Abstract
Background
Contrast‐induced nephropathy (CIN) is associated with increased mortality after primary percutaneous coronary intervention (PCI) for ST‐segment elevation myocardial infarction (STEMI). Recently, fragmented QRS complex (fQRS) on 12‐lead electrocardiography has been introduced as a marker of cardiovascular disease and is associated with increased morbidity and mortality.
Hypothesis
fQRS on ECG is associated with CIN and in‐hospital mortality after primary PCI in patients with STEMI.
Methods
Eight hundred ninety‐five patients with first STEMI treated by primary PCI were enrolled in the study. Patients were divided into 2 groups according to the presence or absence of fQRS as shown by 12‐lead electrocardiography in the first 24 hours. fQRS was defined by presence of an additional R wave (R″), or notching of the S wave, or >1 R′ in 2 contiguous leads. Patients were then reallocated to 2 groups according to presence or absence of postprocedural CIN, which was defined as a rise in serum creatinine of 0.5 mg/dL or a 25% increase from baseline value within 72 hours after the procedure.
Results
Patients with a fQRS were older and had significantly lower left ventricular ejection fraction. CIN occurred in 77 (8.6%) patients. The prevalence of CIN and in‐hospital mortality was significantly higher in the fQRS(+) group. In multivariate analysis, fQRS was found to be an independent predictor of CIN (odds ratio: 3.125, P = 0.029) and in‐hospital mortality (odds ratio: 9.062, P = 0.009).
Conclusions
The fQRS is an independent predictor of postprocedural CIN and in‐hospital mortality in STEMI patients.
Keywords: Fragmented QRS complex, contrast‐induced nephropathy, ST‐segment elevation myocardial infarction, in‐hospital mortality
1. INTRODUCTION
Contrast‐induced nephropathy (CIN) is a serious issue after percutaneous coronary intervention (PCI), and it occurs more frequently after unplanned PCI, especially in acute ST‐elevation myocardial infarction (STEMI).1, 2, 3 The development of CIN is associated with prolonged hospitalization, increased cost, and increased in‐hospital and long‐term morbidity and mortality despite successful early coronary revascularization.4, 5 Although the pathogenesis of CIN is not completely understood, it is widely accepted that systemic inflammation plays an important role in the development of CIN.6, 7, 8
Fragmented QRS complexes (fQRS) can be determined by a routine 12‐lead resting electrocardiogram (ECG) with different QRS morphologies. It is mainly caused by the slowdown of electrical conduction because of myocardial fibrosis/scarring.9, 10, 11 Systemic inflammation has been shown to play a significant role in conduction disturbances.12 The possible reason of cardiac conduction disturbances seems to be related to myocardial inflammation, focal fibrosis, and ischemia within the conduction system.12 It has been shown that fQRS increased even in patients without cardiovascular disease but with rheumatoid arthritis, an inflammatory disease, in which it is speculated that inflammatory processes may play a pivotal role to produce fragmentations on ECG.13 The fQRS have been suggested to be a novel marker of morbidity and mortality in various cardiovascular diseases, including STEMI.14, 15, 16, 17, 18, 19 However, the role of fQRS for predicting the risk of CIN in STEMI patients undergoing primary PCI has not been investigated. Thus, we aimed to investigate whether fQRS is associated with development of CIN and in‐hospital mortality in STEMI patients treated by primary PCI.
2. METHODS
2.1. Patients and definitions
From December 2012 to December 2015, a total of 936 consecutive patients (mean age, 58.48 ± 13.09 years; 75.6% male) with a first acute STEMI who underwent primary PCI within 12 hours after symptom onset were enrolled in our study. The diagnosis of an acute STEMI was made according to an ECG obtained during admission with the presence of clinical symptoms and findings. Patients with chest pain that continued for >30 minutes and with the presence of new or presumed new ST‐segment elevation at the J‐point in ≥2 contiguous leads of ≥0.2 mV in leads V1, V2, or V3 and ≥0.1 mV in other leads were diagnosed as STEMI. Marked ST‐segment depression, which was maximal in leads V1 through V3, without ST‐segment elevation in other leads, was designated as posterior‐wall MI and included in the STEMI diagnosis.20
We excluded patients receiving hemodialysis treatment or administration of metformin and nonsteroidal anti‐inflammatory drugs, as well as patients with a history of recent surgery or trauma within the previous month, known malignancies, febrile conditions, autoimmune disorders, acute or chronic inflammatory disease, or history of recent infection. In addition, we excluded patients if they had contrast exposure 2 weeks before admission, if they died during primary PCI, or if they refused further treatment or were discharged early for various reasons. Patients with prior MI, bundle branch block, cardiomyopathy, organic valvular heart disease, Wolff‐Parkinson‐White syndrome, and permanent pacemakers were also excluded from this study.
Finally, 895 patients were enrolled in our study. Hypertension (HTN) was defined as blood pressure ≥140/90 mm Hg, based on ≥2 measurements, or the use of antihypertensive medications. Diabetes mellitus (DM) was defined as a fasting blood glucose ≥126 mg/dL, based on ≥2 measurements, or the use of hypoglycemic agents. Hypercholesterolemia was defined as a baseline cholesterol level of >200 mg/dL and/or a low‐density lipoprotein cholesterol level of >130 mg/dL, or previously diagnosed and treated hypercholesterolemia. Current smokers were those with regular smoking in the previous 6 months. Family history of coronary artery disease (CAD) was defined as a coronary event occurring before age 55 years and 65 years in first‐degree male and female relatives, respectively. The study protocol was approved by our institutional ethics committee. Informed consent was taken from all patients enrolled before the study.
