subdural, subarachnoid, and intraventricular) and parenchymal lesions (e.g., contusions, lacerations, or diffuse axonal injury [DAI]) (1, 2). If the victim survives, the evolving lesion can develop cerebral edema and swelling, anatomical herniations, secondary hypoxic-ischemic (HI) damage, inflammation, and (with penetrating injury) infection. Vascular lesions frequently accompany trauma, or they can occur in the clinical situation of stroke. These are categorized as ischemic (focal interruption of blood flow) or hemorrhagic (rupture of a subarachnoid or parenchymal blood vessel). Diffuse HI brain injury can follow arterial hypotension, hypoxia, cardiac arrest, or respiratory arrest.
This manuscript is written for forensic pathologists and novice neuropathologists. Both groups are frequently tasked with examination of brains from traumatized or suddenly deceased individuals. Traumatic brain injury, stroke, and HI brain damage following cardiorespiratory arrest are common problems to be addressed (3, 4). The focus is on changes in the mature nervous system. The developing nervous system (i.e., neonatal and pediatric) exhibits a different spectrum of morphological and reactive changes, with differences in the cellular vulnerability. Details of these are beyond the scope of this review and pathologists encountering such situations should consult with an expert in the field.
Discussion
Tissue Sampling
Imaging studies of brain obtained during life should always be reviewed prior to sectioning the fresh or fixed brain. The location, size, and appearance of grossly identifiable lesions should be documented with photographs and detailed diagrams, which may include correlated mapping of lesions and tissues sampled for histologic evaluation. Sections of macroscopic lesions, including hematomas, should be taken from the periphery to show transition to the surrounding parenchyma.
A systematic approach is useful when submitting tissue for evaluation of TBI. A minimum set of blocks includes sections from: the body of the corpus callosum with parasagittal white matter, splenium of the corpus callosum, posterior limb of the internal capsule with adjacent thalamus, rostral pons (including middle cerebellar peduncles), and cerebellum (including the dentate nucleus). Note that these focus on deep white matter lesions. They are less important in the situation of epidural or subdural hematoma associated with low acceleration trauma. In the case of subdural hematoma, evidence of chronic subdural hemorrhage should be sought. Additional brain sections should include hippocampus, dorsal frontal interarterial border zone, midbrain (decussation of the superior cerebellar peduncles), medulla, and spinal cord. Blocks should include sections from both sides but bilaterally paired sections are not necessary (5 –7).
When evaluating for HI injury, the regional susceptibility of the brain in association with vascular anatomy (i.e., in the interarterial boundary zones) and in relation to different neuron types (e.g., relative sensitivity of the cornu ammonis/CA1 hippocampus) must be considered (8). A minimum of the interarterial (“watershed”) zones, basal nuclei (putamen and globus pallidus), thalamus, hippocampus (ideally at the level of the lateral geniculate body), midbrain, pons, medulla oblongata, and cerebellum should be sampled (9). Palpably soft regions of the brain might indicate focal edema.
In cases where an intracerebral hematoma is present and evidence for TBI is questionable (e.g., decedent found at the bottom of a stairwell with minimal extracranial head injury), several sections should be submitted from the periphery of the hematoma to search for nontraumatic sources of hemorrhage including vascular malformation, amyloid angiopathy, neoplasm, or abscess. Slides stained with hematoxylin and eosin (H&E) can be scrutinized for evidence of cerebral atherosclerosis or arteriolosclerosis, which would be indicators of chronic arterial disease. Elastic stains can highlight splitting of the elastic lamina in small vessel disease and saccular aneurysms. Diagnosis of cerebral amyloid angiopathy requires demonstration of amyloid beta protein deposition in cerebral and leptomeningeal vessel walls using Congo red stain or appropriate immunostain (10). Martius scarlet blue (MSB) stain is useful for evaluating sites of hemorrhage (bright red staining of fibrin and yellow-orange staining of recently extravasated red blood cells) (11, 12). Neutrophil aggregates are associated with blood clotting; Gram stain can aid in distinguishing a bacterial abscess with secondary hemorrhage. Close attention to blood cell morphology can raise the possibility of sickle cell disease, malaria, or leukemia as a cause of intracranial hemorrhage.
Neuron Changes Following Hypoxia-Ischemia
Owing to their high metabolic needs and vulnerability to excitotoxicity, neurons are generally more sensitive to HI than macroglia (i.e., astrocytes and oligodendrocytes), microglia, or endothelia (8). In the mature brain, the most sensitive populations (or at least the most obvious victims) are the long-projection large neurons of the third and fifth neocortical layers, the neurons of the hippocampal CA1 regions, and the Purkinje neurons of the cerebellum. Histologic changes following HI are determined by the pathobiology (13). Early subtle changes can be difficult to define in autopsy samples. Also, not all cellular processes are energy dependent and morphology is not immediately frozen after death; passive water movements can continue after death. Swelling with cytoplasmic pallor of neurons and astrocytes occurs within 15-30 minutes of HI because the Na+-K+ exchange pumps are dependent on ATP; water passively follows the altered ion gradients. This can give the tissue a spongy or pale appearance at low magnification as early as one hour after the insult (Image 1). Occasionally, swelling of specific organelles including Golgi apparatus and endoplasmic reticulum can be identified in compromised neurons (Image 1) (14). Experimental studies indicate that shrinkage and scalloping (pyknosis) of dying neurons is apparent as early as 30 minutes after HI and persists for up to one day. Initially, the nucleus is collapsed and dark and the nucleolus is poorly defined. Note that it can be difficult to distinguish these early changes from “dark neuron” artifact (see below). Calcium influx activates a variety of enzyme systems, including calpains, that digest cytoplasmic proteins. Protein homogenization, combined with the loss of ribosomes, renders the cytoplasm of dead neurons brightly eosinophilic. Although typically not identifiable in large quantities earlier than six hours after HI, eosinophilic neurons have been reported as early as one hour (15, 16). The same population of dead neurons can be easily identified with the Fluoro-Jade fluorescent label (17). Loss of cytoskeletal integrity, demonstrated for example by loss of immunoreactivity for the protein MAP2, can help in the early detection of damaged neurons (18). Accompanying cytoplasmic eosinophilia, the nucleus degrades with the chromatin and fragments into fine basophilic particles before disappearing, leaving only the outlines of neurons (Image 1). Large globules of homogenized DNA, characteristic of classic apoptosis, are seen only in fetal and perinatal brains. Dead neurons are phagocytosed by microglia (in circumstances of selective neuron death) or macrophages (in pan-necrosis) within seven to nine days (19, 20). It is important to note that neurons can survive temporarily in a compromised state following a moderately severe HI insult. Neuron death, with all of the associated morphologic changes, can then be delayed for several days (21, 22). Purkinje neurons of the human cerebellum undergo changes similar to those in the cerebrum. However, the disappearance of these may occur more rapidly, with reduced quantity evident at one and a half to two days after HI (23).
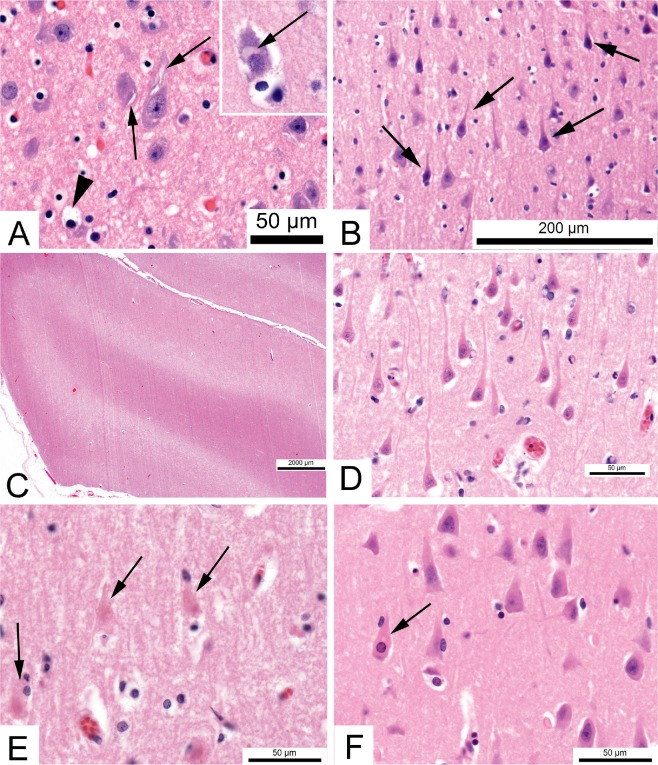
Image 1:
Neuron changes in hypoxia-ischemia. The earliest changes reflect shifts in water accompanying alterations in ion gradients. A) Swelling of the endoplasmic reticulum appears as clefts at the periphery of neurons (arrows). These should not be mistaken for crystalline inclusions. Astroglial cells take up K+ released from neurons and the cytoplasm becomes pale due to water accumulation (arrowhead) (H&E, x600). Inset - Swelling of the Golgi apparatus (arrow) can also reflect early neuron damage. B) In the vicinity of a recent contusion, neurons can appear dark and shrunken (pyknotic, arrows) (H&E, x200). C) If the concentration of swollen cells is sufficient (i.e., cellular edema), regions of the brain may appear pale. This example shows laminar edema of the deep cortex three days after resuscitation following respiratory arrest in a 50-year-old (H&E, x12.5). D) Dead neurons have uniformly eosinophilic cytoplasm. This example shows the hippocampal CA1 sector of a 22-year-old who was in a coma for three days after circulatory arrest (H&E, x600). E) After six days coma following respiratory arrest in a 48-year-old, hippocampal neurons are reduced to ghost cells, with no nuclear staining and a barely visible cell outline (arrows) (H&E, x600). F) However, not all cases are the same. In the hippocampus of this 34-year-old, who was in coma six days following respiratory arrest, only rare neurons are eosinophilic (arrow) (H&E, x600).
The pathogenesis of neuronal damage in the context of severe hypoglycemia partially overlaps with that of HI. However, there are significant differences including local alkalosis (rather than acidosis). The distribution of hippocampal neuron damage differs from HI, with relatively greater dentate neuron loss (24, 25). It should be noted, though, that this assertion is based on a small number of autopsy cases.
Axon Changes Following Traumatic and Hypoxic-Ischemic Brain Injury
Nonpenetrating head injuries associated with acceleration forces can cause distortion and shearing injuries in deep parts of the brain. Axons in the white matter structures must be evaluated. Axon damage is also a common consequence of HI.
The study of fatalities with nonpenetrating “diffuse brain damage of immediate impact type” (26, 27) led to the term “diffuse axonal injury” (DAI), proposed in 1982 (28). This was followed by a grading scheme where grade 1 consists of microscopic changes in white matter of the hemispheres, corpus callosum, and rostral brainstem, grade 2 has an additional focal lesion in the corpus callosum, and grade 3 has a focal lesion in the brainstem (7). One problem with the system is that the “focal lesion” was not clearly defined; “grades 2 and 3 can be said to be severe if the focal lesions are apparent macroscopically” (i.e., with accompanying vascular damage and hemorrhage) (7). The coexistence of axonal and vascular injury is well established (29). It is likely that most changes reported as DAI on computed tomography (CT) or magnetic resonance (MR) imaging studies are influenced more by the vascular pathology (hemorrhage or edema) than actual axon changes. To encompass the axonal and vascular damage, the senior author prefers the more generic term “distortional brain injury.” The concept of DAI is undoubtedly useful in explaining brain dysfunction or sudden traumatic deaths in the absence of extensive contusions or hematomas (30). However, the presence of diffusely damaged axons in a victim who survives hours to days and who does not have hemorrhagic lesions is not necessarily proof of head/brain trauma.
Understanding some axon biology is necessary for understanding the histological features of axon damage. In addition to conducting the electrical action potential from the neuron soma to the synapse, axons physically transport synaptic proteins, neurotransmitter-filled vesicles, and organelles toward the synapse (anterograde); to a lesser extent, they transport materials (including lysosomes) back to the cell body (retrograde) (31). This transport is dependent on physical integrity of the axoskeleton (which consists of microtubules and intermediate filaments, particularly the neurofilament family) and a constant supply of energy. Thus, mitochondria are present throughout axons through which they move and in which they provide ATP locally (32). Physical or metabolic disruption of axons prevents movement of the organelles at that site, leading to accumulation of organelles proximal to the point of damage where axons remain viable. Eventually (6-24 hours), this is microscopically apparent as swelling of the axon (“bulbs”) near the site of damage (Image 2).
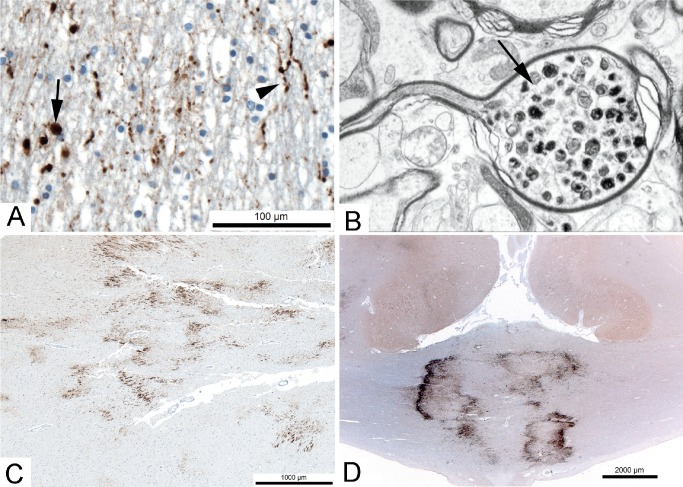
Image 2:
Axon damage. A) Immunostain for amyloid precursor protein (APP) showing damaged axons in the corpus callosum of a person who died 18 hours after a fall. Varicosities (arrowhead) and swollen axons (bulbs; arrow) are evident (x400). B) Electron micrograph showing the structure of an axon bulb on the cell body side of the injury site. Note the accumulation of organelles (x20000). C) APP immunostain showing clusters of damaged axons in the frontal white matter adjacent to an acute infarct in a 58-year-old (x40). D) APP immunostain showing dense collections of damaged axons in the corpus callosum of a 37-year-old who died ˜24 hours after a severe head injury that was associated with a large subdural hematoma and subfalcine herniation (x12.5). In this example, ischemia is likely a greater contributor to the damage.
Because the recognition of axonal damage may be important in the explanation of clinical findings, tools to detect damage earlier have been applied. Immunohistochemical markers of structural degeneration are of value. For example, accumulation of neurofilament (33 –36) and proteolytic fragments of alpha spectrin, one of the proteins at the interface between the axoskeleton and the membrane (37), are reliable indicators of focal damage. Many proteins that undergo anterograde transport to the synapse accumulate can be detected by immunohistochemistry at the site of damage (38, 39).
By far, the most practical and widely used approach is immunohistochemical detection of the amyloid precursor protein (APP; sometimes called beta APP), an important synaptic membrane protein (Image 2). Amyloid precursor protein moves by fast anterograde axonal transport (40). Detection of APP in acutely damaged axons was first reported around cerebral infarcts (41). Amyloid beta (also referred to as Aβ, Abeta), is a cleavage product of APP that is important in the pathogenesis of Alzheimer disease. Early studies suggested that Abeta did not accumulate in damaged axons (42), while more recent studies suggested that both APP and Abeta can be detected (34, 43, 44). Amyloid precursor protein accumulation after TBI was also recognized decades ago (45) and its immunoreactivity is definitely increased within two hours (38, 46), and likely within less than 30 minutes of axonal damage (47 –49). There is a positive relationship between the size of axonal swelling and survival time, which was shown to plateau at ˜85 hours in one study (50).
One report suggested that abnormal immunoreactivity could persist for up to 99 days after mild head injury (51), but that was based on a single subject. Marked immunoreactivity for APP is present for one month after spinal cord injury, while less intense immunoreactivity has been reported for up to 14 months (52). More consistently, axonal bulbs begin to lose APP immunostaining one week after TBI and may not be detectable after 30 days (5). Amyloid precursor protein immunostaining is useful for evaluating optic nerve damage in infants (53).