2.2. Clinical and laboratory data
The baseline clinical characteristics and risk factors for CAD were collected, including age, sex, smoking history, HTN, DM, and stroke. Routine blood samples were obtained from all patients at the time of admission. Baseline creatinine (Cr), estimated glomerular filtration rate (eGFR), white blood cell (WBC) count, platelet count, and hemoglobin levels were measured. High‐sensitivity C‐reactive protein (hs‐CRP) levels were determined by standard methods on admission. Fasting glucose and lipid profile were performed after fasting for ≥8 hours within the first 24 hours. Cardiac biomarker levels including troponin I (cTnI), creatine kinase, and creatine kinase–myocardial band were also measured on admission and 8 hours after admission, and then their peak values were recorded for analysis. eGFR was calculated using the Modification of Diet in Renal Disease study equation.21 CIN was defined as an acute deterioration of renal function in which an increase in serum Cr concentration of 0.5 mg/dL or 25% above the baseline value occurs within 72 hours after the procedure.22
The standard 12‐lead ECG (model ECG‐1550K; Nihon Kohden Corporation, Tokyo, Japan; filter range, 0.15–150 Hz, 25 mm/s, 10 mm/mV) of each patient was obtained on hospital admission, and repeated ECGs were obtained in the 2 hours and 24 hours after primary PCI. ECGs were assessed by 2 independent experienced cardiologists blinded to the clinical data, the cardiac catheterization results, and laboratory data. There was 97% agreement between the 2 readers in defining fQRS. Any disagreement was adjudicated by a third cardiologist. The intraobserver agreement was 96%. The intraobserver and interobserver reliabilities for detecting the presence of fQRS were κ values of 0.967 (P < 0.001) and 0.942 (P < 0.001), respectively. All patients had repeated ECGs during follow‐up. We determined the κ value for intraobserver reproducibility as 0.945 (P < 0.001).
The study patients were divided into 2 groups according to the presence or absence of fQRS as shown by 12‐lead ECGs in the first 24 hours after primary PCI. fQRS was defined by the presence of various RSR′ patterns with or without a Q wave and included an additional R wave, without a typical bundle branch block, notching of the R wave, notching of the downstroke or upstroke of the S wave, or the presence of >1 additional R wave in 2 contiguous leads corresponding to a major coronary artery territory.14 The extent of fQRS in each patient was assessed by counting the number of ECG leads with fQRS. In patients with fQRS, the number of spikes in fQRS configuration was calculated in the lead containing the maximal number of spikes.
Standard 2‐dimensional transthoracic echocardiography was carried out on all patients within 2 days of admission by a single investigator, and the left ventricular ejection fraction (LVEF) was calculated by using the Simpson method in the apical 4‐chamber view (Vivid 3; GE Medical Systems, Horten, Norway).
2.3. Coronary angiography and interventions
Diagnostic coronary angiographies and primary PCI procedures were performed through the femoral artery approach according to standard clinical practice (Axiom Artis zee system; Siemens Healthcare, Forchheim, Germany). Iohexol (Omnipaque 350 mg/mL; GE Healthcare, Cork, Ireland), a nonionic iso‐osmolar contrast medium, was used for all patients. Patients in the emergency department received a bolus of 5000 U of unfractionated heparin, followed by additional intraprocedural boluses to maintain an activated clotting time of 200 to 250 seconds (≥300 seconds when glycoprotein IIb/IIIa receptor inhibitor was not used), an oral aspirin 300 mg, and an oral clopidogrel loading dose of 600 mg. The use of glycoprotein IIb/IIIa inhibitors as well as bare‐metal or drug‐eluting stents was left to the discretion of the interventional cardiologist. Multivessel disease was defined as presence of a stenosis >50% in ≥2 major epicardial coronary arteries. The SYNTAX score for each patient was calculated by scoring all coronary lesions with a diameter stenosis of ≥50%, in vessels ≥1.5 mm, using the SYNTAX score algorithm (available on the SYNTAX score website, http://www.syntaxscore.com). Immediately after the procedure, all patients underwent intravenous hydration with isotonic saline (0.9%) at a rate of 1 mL/kg/h until 12 hours (or 0.5 mL/kg/h for 12 hours in cases of LVEF <40% or overt heart failure).
2.4. Statistical analysis
All statistical analyses were performed using SPSS statistical software version 18.0 (SPSS Inc., Chicago, Illinois). Continuous variables are represented as mean ± SD or as median (interquartile range). The Kolmogorov‐Smirnov criterion was used for the assessment of normality. Differences between the means were compared by unpaired t test when the continuous variables showed normal distribution, or by the Mann‐Whitney U test when they did not. Categorical variables were presented as counts and percentages and compared between the groups using the χ2 test. Intraobserver and interobserver reliability analyses using the κ statistic were applied to evaluate the consistency in determination of fQRS. Receiver operating characteristic (ROC) curve analysis was used to determine the optimum cutoff levels of the presence of fQRS and number of derivations with fQRS to predict the development of CIN and in‐hospital mortality. To identify the independent predictors of CIN and in‐hospital mortality, stepwise univariate and multivariate logistic regression analyses were performed. The goodness‐of‐fit assumption was assessed using the Hosmer‐Lemeshow method and satisfied when P was >0.05. All 2‐sided P values <0.05 were considered to represent statistical significance.
3. RESULTS
Among the 895 patients included in the study, 619 (69.2%) did not have fQRS in ≥2 contiguous leads on the 12‐lead ECG (fQRS[−] group), whereas 276 (30.8%) did (fQRS[+] group). Baseline clinical characteristics, laboratory data, and angiographic findings categorized according to the presence or absence of fQRS are summarized in Table 1. There was no significant difference between fQRS(−) and fQRS(+) groups with regard to baseline clinical characteristics such as sex, current smoking, HTN, DM, hypercholesterolemia, family history of CAD, and time from chest pain to PCI. Patients with fQRS were older, with lower body mass index and systolic blood pressure, compared with patients without fQRS. Heart rate and the prevalence of Killip class ≥2 on admission were significantly higher in the fQRS(+) group. Patients with fQRS had higher cardiac biomarker level including peak cTnI (P < 0.001) and peak creatine kinase–myocardial band level (P < 0.001). They also had a significantly higher level of WBC; higher hs‐CRP, serum Cr, and SYNTAX score; and lower LVEF and eGFR compared with patients without fQRS. Prevalence of multivessel disease, chronic total occlusion, and postprocedural no‐reflow phenomenon was higher in patients with fQRS.
Table 1.
Baseline clinical, laboratory, and angiographic characteristics
| Variable | fQRS(−), n = 619 | fQRS(+), n = 276 | P Value |
|---|---|---|---|
| Age, y | 57.5 ± 12.6 | 60.4 ± 13.8 | 0.002 |
| Male sex | 466 (75.3) | 211 (76.4) | 0.707 |
| BMI, kg/m2 | 28.2 ± 4.3 | 27.3 ± 4.3 | 0.016 |
| HTN | 235 (38.0) | 107 (38.8) | 0.819 |
| DM | 174 (28.1) | 75 (27.2) | 0.773 |
| Current smoker | 333 (53.8) | 155 (56.2) | 0.512 |
| Hypercholesterolemia | 158 (25.6) | 81 (29.3) | 0.374 |
| Family history of CAD | 188 (30.4) | 88 (31.9) | 0.651 |
| Prior CABG | 14 (2.3) | 9 (3.3) | 0.383 |
| SBP, mm Hg | 129 ± 23 | 125 ± 26 | 0.020 |
| DBP, mm Hg | 78 ± 14 | 77 ± 15 | 0.325 |
| Heart rate, bpm | 78 ± 15 | 81 ± 18 | 0.034 |
| Infarct location | 0.138 | ||
| Anterior | 265 (42.8) | 135 (48.9) | |
| Inferior | 298 (48.1) | 124 (44.9) | |
| Other (lateral, true posterior) | 56 (9.0) | 17 (6.2) | |
| LVEF, % | 47 ± 10 | 42 ± 10 | <0.001 |
| Killip class ≥2 on admission | 23 (3.7) | 32 (11.4) | <0.001 |
| Time from chest pain to primary PCI | 0.058 | ||
| 0–6 hours | 501 (80.9) | 208 (75.4) | |
| 6–12 hours | 118 (19.1) | 68 (24.6) | |
| Total ischemic time, min | 150 (120–240) | 180 (120–360) | 0.190 |
| WBC count, ×109/L | 11.5 ± 3.2 | 12.5 ± 4.0 | <0.001 |
| Platelet count, ×109/L | 245 ± 66 | 250 ± 75 | 0.321 |
| Hgb, g/dL | 14.3 ± 1.7 | 14.1 ± 1.9 | 0.140 |
| Glucose, mg/dL | 156 ± 79 | 152 ± 81 | 0.581 |
| Cr, mg/dL | 1.06 ± 0.24 | 1.12 ± 0.31 | 0.005 |
| GFR, mL/min/1.73 m2 | 75.5 ± 19.1 | 71.0 ± 21.2 | 0.002 |
| Peak CK‐MB, U/L | 61 (23–145) | 113 (54–227) | <0.001 |
| Peak TnT, ng/mL | 2300 (737–7312) | 4297 (1659–10000) | <0.001 |
| Total cholesterol, mg/dL | 192 ± 47 | 188 ± 45 | 0.293 |
| HDL‐C, mg/dL | 39 ± 8 | 40 ± 9 | 0.245 |
| LDL‐C, mg/dL | 123 ± 40 | 119 ± 40 | 0.287 |
| TG, mg/dL | 142 (94–204) | 137 (94–185) | 0.338 |
| hs‐CRP, mg/L | 7.21 (2.75–10.6) | 9.91 (3.32–14.6) | 0.001 |
| Initial IRA patency | 290 (46.8) | 69 (25.0) | <0.001 |
| Postprocedural no‐reflow phenomenon | 81 (13.1) | 78 (28.3) | <0.001 |
| Multivessel CAD | 308 (49.8) | 160 (58.0) | 0.023 |
| Presence of chronic total occlusion | 62 (10.0) | 44 (15.9) | 0.011 |
| SYNTAX score | 15.4 ± 8.3 | 19.7 ± 8.7 | <0.001 |
| ST‐segment resolution in ECG | 527 (85.2) | 121 (44.1) | <0.001 |
| Stent diameter, mm | 3.18 ± 0.45 | 3.21 ± 0.42 | 0.348 |
| Total stent length, mm | 25.3 ± 12.0 | 27.6 ± 12.6 | 0.011 |
| CIN | 14 (2.3) | 63 (22.8) | <0.001 |
| In‐hospital mortality | 2 (0.3) | 21 (7.6) | <0.001 |
Abbreviations: BMI, body mass index; CABG, coronary artery bypass surgery; CAD, coronary artery disease; CIN, contrast‐induced nephropathy; CK‐MB, creatine kinase–myocardial band; Cr, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiography; fQRS, fragmented QRS complex; GFR, glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; Hgb, hemoglobin; hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; IQR, interquartile range; IRA, infarct‐related artery; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD, standard deviation; TG, triglycerides; TnT, troponin T; WBC, white blood cell.