Axons are more vulnerable to injury than blood vessels (54); therefore, the absence of hemorrhage is not necessarily an indicator of the absence of trauma. The absence of APP immunostaining can be useful in ruling out traumatic brain injury in individuals with survival times greater than one hour after an event that renders them unconscious. However, in individuals with survival intervals less than 30 minutes it would be difficult to completely exclude TBI as a cause of death due to known limits of APP staining in very short survival intervals (5, 55, 56).
The pattern and distribution of APP immunostaining has been proposed to distinguish TBI from HI. In general, TBI is described as having single or small clusters of APP positive axons arranged in linear or bundled patterns along white matter tracts with fusiform or beaded patterns (“varicosities”) (Image 2). Axon injury associated with HI is described as having granular APP immunostaining of axon groups resulting in circumscribed, irregular foci with a “zigzag” pattern, often at the edge of ischemic tissue or around blood vessels (5, 57 –64). However, there are overlaps in the patterns and correlation with the anatomical distribution and gross findings is necessary. Victims of TBI who survive a few days usually have accompanying HI brain damage as a consequence of respiratory compromise or raised intracranial pressure. Graham et al. concluded:
…that the proper interpretation of cases requires the examination of a sufficient number of blocks (n = 15), processing using standardized protocols including βAPP immunohistochemistry and in some cases the mapping of any [immunoreactive] on anatomical line diagrams. βAPP carried out on a small number of randomly taken blocks is likely to lead to misinterpretation of the clinico-pathological correlations and possibly to a miscarriage of justice (55, 65).
Even among victims of known falls, the extent of axonal injury was not correlated with the height of the fall (66). Some authors, including the senior author of this paper, contest the practical utility of pattern distinction in individual cases (67 –70). In summary, interpretation of APP immunostaining must not be performed in isolation. Its presence, no matter the distribution, should not be the sole evidence used to conclude that TBI has occurred.
Increased Permeability of Blood Vessels
The cerebral vasculature possesses a blood-brain barrier (BBB) that is necessary for homeostasis of the neuronal extracellular environment. So-called “vasogenic edema” is the result of a disrupted BBB and subsequent movement of plasma proteins into the brain. Histologically, enlarged extracellular spaces are expanded by acellular, faintly eosinophilic material. Macroglia can ingest plasma proteins, presumably by pinocytosis, whereupon they become engorged and uniformly eosinophilic (Image 3). Albumin and immunoglobulins can be detected by immunohistochemistry in swollen astrocytes and oligodendrocytes (71, 72). Damaged neurons may be immunoreactive for fibrinogen (73); however, albumin and other proteins that leak from blood vessels postmortem can enter otherwise normal neurons (74, 75).
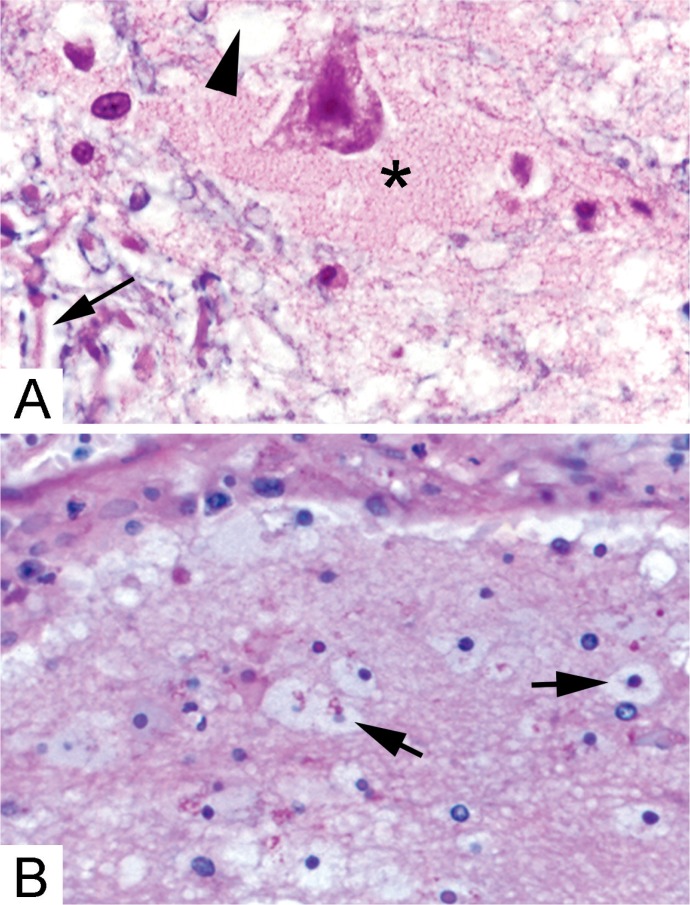
Image 3:
Histologic features of brain edema. A) Near sites of hemorrhage or blood-brain barrier disruption, plasma proteins fill the expanded extracellular space surrounding neurons (*). Intracellular edema can manifest as empty appearing glial cell cytoplasm (arrowhead) or separation of myelin (blue) from the surrounding axons (arrow) (Solochrome cyanin & eosin, x400). B) Plasma proteins are ingested by astrocytes and oligodendrocytes leading to a swollen, eosinophilic appearance (arrows) (H&E, x200).
Inflammation in Association with Cell Death and Hemorrhage
Microglia are the brain’s resident macrophages. They are activated in a range of systemic and local brain disorders. Detection of the activated state may be the only evidence of early neuronal damage. Activation is associated with transformation from a bipolar cell with delicate processes to one with shorter thicker cell projections. These cells often wrap themselves around dead neurons (Image 4). As phagocytosis progresses, the microglia acquire a spherical macrophage morphology. Shape changes are associated with expression of new cell surface markers that are demonstrable by lectin histochemistry or, more practically, with immunostaining (e.g., with anti-CD68 or anti-HLA-DR) (76). Circulating leukocytes are minimally involved in the response to selective HI neuron damage (77). In contrast, neutrophils, monocytes, and, to a lesser extent, T lymphocytes are prominent in the reaction to pan-infarction and hemorrhagic brain lesions (78) (Image 4).
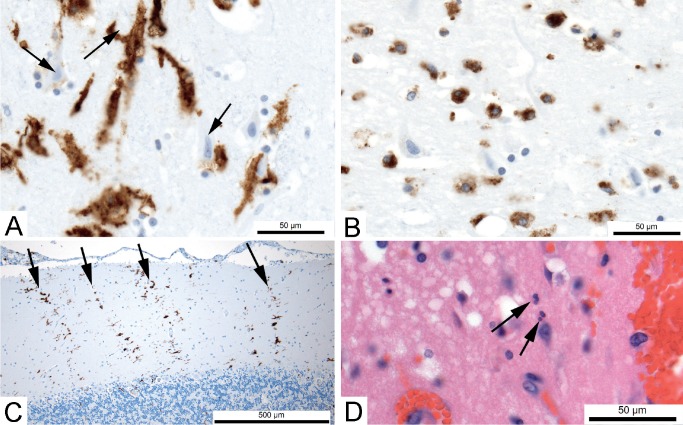
Image 4:
Inflammation associated with brain damage. A) Immunostain for HLA-DR showing activated microglia (brown); many are in contact with dead neurons (arrows) in the hippocampus of a 22-year-old with severe hypoxic-ischemic brain damage and in deep coma for three days after resuscitation following cardiac arrest (x600). B) Immunostain for HLA-DR showing round macrophages (brown) in the same case (x600). C) After neurons have been phagocytosed and are no longer visible, microglial activation can persist in patterns that indicate the loss of neurons. In this example, immunostain for HLA-DR shows columns (arrows) corresponding to the dendrites of lost Purkinje neurons in the cerebellum of a 60-year-old who had been in coma for three weeks after severe hypoxic brain injury (x100). D) Circulating leukocytes more commonly enter brain in hemorrhagic lesions. In this example, rare neutrophils (arrow) have moved into the brain parenchyma from the site of hemorrhage in frontal lobe of a 42-year-old who died three days after brain trauma with contusions (H&E, x600).