Data are presented as n (%), mean ± SD, or median (IQR).
The incidence of CIN and in‐hospital mortality was significantly higher in the fQRS(+) group, compared with the fQRS(−) group. Table 2 lists the clinical, laboratory, and angiographic characteristics of patients with and without CIN. Of 895 patients, 77 (8.6%) developed CIN after primary PCI. Patients with CIN tended to be older, were more often female, had a higher prevalence of HTN, DM, and Killip class ≥2 on admission, and had lower prevalence of smoking, lower LVEF, and were more likely to present with higher Cr, glucose, WBC, hs‐CRP, and peak Tn values than patients without CIN. Hemoglobin and eGFR levels were significantly lower in patients with CIN. Patients with CIN had a higher SYNTAX score and total stent length than those without CIN. In addition, patients developing CIN more frequently had multivessel disease and postprocedural no‐reflow, and less frequently had complete ST‐segment resolution on ECG. The rate of presence of fQRS and in‐hospital mortality was also higher in patients with CIN.
Table 2.
Hematological and biochemical measurements of the study population
| Variable | CIN | P Value | |
|---|---|---|---|
| No, n = 818 | Yes, n = 77 | ||
| Age, y | 57.4 ± 12.5 | 69.0 ± 14.0 | <0.001 |
| Female sex | 187 (22.9) | 31 (40.3) | 0.001 |
| BMI, kg/m2 | 28.0 ± 4.3 | 27.3 ± 4.5 | 0.218 |
| HTN | 297 (36.3) | 45 (58.4) | <0.001 |
| DM | 217 (26.5) | 32 (41.6) | 0.005 |
| Current smoker | 471 (57.6) | 17 (22.1) | <0.001 |
| Hypercholesterolemia | 220 (26.9) | 19 (24.7) | 0.879 |
| Prior CABG | 19 (2.3) | 4 (5.2) | 0.128 |
| SBP, mm Hg | 128 ± 24 | 126 ± 29 | 0.612 |
| DBP, mm Hg | 78 ± 14 | 77 ± 17 | 0.461 |
| Infarct location | 0.113 | ||
| Anterior | 360 (44.0) | 40 (51.9) | |
| Inferior | 394 (48.2) | 28 (36.4) | |
| Other (lateral, true posterior) | 64 (7.8) | 9 (11.7) | |
| LVEF, % | 46 ± 10 | 40 ± 11 | <0.001 |
| Killip class ≥2 on admission | 45 (5.5) | 10 (13.0) | 0.009 |
| Time from chest pain to primary PCI | 0.240 | ||
| 0–6 hours | 652 (79.7) | 57 (74.0) | |
| 6–12 hours | 166 (20.3) | 20 (26.0) | |
| WBC count, ×109/L | 11.7 ± 3.4 | 12.6 ± 3.9 | 0.034 |
| Platelet count, ×109/L | 246 ± 67 | 250 ± 90 | 0.653 |
| Hgb, g/dL | 14.4 ± 1.7 | 12.6 ± 3.9 | <0.001 |
| Glucose, mg/dL | 153 ± 78 | 174 ± 90 | 0.030 |
| Cr, mg/dL | 1.06 ± 0.24 | 1.29 ± 0.40 | <0.001 |
| GFR, mL/min/1.73 m2 | 75.9 ± 18.8 | 55.1 ± 21.1 | <0.001 |
| Peak TnT, ng/mL | 2758 (843–7824) | 5967 (1596–10 000) | 0.008 |
| Postprocedural no‐reflow phenomenon | 132 (16.1) | 27 (35.1) | <0.001 |
| Multivessel CAD | 418 (51.1) | 50 (64.9) | 0.020 |
| Total procedure time, min | 36.7 ± 16.4 | 38.9 ± 17.9 | 0.406 |
| Total amount of contrast, mL | 170 ± 73 | 168 ± 75 | 0.862 |
| SYNTAX score | 16.4 ± 8.5 | 20.8 ± 9.6 | <0.001 |
| Stent diameter, mm | 3.19 ± 0.44 | 3.12 ± 0.39 | 0.177 |
| Total stent length, mm | 25.8 ± 12.4 | 28.9 ± 13.3 | 0.038 |
| ST‐segment resolution in ECG | 573 (70.1) | 32 (42.5) | <0.001 |
| Presence of fQRS | 213 (26.0) | 63 (81.8) | <0.001 |
| In‐hospital mortality | 8 (1.0) | 15 (19.5) | <0.001 |
| hs‐CRP, mg/L | 7.36 (2.81–10.60) | 10.6 (5.08–14.92) | 0.005 |
| Prior medications | |||
| ASA | 81 (10.0) | 10 (13.3) | 0.557 |
| β‐Blocker | 157 (19.3) | 18 (23.3) | 0.398 |
| Angiotensin‐aldosterone system antagonist | 228 (27.9) | 28 (36.7) | 0.210 |
| Statin | 56 (6.9) | 7 (9.3) | 0.590 |
Abbreviations: ASA, acetylsalicylic acid (aspirin); BMI, body mass index; CABG, coronary artery bypass surgery; CAD, coronary artery disease; CIN, contrast‐induced nephropathy; Cr, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiography; fQRS, fragmented QRS complex; GFR, glomerular filtration rate; Hgb, hemoglobin; hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; IQR, interquartile range; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD, standard deviation; TnT, troponin T; WBC, white blood cell.
Data are presented as n (%), mean ± SD, or median (IQR).
In ROC curve analysis, the area under the curve for predicting postprocedural CIN was 0.779 (sensitivity 81.8%, specificity 74.0%, 95% confidence interval [CI]: 0.726‐0.832) for presence of fQRS, and it was 0.794 (sensitivity 80.5%, specificity 78.2%, 95% CI: 0.740‐0.847) for the total number of fQRS ≥3 leads (Figure, 1A). ROC curve analysis also revealed that the area under the curve for predicting in‐hospital mortality was 0.810 (sensitivity 91.3%, specificity 70.8%, 95% CI: 0.737‐0.883) for presence of fQRS, and it was 0.831 (sensitivity 91.3%, specificity 74.9%, 95% CI: 0.760‐0.902) for the total number of fQRS ≥3 leads (Figure, 1B).
Figure 1.
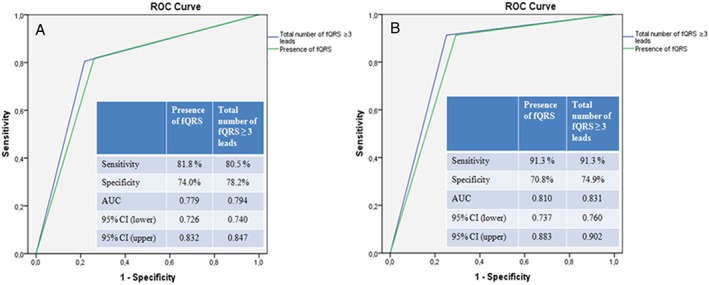
(A) ROC curve analysis of presence of fQRS and total number of fQRS ≥3 leads for predicting CIN. (B) ROC curve analysis of presence of fQRS and total number of fQRS ≥3 leads for predicting in‐hospital mortality. Abbreviations: AUC, area under the curve; CI, confidence interval; CIN, contrast‐induced nephropathy; fQRS, fragmented QRS complex; ROC, receiver operating characteristic.
In multivariate logistic regression analysis, after adjusting for baseline variables, presence of fQRS (odds ratio [OR]: 3.125, 95% CI: 1.127‐8.695, P = 0.029), older age (OR: 1.057, 95% CI: 1.013‐1.104, P = 0.012), Cr (OR: 5.476, 95% CI: 1.410‐21.268, P = 0.014), and WBC count (OR: 1.160, 95% CI: 1.005‐1.340, P = 0.043) all were determined as independent predictors of CIN (Table 3). On final logistic regression analysis, after adjusting for baseline variables, the presence of fQRS complex ≥3 leads (OR: 9.062, 95% CI: 1.722‐47.681, P = 0.009), LVEF (OR: 0.920, 95% CI: 0.852‐0.995, P = 0.036), and multivessel disease (OR: 7.981, 95% CI: 1.080‐58.990, P = 0.042) were all independent predictors of in‐hospital mortality (Table 4).
Table 3.