Inflammatory reactions are somewhat helpful in estimating the stage of intracranial lesions. Histologic aging can help determine if an insult occurred within an alleged time frame. This involves estimating the time between when an insult occurred and death (the posttraumatic interval or survival interval). The general tempo of changes is similar in intracerebral and meningeal (i.e., subdural and epidural) hematomas, but there are major cytologic differences. The meninges lack the microglial reaction but exhibit a much more exuberant fibrovascular proliferation in the resolution phase. The phases of healing after traumatic tissue injury can be categorized by distinct, chronologic, partially overlapping morphological changes that have been established by numerous experimental investigations (79).
Degradation of Hemoglobin After Hemorrhage
Rupture of blood vessels permits extravasation of blood cells and plasma into intracranial compartments and extracellular spaces. Acutely extravasated erythrocytes are easily detected on routine H&E stains immediately after injury. In a large hematoma, they can remain intact for months because their ultimate degradation is dependent on macrophages delivered via the vasculature of live tissue (15, 79, 80). Martius scarlet blue stain can provide better contrast than H&E; the cytoplasm of fresh red blood cells stains yellow-orange, fibrin stains red, and collagen stains blue. As erythrocytes begin to degenerate, the intensity of yellow staining diminishes and the cells appear as empty “ghost” cells (11). The chemistry and exact timing of this change is unclear. Martius scarlet blue stain cannot distinguish between antemortem hemorrhage and postmortem blood leakage. Note that blood vessels are disrupted during the autopsy and organ removal and it is possible that some seemingly hemorrhagic lesions (e.g., intradural blood near the superior sagittal sinus) simply reflect the forces necessary for blunt separation of the tissues after death.
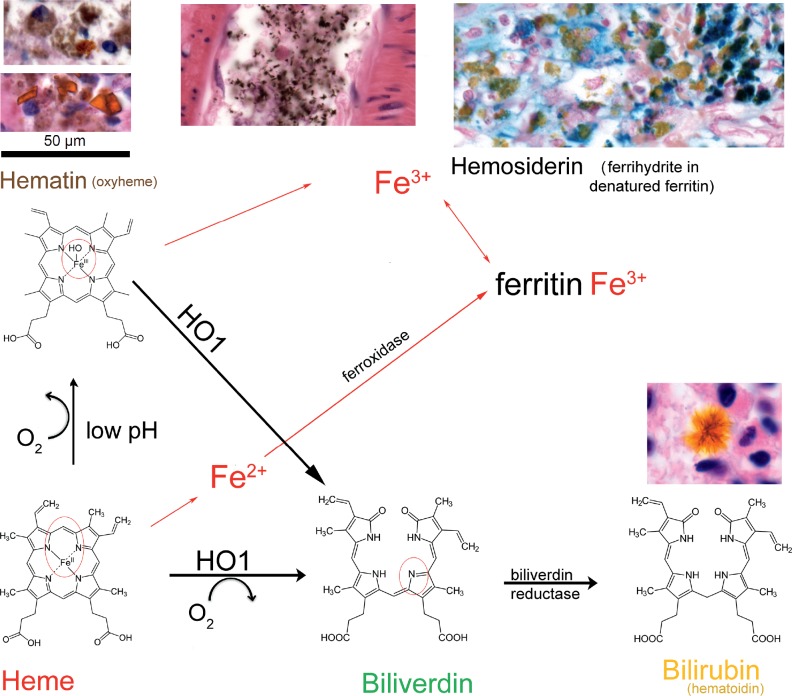
Hemosiderin, an iron-storage complex that results from hemoglobin degradation, is often used in estimating the timing of hemorrhage (Image 5). After hemoglobin is released from lysed red blood cells, the heme molecule is converted to hematin by oxidization of the ferrous iron (Fe2+) to ferric iron (Fe3+). Both hemoglobin and hematin are bound by proteins that facilitate phagocytosis by macrophages. When in sufficient concentration and in the right environment, hematin aggregates into yellow-orange triclinic (tilted rectangular) crystals (81). Hematin is converted by heme oxygenase 1 to biliverdin by release of ferric iron. Biliverdin is converted to bilirubin (also known as hematoidin) by the action of biliverdin reductase. Bilirubin can form radiating needle-like crystals (82, 83). Ferric iron is sequestered in ferritin whose denaturation forms the ferrihydrite compounds that make up hemosiderin, which appears as coarse brown granules histologically. Perls’ Prussian blue method can be used to stain for non-heme ferric iron in hemosiderin. The appearance of detectable hemosiderin at the site of cortical contusions takes at least 48 hours (15, 79). Hemosiderin in macrophages (siderophages) can persist for months and in glial cells for years. The time course for the appearance and resolution of Perls’
Image 5:
Heme degradation following hemorrhage. After hemorrhage, the heme component (bottom left) of hemoglobin is released from erythrocytes. In low extracellular pH conditions, the iron atom becomes oxidized to form hematin (oxyheme), which is bright orange and has a rhomboid crystal structure (top left lower; hemosiderin is brown and globular). Following formalin fixation and paraffin embedding, the crystals usually lose their distinct structure, but remain orange (top left upper). In some circumstances, the action of formaldehyde on heme that is not associated with a hemorrhagic process can create hematin. In this situation, the coarse brown granules are called “formalin pigment”, which can appear in blood vessel lumens (top middle) or within cells. Phagocytosis of heme or hematin, usually by macrophages but also potentially by astrocytes, allows heme oxygenase 1 (HO1) to remove the iron ion, yielding biliverdin. Biliverdin, in turn, is converted to bilirubin (also called hematoidin) by biliverdin reductase. Bilirubin is usually removed from the tissue into blood but can form radiating needle-like extracellular crystals (lower right). Ferrous iron (Fe2+) from heme is complexed with intracellular ferritin, which has ferroxidase activity that converts iron to the ferric form (Fe3+). Hemosiderin is a ferrihydrite (hydrous ferric oxyhydroxide) compound bound to denatured ferritin. It gives the yellow color to sites of old hemorrhage. Ferric iron can be detected with the Perls’ Prussian blue stain (top right; note that the hematin does not stain because the ferric iron remains chelated).
Prussian blue stainable material has been reviewed in detail recently (11). Typically, macrophages stain with Perls’ method no earlier than two days. Note that the Prussian blue stain can fail in tissue that has been decalcified in acidic solution (84). Also, it can give a positive result (typically a smeared appearance) unrelated to hemoglobin breakdown in putrefied tissue where cyanide has been generated (85).
Subdural Hemorrhage/Hematoma
The histologic evolution of subdural hemorrhage/hematoma is well documented (86 –88). Briefly, a layer of fibrin forms on the dural and arachnoid surfaces early during clotting. Neutrophils in the extravasated blood tend to aggregate during clotting; as a reactive phenomenon, they likely peak along the interfaces with viable tissue at two to three days. Although rare macrophages may be seen as early as 12-17 hours, hemosiderin is typically not seen until three to five days at the dural surface (3). Because the arachnoid mater itself has few blood vessels, delivery of macrophages to this surface is slower. Reactive fibroblasts appear at the dural interface within three to five days and neocapillary formation (granulation tissue) is evident along the dural surface by five to ten days (3). By three to four weeks, the fibrovascular membrane can equal the dural thickness. If the hematoma is thick, clotted blood persists between the dura and arachnoid for weeks to months. In this situation, a thin fibrovascular membrane forms at the arachnoid surface beginning at 10-20 days and gradually thickens (89). As a consequence of repeated hemorrhage, chronic subdural hematoma membranes often have a mixed inflammatory infiltrate with lymphocytes and eosinophils (90). The histologic evolution of subarachnoid hemorrhage follows a similar time course (91, 92).
Cortical Contusions and Hematomas
Cortical contusions are more complex lesions than meningeal hematomas because the blood collections are interspersed with viable and damaged brain parenchyma. Immediately after trauma, ruptured blood vessels release blood cells and plasma into the extracellular spaces. Some neurons are subject to HI damage because the vascular supply is interrupted while others are compromised by plasma enzymes (e.g., thrombin and plasmin) (93). The temporal course of histologic changes has been described in several detailed studies and reviews (15, 79, 80, 94 –98). Note that there is some variability in the observations; this could reflect the patient populations studied or the criteria used for reporting cellular details. Eosinophilic macroglia become engorged by plasma proteins within hours (see above) (71). Pyknotic neurons can be seen less than one hour post-trauma. Neuron eosinophilia follows the same time course seen in HI (see above). Neutrophils begin to enter the tissue in one to three hours, while lymphocytes appear at 18 hours. Swollen axons are typically obvious at 10-24 hours (although note detailed consideration of APP immunostaining above). Mitotic figures among astrocytes and endothelial cells can be seen at two to three days and hypertrophic astrocytes are obvious by five to seven days. Hemosiderin-laden macrophages begin to appear at two days and increase gradually. Hemosiderin-containing astrocytes appear at five days. All of these features can appear over a protracted time course because of delayed HI from compromised blood flow due to herniation or raised intracranial pressure, or the toxicity of plasma proteins.