Independent predictors of postprocedural CIN in patients with STEMI
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | 1.070 (1.050‐1.091) | <0.001 | 1.057 (1.013‐1.104) | 0.012 |
| Female sex | 2.274 (1.402‐3.689) | 0.001 | 1.454 (0.465‐4.541) | 0.520 |
| HTN | 2.469 (1.533‐3.968) | <0.001 | 1.091 (0.440‐2.703) | 0.851 |
| DM | 1.968 (1.219‐3.184) | 0.006 | 1.160 (0.441‐3.048) | 0.763 |
| Current smoker | 0.208 (0.119‐0.364) | <0.001 | 0.284 (0.079‐1.009) | 0.052 |
| Killip class ≥2 on admission | 2.564 (1.236‐5.319) | 0.011 | 1.500 (0.384‐3.857) | 0.559 |
| LVEF | 0.939 (0.917‐0.963) | <0.001 | 0.999 (0.951‐1.049) | 0.967 |
| Hgb | 0.725 (0.646‐0.815) | <0.001 | 0.818 (0.636‐1.052) | 0.118 |
| Cr | 9.881 (4.842‐20.162) | <0.001 | 5.476 (1.410‐21.268 | 0.014 |
| Postprocedural no‐reflow | 0.356 (0.215‐0.589) | <0.001 | 1.298 (0.445‐3.787) | 0.632 |
| fQRS complex | 12.820 (6.993‐23.255) | <0.001 | 3.125 (1.127‐8.695) | 0.029 |
| Total stent length | 1.017 (1.001‐1.044) | 0.040 | 1.003 (0.971‐1.037) | 0.839 |
| hs‐CRP | 1.038 (1.009‐1.068) | 0.009 | 1.037 (0.958‐1.122) | 0.362 |
| WBC count | 1.068 (1.005‐1.135) | 0.035 | 1.160 (1.005‐1.340) | 0.043 |
| SYNTAX score | 1.055 (1.028‐1.083) | <0.001 | 1.026 (0.971‐1.085) | 0.356 |
| ST‐segment resolution in ECG | 0.314 (0.163‐0.606) | 0.001 | 0.584 (0.210‐1.623) | 0.302 |
Abbreviations: CI, confidence interval; CIN, contrast‐induced nephropathy; Cr, creatinine; DM, diabetes mellitus; ECG, electrocardiography; fQRS, fragmented QRS complex; Hgb, hemoglobin; hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; IQR, interquartile range; LVEF, left ventricular ejection fraction; OR, odds ratio; STEMI, ST‐segment elevation myocardial infarction; WBC, white blood cell.
Table 4.
Independent predictors of in‐hospital mortality in patients with STEMI
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | 1.077 (1.042‐1.113) | <0.001 | 1.052 (0.983‐1.126) | 0.145 |
| Female sex | 2.039 (0.870‐4.779) | 0.101 | ||
| HTN | 1.498 (0.654‐3.434) | 0.339 | ||
| DM | 1.139 (0.463‐2.803) | 0.777 | ||
| Current smoker | 0.356 (0.145‐0.873) | 0.024 | 0.626 (0.111‐3.523) | 0.595 |
| Killip class ≥2 on admission | 17.250 (7.209‐41.278) | <0.001 | 2.067 (0.458‐9.335) | 0.345 |
| LVEF | 0.877 (0.836‐0.920) | <0.001 | 0.920 (0.852‐0.995) | 0.036 |
| Hgb | 0.763 (0.627‐0.928) | 0.007 | 0.755 (0.558‐1.021) | 0.068 |
| Cr | 10.427 (3.994‐27.220) | <0.001 | 2.358 (0.375‐14.807) | 0.360 |
| Glucose | 1.005 (1.002‐1.009) | 0.005 | 1.003 (0.996‐1.010) | 0.399 |
| Postprocedural no‐reflow | 7.799 (3.313‐18.360) | <0.001 | 3.124 (0.794‐12.281) | 0.103 |
| fQRS complex ≥3 leads | 31.308 (7.282‐134.603) | <0.001 | 9.062 (1.722‐47.681) | 0.009 |
| Multivessel disease | 2.645 (1.033‐6.772) | 0.043 | 7.981 (1.080‐58.990) | 0.042 |
| Chronic total occlusion | 4.212 (1.741‐10.190) | 0.001 | 1.921 (0.384‐9.610) | 0.427 |
| WBC count | 1.219 (1.116‐1.330) | <0.001 | 1.085 (0.900‐1.310) | 0.392 |
| Peak CK‐MB | 1.003 (0.999‐1.007) | 0.168 | ||
| hs‐CRP | 1.048 (1.010‐1.088) | 0.013 | 1.000 (0.954‐1.047) | 0.992 |
Abbreviations: CI, confidence interval; CK‐MB, creatine kinase–myocardial band; Cr, creatinine; DM, diabetes mellitus; fQRS, fragmented QRS complex; Hgb, hemoglobin; hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; LVEF, left ventricular ejection fraction; OR, odds ratio; STEMI, ST‐segment elevation myocardial infarction; WBC, white blood cell.
4. DISCUSSION
To the best of our knowledge, our study is the first that investigates the predictive significance of the fQRS for CIN in patients who underwent primary PCI. The main findings of our study are as follows: (1) the presence of fQRS was demonstrated as an independent predictor of CIN in patients with STEMI who were treated with primary PCI; and (2) the presence of fQRS ≥3 leads was found as the cutoff point in discriminating patients with CIN and also in‐hospital mortality.
Despite improvements in treatment modalities, CIN is still a major issue after PCI. Patients with acute STEMI are more prone to this complication. CIN is significantly associated with poor clinical outcomes, including mortality, after primary PCI for STEMI.23, 24 Although the exact mechanism of CIN is still not completely understood, there is increasing evidence to support the critical role of inflammatory response in the pathogenesis of CIN.6 Patti et al7 demonstrated that short‐term pretreatment with high‐dose atorvastatin load prevents CIN and shortens hospital stay in patients with acute coronary syndrome undergoing PCI, and they suggested that anti‐inflammatory effects may be involved in this renal protection. In addition, Liu et al8 showed that hs‐CRP predicts CIN after primary PCI. In our study population, hs‐CRP was not an independent variable for predicting CIN; but WBC count, as another inflammatory marker, was independently associated with CIN development.
The fQRS on 12‐lead ECG is defined by the presence of an additional R wave or notching in the nadir of the S wave, or the presence of >1 additional R wave (fragmentation) in 2 contiguous leads corresponding to a major coronary artery territory.14 The presence of fQRS, which include various RSR′ patterns without typical bundle branch block on routine 12‐lead ECG, has recently been proposed as a reliable and sensitive prognostic marker in numerous cardiovascular diseases.14, 15, 16, 17, 18, 19 A standard resting 12‐lead ECG is an important part of the evaluation of acute STEMI. In patients with STEMI, fQRS on the 12‐lead ECG is a significant predictor of long‐term mortality.9, 17 Das et al16 concluded that fQRS on 12‐lead ECG is a moderately sensitive but highly specific sign for STEMI and non–ST‐segment elevation myocardial infarction, and presence of fQRS is an independent predictor of mortality in these patients. A recently published study has shown that the presence of fQRS is a predictor in STEMI patients undergoing primary PCI, and the occurrence of fQRS is beneficial to identify the patients with severe coronary lesion, left ventricular contraction dysfunction, and larger areas of ischemic injury.18
Although the exact mechanisms of fQRS are still unknown, the widely accepted hypotheses are conduction abnormalities or peri‐infarction conduction block due to myocardial necrosis, scarring, and inflammation.10, 11, 12, 13, 25, 26 Systemic inflammation has been shown to play a significant role in cardiac arrhythmias and conduction disturbances. The possible reason for cardiac arrhythmias and conduction disturbances seems to be related to myocardial inflammation, focal fibrosis, and ischemia within the conduction system.12 In a previous study, Kadi et al13 showed that fQRS increased even in patients with rheumatoid arthritis but without cardiovascular disease, speculating that inflammatory processes may play a pivotal role in producing fragmentations on ECG. With regard to cardiovascular diseases, Zhang et al27 demonstrated that CRP, as an inflammatory marker, is able to directly induce cardiac fibrosis and inflammation on cardiac fibroblasts and also promotes angiotensin II–mediated cardiac remodeling. In 2 previously published studies, Çetin et al25, 26 showed that fQRS was independently related with increased CRP in patients with stable CAD and acute coronary syndrome, so the development of fQRS may be associated with systemic inflammation in these patients. Our results confirmed a relationship between fQRS and hs‐CRP that probably results from structural and conduction alterations in myocardial tissue because of increased inflammatory response. In addition, presence of fQRS and WBC count were independent predictors of CIN in our population, whereas hs‐CRP was not.
Because increased inflammatory response is associated with the development of both of fQRS and CIN, we hypothesized that fQRS would be associated with CIN after contrast‐agent exposure. Therefore, we investigated whether fQRS on ECG is independently associated with CIN and in‐hospital mortality in patients with STEMI who underwent primary PCI. We speculated that the sequence of ventricular depolarization was altered due to myocardial conduction delay, owing to increased systemic inflammation together with myocardial scar and ischemia, and increased inflammatory response may also simultaneously trigger the development of CIN. In our study, we found that the fQRS was, together with older age, Cr, and WBC count, independently associated with the development of CIN after primary PCI. We also demonstrated that the presence of fQRS complex ≥3 leads was, together with LVEF and multivessel disease, an independent predictor of in‐hospital mortality in the study population. Consequently, fQRS can be used to identify patients with high risk for CIN development and in‐hospital mortality at an early stage. Overall, we have suggested that fQRS is a new tool for evaluating STEMI patients and it allows for a quick risk‐stratification in a select group of patients.
4.1. Study limitations
The present study has several limitations. First, to analyze QRS fragmentation, we included only patients without previous MI. Second, we only included patients with a QRS duration <120 ms in the study. We excluded patients with complete bundle branch block, intraventricular conduction delay, and permanent pacemakers; therefore, our results do not apply to patients with wide QRS complexes. Finally, some high‐risk patients were excluded from the study because the patients died during or shortly after primary PCI or shortly after the procedure. Therefore, postprocedural event rates in real clinical situations would be higher than the rates found during the present study.
5. CONCLUSION
The presence of fQRS was strongly associated with development of CIN in patients with STEMI treated by primary PCI. Furthermore, total number of fQRS ≥3 leads was identified as an independent predictor of in‐hospital mortality in STEMI patients.
Conflicts of interest
The authors declare no potential conflicts of interest.