Special histochemical stains and immunohistochemistry can be used to highlight the evolution of cortical contusions. Anti-HLA-DR can show early microglial reaction and anti-CD68 can highlight macrophages (99). Anti-GFAP and anti-vimentin can be used to evaluate astrocyte changes. Anti-Ki67 shows cell proliferation from 3-15 days after contusion (100). Modified phosphotungstic acid-hematoxylin (PTAH) stain is very useful for demonstration of chronic, persistent alterations of the astrocyte cytoskeleton, even years after the hypertrophic forms have resolved (101).
Artifacts
Decomposition can make the evaluation of TBI or HI challenging (102, 103). Autolysis (i.e., digestion and degradation by unregulated enzymes from local cells) and putrefaction (i.e., decomposition as a consequence of bacterial growth) can create gross and cytologic changes (104). The brain should be carefully examined in situ to ascertain macroscopic changes that might not be obvious once the softened brain is removed from the skull. Many anaerobic bacteria produce endonucleases that can digest the DNA of brain cells (105), leading to a loss of nuclear staining that can be mistaken for ischemic damage. Careful microscopic inspection of blood vessel lumina or parenchyma for bacterial forms (usually bacilli) must be done to help interpret degenerative cell changes.
Dark neurons are commonly identified in histologic sections of the brain. They are characterized by shrunken cell bodies with hyperbasophilic staining of the nuclei and convoluted dendrites. Their cause and significance have been debated for over a century (106, 107). Dark neurons can be produced by mechanical handling of tissue prior to fixation (108). Physiologic changes prior to death can also cause them. In surgically excised tissue, prior depolarization seems to be important (109). Dark neurons appear at sites of contusion and areas of secondary compression in cases with short survival intervals (15). Damaged dark neurons may be indistinguishable from artifactual dark neurons (110). If the nucleoli are of normal size despite the shrunken cell, the change is more likely an artifact (111).
Conclusion
Forensic pathologists and neuropathologists predominantly rely on gross findings to diagnose TBI. However, histologic slides and paraffin blocks can serve as forensic evidence even in the absence of gross photographs. Histology should be used to augment macroscopic diagnoses, particularly in the absence of gross evidence of intracranial trauma. Histology can detect nontraumatic causes of seemingly traumatic lesions. Histology can help estimate the survival interval after an insult. However, even detailed documentation of cytologic, inflammatory, and immunohistochemical (including APP) changes is at best an approximation. Note that the legal time of death might not be the same as the biological time of death. In individuals who have been declared brain dead and whose bodies are kept alive until transplant harvesting occurs, the pathologist should consider the time of cardiac cessation. Regardless, it is crucial to be aware of the limitations of such an evaluation. An integrated consideration of the macroscopic and microscopic features, along with historical and medical evidence, are necessary to distinguish TBI from HI from artifact. Even if TBI is definite, examination of the brain in isolation cannot help distinguish between accidental and nonaccidental etiologies.
Authors
Petra Rahaman MD, Shared Health Manitoba - Pathology
Roles: Data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work.
Marc R. Del Bigio MD PhD FRCPC, University of Manitoba - Pathology
Roles: Project conception and/or design, data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work, principal investigator of the current study.
Footnotes
Ethical Approval: As per Journal Policies, ethical approval was not required for this manuscript
Statement of Human and Animal Rights: This article does not contain any studies conducted with animals or on living human subjects
Statement of Informed Consent: No identifiable personal data were presented in this manuscript
Disclosures & Declaration of Conflicts of Interest: The authors, reviewers, editors, and publication staff do not report any relevant conflicts of interest
Financial Disclosure: The authors have indicated that they do not have financial relationships to disclose that are relevant to this manuscript
References
- 1). Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012. December 6; 76(5):886–99. PMID: 23217738 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 2). Finnie JW. Forensic pathology of traumatic brain injury. Vet Pathol. 2016. September; 53(5):962–78. PMID: 26578643 10.1177/0300985815612155. [DOI] [PubMed] [Google Scholar]
- 3). Dettmeyer RB. Forensic histopathology. Berlin: Springer-Verlag; c2011. Chapter 20, Forensic neuropathology; p. 413–38. [Google Scholar]
- 4). Oehmichen M, Meissner C, von Wurmb-Schwark N, Schwark T. Methodical approach to brain hypoxia/ischemia as a fundamental problem in forensic neuropathology. Leg Med (Tokyo). 2003. December; 5(4):190–201. PMID: 14602162 10.1016/s1344-6223(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 5). Geddes JF, Whitwell HL, Graham DI. Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol Appl Neurobiol. 2000. April; 26(2):105–16. PMID: 10840273 10.1046/j.1365-2990.2000.026002105.x. [DOI] [PubMed] [Google Scholar]
- 6). Kalimo H, Saukko P, Graham D. Neuropathological examination in forensic context. Forensic Sci Int. 2004. December 16; 146(2-3):73–81. PMID: 15542266 10.1016/j.forsciint.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 7). Adams JH, Doyle D, Ford I, et al. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989. July; 15(1):49–59. PMID: 2767623 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 8). Schmidt-Kastner R. Genomic approach to selective vulnerability of the hippocampus in brain ischemia-hypoxia. Neuroscience. 2015. November 19; 309:259–79. PMID: 26383255 10.1016/j.neuroscience.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 9). Love S. Autopsy approach to stroke. Histopathology. 2011. February; 58(3):333–51. PMID: 20666847 10.1111/j.1365-2559.2010.03614.x. [DOI] [PubMed] [Google Scholar]
- 10). Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol. 2010. March; 119(3):277–90. PMID: 20155424. PMCID: PMC2831184 10.1007/s00401-010-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Del Bigio MR, Phillips SM. Retroocular and subdural hemorrhage or hemosiderin deposits in pediatric autopsies. J Neuropathol Exp Neurol. 2017. April 1; 76(4):313–322. PMID: 28340081 10.1093/jnen/nlx010. [DOI] [PubMed] [Google Scholar]
- 12). Lendrum AC, Fraser DS, Slidders W, Henderson R. Studies on the character and staining of fibrin. J Clin Pathol. 1962. September; 15:401–13. PMID: 13929601. PMCID: PMC480427 10.1136/jcp.15.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Garcia JH, Lossinsky AS, Kauffman FC, Conger KA. Neuronal ischemic injury: light microscopy, ultrastructure and biochemistry. Acta Neuropathol. 1978. August 7; 43(1-2):85–95. PMID: 97917 10.1007/bf00685002. [DOI] [PubMed] [Google Scholar]
- 14). Petito CK, Pulsinelli WA. Sequential development of reversible and irreversible neuronal damage following cerebral ischemia. J Neuropathol Exp Neurol. 1984. March; 43(2):141–53. PMID: 6707703 10.1097/00005072-198403000-00004. [DOI] [PubMed] [Google Scholar]
- 15). Anderson R. Timing of early changes in brain trauma. Am J Forensic Med Pathol. 1998. March; 19(1):1–9. 10.1097/00000433-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 16). Garcia JH, Liu KF, Ho KL. Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke. 1995. April; 26(4):636–42; discussion 643. PMID: 7709411 10.1161/01.str.26.4.636. [DOI] [PubMed] [Google Scholar]
- 17). Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005. February 21; 1035(1):24–31. PMID: 15713273 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 18). Kuhn J, Meissner C, Oehmichen M. Microtubule-associated protein 2 (MAP2)--a promising approach to diagnosis of forensic types of hypoxia-ischemia. Acta Neuropathol. 2005. December; 110(6):579–86. PMID: 16328528 10.1007/s00401-005-1090-9. [DOI] [PubMed] [Google Scholar]
- 19). Sager TN, Hansen AJ, Laursen H. Correlation between N-acetylaspartate levels and histopathologic changes in cortical infarcts of mice after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2000. May; 20(5):780–8. PMID: 10826528 10.1097/00004647-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 20). Chuaqui R, Tapia J. Histologic assessment of the age of recent brain infarcts in man. J Neuropathol Exp Neurol. 1993. September; 52(5):481–9. PMID: 8360701 10.1097/00005072-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 21). Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987. August; 37(8):1281–6. PMID: 3614648 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- 22). Kirino T. Delayed neuronal death. Neuropathology. 2000. September; 20 Suppl: S95–7. PMID: 11037198 10.1046/j.1440-1789.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- 23). Hausmann R, Seidl S, Betz P. Hypoxic changes in Purkinje cells of the human cerebellum. Int J Legal Med. 2007. May; 121(3):175–83. PMID: 17031692 10.1007/s00414-006-0122-x. [DOI] [PubMed] [Google Scholar]
- 24). Auer RN, Hugh J, Cosgrove E, Curry B. Neuropathologic findings in three cases of profound hypoglycemia. Clin Neuropathol. 1989. Mar-Apr; 8(2):63–8. PMID: 2721042. [PubMed] [Google Scholar]
- 25). Auer RN. Hypoglycemic brain damage. Forensic Sci Int. 2004. December 16; 146(2-3):105–10. PMID: 15542270 10.1016/j.forsciint.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 26). Adams H, Mitchell DE, Graham DI, Doyle D. Diffuse brain damage of immediate impact type. Its relationship to ‘primary brain-stem damage’ in head injury. Brain. 1977. September; 100(3):489–502. PMID: 589428 10.1093/brain/100.3.489. [DOI] [PubMed] [Google Scholar]
- 27). Adams JH, Graham DI, Scott G, et al. Brain damage in fatal nonmissile head injury. J Clin Pathol. 1980. December; 33(12):1132–45. PMID: 7451661. PMCID: PMC1146364 10.1136/jcp.33.12.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982. December; 12(6):557–63. PMID: 7259059 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 29). Pittella JE, Gusmao SN. Diffuse vascular injury in fatal road traffic accident victims: its relationship to diffuse axonal injury. J Forensic Sci. 2003. May; 48(3):626–30. PMID: 12762535 10.1520/jfs2002244. [DOI] [PubMed] [Google Scholar]
- 30). Davceva N, Sivevski A, Basheska N. Traumatic axonal injury, a clinical-pathological correlation. J Forensic Leg Med. 2017. May; 48:35–40. PMID: 28437717 10.1016/j.jflm.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 31). Ferguson SM. Axonal transport and maturation of lysosomes. Curr Opin Neurobiol. 2018. March 9; 51:45–51. PMID: 29529416 10.1016/j.conb.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J Cell Sci. 2012. May 1; 125(Pt 9):2095–104. PMID: 22619228. PMCID: PMC3656622 10.1242/jcs.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Marmarou CR, Walker SA, Davis CL, Povlishock JT. Quantitative analysis of the relationship between intraaxonal neurofilament compaction and impaired axonal transport following diffuse traumatic brain injury. J Neurotrauma. 2005. October; 22(10):1066–80. PMID: 16238484 10.1089/neu.2005.22.1066. [DOI] [PubMed] [Google Scholar]
- 34). Uryu K, Chen XH, Martinez D, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007. December; 208(2):185–92. PMID: 17826768. PMCID: PMC3979356 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Grady MS, McLaughlin MR, Christman CW, et al. The use of antibodies targeted against the neurofilament subunits for the detection of diffuse axonal injury in humans. J Neuropathol Exp Neurol. 1993. March; 52(2):143–52. PMID: 8440996 10.1097/00005072-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 36). Kobek M, Skowronek R, Jankowski Z, Palasz A. Neurofilaments and traumatic brain injury. Arch Med Sadowej Kryminol. 2014; 64(4):268–79. PMID: 25909921 10.5114/amsik.2014.50531. [DOI] [PubMed] [Google Scholar]
- 37). Johnson VE, Stewart W, Weber MT, et al. SNTF immunostaining reveals previously undetected axonal pathology in traumatic brain injury. Acta Neuropathol. 2016. January; 131(1):115–35. PMID: 26589592. PMCID: PMC4780426 10.1007/s00401-015-1506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Sherriff FE, Bridges LR, Gentleman SM, et al. Markers of axonal injury in post mortem human brain. Acta Neuropathol. 1994; 88(5):433–9. PMID: 7847072 10.1007/s004010050181. [DOI] [PubMed] [Google Scholar]
- 39). Ogata M, Tsuganezawa O. Neuron-specific enolase as an effective immunohistochemical marker for injured axons after fatal brain injury. Int J Legal Med. 1999; 113(1):19–25. PMID: 10654234 10.1007/s004140050273. [DOI] [PubMed] [Google Scholar]
- 40). Koo EH, Sisodia SS, Archer DR, et al. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990. February; 87(4):1561–5. PMID: 1689489. PMCID: PMC53515 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Cochran E, Bacci B, Chen Y, et al. Amyloid precursor protein and ubiquitin immunoreactivity in dystrophic axons is not unique to Alzheimer’s disease. Am J Pathol. 1991. September; 139(3):485–9. PMID: 1716043. PMCID: PMC1886228. [PMC free article] [PubMed] [Google Scholar]
- 42). Ohgami T, Kitamoto T, Tateishi J. Alzheimer’s amyloid precursor protein accumulates within axonal swellings in human brain lesions. Neurosci Lett. 1992. February 17; 136(1):75–8. PMID: 1635670. [DOI] [PubMed] [Google Scholar]
- 43). Nukina N, Kanazawa I, Mannen T, Uchida Y. Accumulation of amyloid precursor protein and beta-protein immunoreactivities in axons injured by cerebral infarct. Gerontology. 1992; 38 Suppl 1: 10–4. PMID: 1459467 10.1159/000213357. [DOI] [PubMed] [Google Scholar]
- 44). Smith DH, Chen XH, Iwata A, Graham DI. Amyloid beta accumulation in axons after traumatic brain injury in humans. J Neurosurg. 2003. May; 98(5):1072–7. PMID: 12744368 10.3171/jns.2003.98.5.1072. [DOI] [PubMed] [Google Scholar]
- 45). Gentleman SM, Nash MJ, Sweeting CJ, et al. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett. 1993. October 1; 160(2):139–44. PMID: 8247344 10.1016/0304-3940(93)90398-5. [DOI] [PubMed] [Google Scholar]
- 46). McKenzie KJ, McLellan DR, Gentleman SM, et al. Is beta-APP a marker of axonal damage in short-surviving head injury? Acta Neuropathol. 1996. December; 92(6):608–13. PMID: 8960319 10.1007/s004010050568. [DOI] [PubMed] [Google Scholar]
- 47). Morrison C, Mackenzie JM. Axonal injury in head injuries with very short survival times. Neuropathol Appl Neurobiol. 2008. February; 34(1): 124–5. PMID: 17971077 10.1111/j.1365-2990.2007.00876.x. [DOI] [PubMed] [Google Scholar]