Kurtul A and Duran M. Fragmented QRS complex predicts contrast‐induced nephropathy and in‐hospital mortality after primary percutaneous coronary intervention in patients with ST‐segment elevation myocardial infarction, Clin Cardiol, 2017. 10.1002/clc.22651
REFERENCES
- 1. Sadeghi HM, Stone GW, Grines CL, et al. Impact of renal insufficiency in patients undergoing primary angioplasty for acute myocardial infarction. Circulation. 2003;108:2769–2775. [DOI] [PubMed] [Google Scholar]
- 2. Marenzi G, Lauri G, Assanelli E, et al. Contrast‐induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. [DOI] [PubMed] [Google Scholar]
- 3. Liu YH, Liu Y, Zhou YL, et al. Comparison of different risk scores for predicting contrast induced nephropathy and outcomes after primary percutaneous coronary intervention in patients with ST elevation myocardial infarction. Am J Cardiol. 2016;117:1896–1903. [DOI] [PubMed] [Google Scholar]
- 4. Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. [DOI] [PubMed] [Google Scholar]
- 5. Senoo T, Motohiro M, Kamihata H, et al. Contrast‐induced nephropathy in patients undergoing emergency percutaneous coronary intervention for acute coronary syndrome. Am J Cardiol. 2010;105:624–628. [DOI] [PubMed] [Google Scholar]
- 6. Kwasa EA, Vinayak S, Armstrong R. The role of inflammation in contrast‐induced nephropathy. Br J Radiol. 2014;87:20130738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patti G, Ricottini E, Nusca A, et al. Short‐term, high‐dose atorvastatin pretreatment to prevent contrast‐induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA‐CIN [Atorvastatin for Reduction of Myocardial Damage During Angioplasty‐‐Contrast‐Induced Nephropathy] trial. Am J Cardiol. 2011;108:1–7. [DOI] [PubMed] [Google Scholar]
- 8. Liu Y, Tan N, Zhou YL, et al. High‐sensitivity C‐reactive protein predicts contrast‐induced nephropathy after primary percutaneous coronary intervention . J Nephrol. 2012;25:332–340. [DOI] [PubMed] [Google Scholar]
- 9. Chatterjee S, Changawala N. Fragmented QRS complex: a novel marker of cardiovascular disease. Clin Cardiol. 2010;33:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castle CH, Keane WM. Electrocardiographic “peri‐infarction block”: a clinical and pathologic correlation. Circulation. 1965;31:403–408. [DOI] [PubMed] [Google Scholar]
- 11. Das MK, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12‐lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008;1:258‐268. [DOI] [PubMed] [Google Scholar]
- 12. Eisen A, Arnson Y, Dovrish Z, et al. Arrhythmias and conduction defects in rheumatological diseases—a comprehensive review. Semin Arthritis Rheum. 2009;39:145–156. [DOI] [PubMed] [Google Scholar]
- 13. Kadi H, Inanir A, Habiboglu A, et al. Frequency of fragmented QRS on ECG is increased in patients with rheumatoid arthritis without cardiovascular disease: a pilot study. Mod Rheumatol. 2012;22:238–242. [DOI] [PubMed] [Google Scholar]
- 14. Das MK, Saha C, El Masry H, et al. Fragmented QRS on a 12‐lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385–1392. [DOI] [PubMed] [Google Scholar]
- 15. Das MK, Maskoun W, Shen C, et al. Fragmented QRS on twelve‐lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010;7:74–80. [DOI] [PubMed] [Google Scholar]
- 16. Das MK, Michael MA, Suradi H, et al. Usefulness of fragmented QRS on a 12‐lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol. 2009;104:1631–1637. [DOI] [PubMed] [Google Scholar]
- 17. Pietrasik G, Goldenberg I, Zdzienicka J, et al. Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q‐wave myocardial infarction. Am J Cardiol. 2007;100:583–586. [DOI] [PubMed] [Google Scholar]
- 18. Ma X, Duan W, Poudel P, et al. Fragmented QRS complexes have predictive value of imperfect ST‐segment resolution in patients with STEMI after primary percutaneous coronary intervention. Am J Emerg Med. 2016;34:398–402. [DOI] [PubMed] [Google Scholar]
- 19. Çetin MS, Ozcan Çetin EH, Canpolat U, et al. Usefulness of fragmented QRS complex to predict arrhythmic events and cardiovascular mortality in patients with noncompaction cardiomyopathy. Am J Cardiol. 2016;117:1516–1523. [DOI] [PubMed] [Google Scholar]
- 20. Thygesen K, Alpert JS, Jaffe AS, et al. Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Glob Heart . 2012;7:275–295. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 22. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 23. Narula A, Mehran R, Weisz G, et al. Contrast‐induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS‐AMI substudy. Eur Heart J. 2014;35:1533–1540. [DOI] [PubMed] [Google Scholar]
- 24. Centola M, Lucreziotti S, Salerno‐Uriarte D, et al. A comparison between two different definitions of contrast‐induced acute kidney injury in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol. 2016;210:4–9. [DOI] [PubMed] [Google Scholar]
- 25. Çetin M, Kocaman SA, Canga A, et al. The independent relationship between systemic inflammation and fragmented QRS complexes in patients with stable angina pectoris. Kardiol Pol. 2012;70:668–675. [PubMed] [Google Scholar]
- 26. Çetin M, Kocaman SA, Erdoğan T, et al. The independent relationship of systemic inflammation with fragmented QRS complexes in patients with acute coronary syndromes. Korean Circ J. 2012;42:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang R, Zhang YY, Huang XR, et al. C‐reactive protein promotes cardiac fibrosis and inflammation in angiotensin II‐induced hypertensive cardiac disease. Hypertension. 2010;55:953–960. [DOI] [PubMed] [Google Scholar]