- 48). Hortobagyi T, Wise S, Hunt N, et al. Traumatic axonal damage in the brain can be detected using beta-APP immunohistochemistry within 35 min after head injury to human adults. Neuropathol Appl Neurobiol. 2007. April; 33(2):226–37. PMID: 17359363 10.1111/j.1365-2990.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- 49). Stone JR, Okonkwo DO, Dialo AO, et al. Impaired axonal transport and altered axolemmal permeability occur in distinct populations of damaged axons following traumatic brain injury. Exp Neurol. 2004. November; 190(1):59–69. PMID: 16473980 10.1016/j.expneurol.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 50). Wilkinson AE, Bridges LR, Sivaloganathan S. Correlation of survival time with size of axonal swellings in diffuse axonal injury. Acta Neuropathol. 1999. August; 98(2):197–202. PMID: 10442560 10.1007/s004010051069. [DOI] [PubMed] [Google Scholar]
- 51). Blumbergs PC, Scott G, Manavis J, et al. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994. October 15; 344(8929):1055–6. PMID: 7523810 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- 52). Ahlgren S, Li GL, Olsson Y. Accumulation of beta-amyloid precursor protein and ubiquitin in axons after spinal cord trauma in humans: immunohistochemical observations on autopsy material. Acta Neuropathol. 1996. July; 92(1):49–55. PMID: 8811125 10.1007/s004010050488. [DOI] [PubMed] [Google Scholar]
- 53). Gleckman AM, Evans RJ, Bell MD, Smith TW. Optic nerve damage in shaken baby syndrome: detection by beta-amyloid precursor proteinimmunohistochemistry. ArchPatholLabMed. 2000. February; 124(2):251–6. PMID: 10645735 10.1016/s0002-9394(00)00508-0. [DOI] [PubMed] [Google Scholar]
- 54). Blumbergs PC, Scott G, Manavis J, et al. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma. 1995. August; 12(4):565–72. PMID: 8583607 10.1089/neu.1995.12.565. [DOI] [PubMed] [Google Scholar]
- 55). Johnson MW, Stoll L, Rubio A, et al. Axonal injury in young pediatric head trauma: a comparison study of β-amyloid precursor protein (β-APP) immunohistochemical staining in traumatic and nontraumatic deaths. J Forensic Sci. 2011. September; 56(5):1198–205. PMID: 21595698. PMCID: PMC4033509 10.1111/j.1556-4029.2011.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Geddes JF, Vowles GH, Beer TW, Ellison DW. The diagnosis of diffuse axonal injury: implications for forensic practice. Neuropathol Appl Neurobiol. 1997. August; 23(4):339–47. PMID: 9292874 10.1111/j.1365-2990.1997.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 57). Reichard RR, White CL, 3rd, Hladik CL, Dolinak D. Beta-amyloid precursor protein staining of nonaccidental central nervous system injury in pediatric autopsies. J Neurotrauma. 2003. April; 20(4):347–55. PMID: 12866814 10.1089/089771503765172309. [DOI] [PubMed] [Google Scholar]
- 58). Reichard RR, Smith C, Graham DI. The significance of beta-APP immunoreactivity in forensic practice. Neuropathol Appl Neurobiol. 2005; 31(3):304–13. PMID: 15885067 10.1111/j.1365-2990.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- 59). Hayashi T, Ago K, Ago M, Ogata M. Two patterns of beta-amyloid precursor protein (APP) immunoreactivity in cases of blunt head injury. Leg Med (Tokyo). 2009. April; 11 Suppl 1: S171–3. PMID: 19251455 10.1016/j.legalmed.2009.01.076. [DOI] [PubMed] [Google Scholar]
- 60). Hayashi T, Ago K, Nakamae T, et al. Two different immunostaining patterns of beta-amyloid precursor protein (APP) may distinguish traumatic from nontraumatic axonal injury. Int J Legal Med. 2015. September; 129(5):1085–90. PMID: 26249371 10.1007/s00414-015-1245-8. [DOI] [PubMed] [Google Scholar]
- 61). Davceva N, Basheska N, Balazic J. Diffuse axonal injury-a distinct clinicopathological entity in closed head injuries. Am J Forensic Med Pathol. 2015. September; 36(3):127–33. PMID: 26010053 10.1097/paf.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 62). Davceva N, Janevska V, Ilievski B, et al. Dilemmas concerning the diffuse axonal injury as a clinicopathological entity in forensic medical practice. J Forensic Leg Med. 2012. October; 19(7):413–8. PMID: 22920765 10.1016/j.jflm.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 63). Gleckman AM, Bell MD, Evans RJ, Smith TW. Diffuse axonal injury in infants with nonaccidental craniocerebral trauma: enhanced detection by beta-amyloid precursor protein immunohistochemical staining. Arch Pathol Lab Med. 1999. February; 123(2):146–51. PMID: 10050789. [DOI] [PubMed] [Google Scholar]
- 64). Lambri M, Djurovic V, Kibble M, et al. Specificity and sensitivity of betaAPP in head injury. Clin Neuropathol. 2001. Nov-Dec; 20(6):263–71. PMID: 11758782. [PubMed] [Google Scholar]
- 65). Graham DI, Smith C, Reichard R, et al. Trials and tribulations of using beta-amyloid precursor protein immunohistochemistry to evaluate traumatic brain injury in adults. Forensic Sci Int. 2004. December 16; 146(2-3):89–96. PMID: 15542268 10.1016/s0379-0738(03)00274-3. [DOI] [PubMed] [Google Scholar]
- 66). Abou-Hamden A, Blumbergs PC, Scott G, et al. Axonal injury in falls. J Neurotrauma. 1997. October; 14(10):699–713. PMID: 9383089 10.1089/neu.1997.14.699. [DOI] [PubMed] [Google Scholar]
- 67). Harrington D, Rutty GN, Timperley WR. β-amyloid precursor protein positive axonal bulbs may form in non-head-injured patients. J Clin Forensic Med. 2000; 7(1):19–25. PMID: 16083644 10.1054/jcfm.2000.0359. [DOI] [PubMed] [Google Scholar]
- 68). Oehmichen M, Meissner C, Schmidt V, et al. Axonal injury--a diagnostic tool in forensic neuropathology? A review. Forensic Sci Int. 1998. July 6; 95(1):67–83. PMID: 9718672 10.1016/s0379-0738(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 69). MacKenzie JM. Axonal injury in stroke: a forensic neuropathology perspective. Am J Forensic Med Pathol. 2015. September; 36(3):172–5. PMID: 26266889 10.1097/paf.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 70). Kaur B, Rutty GN, Timperley WR. The possible role of hypoxia in the formation of axonal bulbs. J Clin Pathol. 1999. March; 52(3):203–9. PMID: 10450180. PMCID: PMC501080 10.1136/jcp.52.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). Del Bigio MR, Deck JH, Davidson GS. Glial swelling with eosinophilia in human post-mortem brains: a change indicative of plasma extravasation. Acta Neuropathol. 2000. December; 100(6):688–94. PMID: 11078221 10.1007/s004010000236. [DOI] [PubMed] [Google Scholar]
- 72). Liu HM, Sturner WQ. Extravasation of plasma proteins in brain trauma. Forensic Sci Int. 1988. September; 38(3-4):285–95. PMID: 3056799 10.1016/0379-0738(88)90174-0. [DOI] [PubMed] [Google Scholar]
- 73). Loberg EM, Torvik A. Neuronal uptake of plasma proteins in brain contusions. An immunohistochemical study. Acta Neuropathol. 1992; 84(3):234–7. PMID: 1414276 10.1007/bf00227814. [DOI] [PubMed] [Google Scholar]
- 74). Loberg EM, Torvik A. Plasma proteins in normal neurons. Immunohistochemical studies on autopsy material and experimental animals. APMIS. 1992. May; 100(5):431–6. PMID: 1586480 10.1111/j.1699-0463.1992.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 75). Liu HM, Atack JR, Rapoport SI. Immunohistochemical localization of intracellular plasma proteins in the human central nervous system. Acta Neuropathol. 1989; 78(1):16–21. PMID: 2735186 10.1007/bf00687397. [DOI] [PubMed] [Google Scholar]
- 76). Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013. February; 39(1):3–18. PMID: 23252647 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- 77). Zrzavy T, Machado-Santos J, Christine S, et al. Dominant role of microglial and macrophage innate immune responses in human ischemic infarcts. Brain Pathol. 2017. December 8 PMID: 29222823 10.1111/bpa.12583. [DOI] [PMC free article] [PubMed]
- 78). Xue M, Balasubramaniam J, Del Bigio MR. Brain inflammation following intracerebral hemorrhage. Curr Neuropharmacol. 2003; 1(4):325–32. 10.2174/1570159033477008. [DOI] [Google Scholar]
- 79). Hausmann R. Timing of cortical contusions in human brain injury. Forensic Pathol Rev. 2004; 1:53–75. 10.1007/978-1-59259-786-4_3. [DOI] [Google Scholar]
- 80). Oehmichen M, Raff G. Timing of cortical contusion. Correlation between histomorphologic alterations and post-traumatic interval. Z Rechtsmed. 1980. January; 84(2):79–94. PMID: 7376745 10.1007/bf02114577. [DOI] [PubMed] [Google Scholar]
- 81). Olafson KN, Rimer JD, Vekilov PG. Growth of large hematin crystals in biomimetic solutions. Cryst Growth Des. 2014. May 7; 14(5):2123–2127. PMID: 24839403. PMCID: PMC4018177 10.1021/cg5002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82). Silberberg DH, Johnson L, Ritter L. Factors influencing toxicity of bilirubin in cerebellum tissue culture. J Pediatr. 1970. September; 77(3):386- 96. PMID: 5502088 10.1016/s0022-3476(70)80005-1 [DOI] [PubMed] [Google Scholar]
- 83). Ostrow JD, Hammaker L, Schmid R. The preparation of crystalline bilirubin-C14. J Clin Invest. 1961. August; 40:1442–52. PMID: 13731540. PMCID: PMC292520 10.1172/jci104375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84). Byard RW, Bellis M. The effect of decalcifying solutions on hemosiderin staining. J Forensic Sci. 2010. September; 55(5):1356–8. PMID: 20487146 10.1111/j.1556-4029.2010.01442.x. [DOI] [PubMed] [Google Scholar]
- 85). Curry AS, Price DE, Rutter ER. The production of cyanide in post mortem material. Acta Pharmacol Toxicol (Copenh). 1967;25(3):339–44. PMID: 5630491 10.1111/j.1600-0773.1967.tb01442.x. [DOI] [PubMed] [Google Scholar]
- 86). Rao MG, Singh D, Vashista RK, Sharma SK. Dating of acute and subacute subdural haemorrhage: a histo-pathological study. J Clin Diagn Res. 2016. July; 10(7): HC01-7 PMID: 27630864. PMCID: PMC5020299 10.7860/jcdr/2016/19783.8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87). Walter T, Meissner C, Oehmichen M. Pathomorphological staging of subdural hemorrhages: statistical analysis of posttraumatic histomorphological alterations. Leg Med (Tokyo). 2009. April; 11 Suppl 1: S56–62. PMID: 19299189 10.1016/j.legalmed.2009.01.112. [DOI] [PubMed] [Google Scholar]
- 88). Squier W, Mack J. The neuropathology of infant subdural haemorrhage. Forensic Sci Int. 2009. May 30; 187(1-3):6–13. PMID: 19303229 10.1016/j.forsciint.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 89). Leestma JE. Forensic neuropathology. Boca Raton: CRC Press; 2014. 811 p. [Google Scholar]
- 90). Castellani RJ, Mojica-Sanchez G, Schwartzbauer G, Hersh DS. Symptomatic acute-on-chronic subdural hematoma: a clinicopathological study. Am J Forensic Med Pathol. 2017. June; 38(2):126–130. PMID: 28319470 10.1097/paf.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 91). Ross JL, Sandberg GD, Powell SZ. Forensic evaluation of subarachnoid hemorrhage. Acad Forensic Pathol. 2012; 2(1):30–5. 10.23907/2012.004. [DOI] [Google Scholar]
- 92). Massicotte EM, Del Bigio MR. Human arachnoid villi response to subarachnoid hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurg. 1999. July; 91(1):80–4.PMID: 10389884 10.3171/jns.1999.91.1.0080. [DOI] [PubMed] [Google Scholar]
- 93). Xue M, Del Bigio MR. Intracortical hemorrhage injury in rats: relationship between blood fractions and brain cell death. Stroke. 2000. July; 31(7):1721–7. PMID: 10884479 10.1161/01.str.31.7.1721. [DOI] [PubMed] [Google Scholar]
- 94). Kobek M, Skowronek R, Jankowski Z, Palasz A. Angiogenesis in brain contusion. Arch Med Sadowej Kryminol. 2015; 65(2):112–24. PMID: 26284969 10.5114/amsik.2015.53227. [DOI] [PubMed] [Google Scholar]
- 95). Loberg EM, Torvik A. Brain contusions: the time sequence of the histological changes. Med Sci Law. 1989. April; 29(2):109–15. PMID; 2747472 10.1177/002580248902900205. [DOI] [PubMed] [Google Scholar]
- 96). Hausmann R, Kaiser A, Lang C, et al. A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. Int J Legal Med. 1999; 112(4):227–32. PMID: 10433032 10.1007/s004140050241. [DOI] [PubMed] [Google Scholar]
- 97). Hausmann R. Age determination of brain contusions. Forensic Sci Med Pathol. 2006. June; 2(2):85–93. PMID; 25868586 10.1385/fsmp:2:2:85. [DOI] [PubMed] [Google Scholar]
- 98). Mackenzie JM, Clayton JA. Early cellular events in the penumbra of human spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 1999. Jan-Feb; 8(1):1–8. PMID: 17895130 10.1016/s1052-3057(99)80032-9. [DOI] [PubMed] [Google Scholar]
- 99). Oehmichen M, Jakob S, Mann S, et al. Macrophage subsets in mechanical brain injury (MBI) -- a contribution to timing of MBI based on immunohistochemical methods: a pilot study. Leg Med (Tokyo). 2009. May; 11(3):118–24. PMID: 19121970 10.1016/j.legalmed.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 100). Hausmann R, Betz P. The course of MIB-1 expression by cerebral macrophages following human brain injury. Leg Med (Tokyo). 2002. June; 4(2):79–83. PMID: 12935673 10.1016/s1344-6223(02)00003-2. [DOI] [PubMed] [Google Scholar]
- 101). Manlow A, Munoz DG. A non-toxic method for the demonstration of gliosis. J Neuropathol Exp Neurol. 1992. May; 51(3):298–302. PMID: 1374794 10.1097/00005072-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 102). MacKenzie JM. Examining the decomposed brain. Am J Forensic Med Pathol. 2014. December; 35(4):265–70. PMID: 25384305 10.1097/paf.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 103). McInnes E. Artefacts in histopathology. Comp Clin Pathol. 2005; 13(3):100–8. 10.1007/s00580-004-0532-4. [DOI] [Google Scholar]
- 104). Pope A, Nixon RA. Proteases of human brain. Neurochem Res. 1984. March; 9(3):291–323. PMID: 6377107 10.1007/bf00963980. [DOI] [PubMed] [Google Scholar]
- 105). Bar W, Kratzer A, Machler M, Schmid W. Postmortem stability of DNA. Forensic Sci Int. 1988. October; 39(1):59–70. PMID: 2905319 10.1016/0379-0738(88)90118-1. [DOI] [PubMed] [Google Scholar]
- 106). Cammermeyer J. The importance of avoiding “dark” neurons in experimental neuropathology. Acta Neuropathol. 1961; 1(3):245–70. 10.1007/bf00687191. [DOI] [Google Scholar]
- 107). Cammermeyer J. I. An evaluation of the significance of the “dark” neuron. Ergeb Anat Entwicklungsgesch. 1962; 36:1–61. PMID: 14018067. [PubMed] [Google Scholar]
- 108). de Souza Queiroz L, de Paula Eduardo RM. Occurrence of dark neurons in living mechanically injured rat neocortex. Acta Neuropathol. 1977. April 29; 38(1):45–8. PMID: 855651 10.1007/bf00691275. [DOI] [PubMed] [Google Scholar]
- 109). Kherani ZS, Auer RN. Pharmacologic analysis of the mechanism of dark neuron production in cerebral cortex. Acta Neuropathol. 2008. October; 116(4):447–52. PMID: 18521615 10.1007/s00401-008-0386-y. [DOI] [PubMed] [Google Scholar]
- 110). Loberg EM, Torvik A. Distinction between artefactually shrunken and truly degenerated ‘dark’ neurons by in situ fixation with microwave irradiation. Neuropathol Appl Neurobiol. 1993. August; 19(4):359–63. PMID: 8232757 10.1111/j.1365-2990.1993.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 111). Cammermeyer J. The post-mortem origin and mechanism of neuronal hyperchromatosis and nuclear pyknosis. Exp Neurol. 1960. August; 2:379–405. PMID: 13807188 10.1016/0014-4886(60)90022-4. [DOI] [PubMed] [Google Scholar]