Abstract
Background
CHADS2 (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke) and CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke, vascular disease, age 65 to 74 years, sex category) scores showed just moderate discrimination ability in predicting thromboembolic complications in patients with nonvalvular atrial fibrillation (AF).
Hypothesis
To determine the association of antithrombin III (AT‐III) deficiency and mean platelet volume (MPV) with the development of stroke or left atrial (LA) thrombus in patients with AF.
Methods
AT‐III and MPV were analyzed in 352 patients with AF. The primary endpoint was a composite of ischemic stroke event and incidental LA thrombus.
Results
There were 50 events (14.2%) during a mean 35.4 months of follow‐up. A significantly higher stroke or LA thrombus rate was observed in the low–AT‐III group (<70%) than that in the high–AT‐III group (≥70%). A significantly higher stroke or LA thrombus rate was observed in the high‐MPV group (≥7.0 fL) than that in the low‐MPV group (<7.0 fL). AF patients with an MPV ≥7.0 fL and AT‐III deficiency had higher stroke or LA thrombus risk than those without an MPV ≥7.0 fL and AT‐III deficiency. In the Cox proportional hazard analysis, high MPV was found to be an independent predictor of stroke or LA thrombus risk (hazard ratio: 6.408; 95% confidence interval: 2.874‐14.286). Although AT‐III deficiency was not an independent predictor of stroke or LA thrombus risk, a trend was observed.
Conclusions
High MPV and AT‐III deficiency were predictive markers for stroke or LA thrombus. Their predictive power for stroke was independent of antiplatelet treatment, anticoagulation therapy, and a high CHA2DS2‐VASc score in patients with AF.
Keywords: Antithrombin III, Atrial Fibrillation, Left Atrial Thrombus, Mean Platelet Volume, Stroke
1. INTRODUCTION
Atrial fibrillation (AF) is the most common type of arrhythmia observed in clinical practice. It is associated with substantial morbidity and mortality from stroke events.1, 2 Currently, the risk for stroke and the indication for anticoagulant therapy in AF can be estimated using the CHADS2 (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke) and CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke, vascular disease, age 65 to 74 years, sex category) scores.3 Left atrial (LA) thrombus is a risk factor for stroke in patients with AF. It has been estimated that at least two‐thirds of the stroke events in patients with nonvalvular AF occur due to LA thrombus drop‐off.4
Mean platelet volume (MPV), the most frequently used measure of platelet size, is a surrogate marker of platelet function and a potential link between thrombosis and inflammation.5 It can be measured in an inpatient or outpatient situation and is a low‐cost examination. Bigger platelets are metabolically and enzymatically more active and have greater prothrombotic potential. In addition, evidence demonstrates that MPV is an independent predictor of the risk of stroke among individuals with a history of stroke or transient ischemic attacks and AF.6, 7, 8
Antithrombin‐III (AT‐III) is a naturally occurring anticoagulant protein that plays a key role in controlling thrombus development and spread.9 AT‐III is produced by the endothelium of blood vessels and in the liver, which have linking sites for heparin and thrombin. Once thrombin is produced, it links with AT‐III and forms thrombin‐AT III complex, which impedes thrombosis.10 AT‐III deficiency is associated with hypercoagulability, causing deep vein thrombosis and pulmonary embolism.11 However, the association of AT‐III deficiency and MPV with the development of an ischemic stroke event and incidental LA thrombus in patients with AF has not been investigated.
The aim of this study was to determine the association of AT‐III deficiency and MPV with the development of ischemic stroke event and incidental LA thrombus in patients with AF.
2. METHODS
2.1. Study population
A total of 352 consecutive patients with AF who underwent measurements of AT‐III activity and MPV between September 2009 and October 2014 were included in this study. This study was approved by the Chosun University Hospital Research Ethics Committee (CHOSUN 2014‐09‐011). The primary endpoint was a composite of ischemic stroke event and incidental LA thrombus.
2.2. Blood collection and biomarker measurements
Venous blood samples were collected in K2‐EDTA tubes (Becton Dickinson, Franklin Lakes, NJ). MPV was analyzed using an Advia 2120 hematology analyzer (Siemens Healthcare Diagnostics GmbH, Eschborn, Germany) within 2 hours of sample collection. AT‐III activity was measured using a HemosIL Liquid Antithrombin kit (Instrumentation Laboratory, Bedford, MA). According to the manufacturer's recommendations, the normal range of AT‐III activity is 83% to 128%. Because the risk of certain hypercoagulability disorders, such as venous thromboembolism, is generally documented in patients with an AT‐III level of <70%,12 we stratified our study population as follows: low–AT‐III group (<70%) and high–AT‐III group (≥70%).
2.3. Calculation of CHA2DS2‐VASc score
We calculated the CHA2DS2‐VASc score as follows: 2 points were assigned for a history of stroke or transient ischemic attack, or age ≥ 75 years; 1 point was assigned for age 65 to 74 years, history of hypertension, diabetes mellitus, recent cardiac failure, vascular disease, and female sex.3 A patient was considered to have diabetes if it was reported by a physician, or the fasting blood glucose level was ≥126 mg/dL, or the patient was on antidiabetic medication. Hypertension was confirmed if the systolic blood pressure was ≥140 mm Hg, diastolic blood pressure was ≥90 mm Hg, or the patient was on antihypertensive medication. The presence of heart failure and history of stroke/transient ischemic attack was determined from the patients' medical records.
2.4. Detection of LA thrombus
Incidental LA thrombus was defined as a circumscribed homogeneous mass with a nonmyocardial texture, which was detected by transesophageal echocardiography (TEE) or multidetector computed tomography (MDCT). TEE was performed using an iE33 system with a multiplane 5 MHz probe (Philips Medical Systems, Bothell, WA) or a VIVID E9 echocardiography system (GE Medical Systems, Horten, Norway). In computed tomography (CT), LA thrombus appears as a nonenhanced, well‐circumscribed nodule with irregular margins. For detecting LA thrombus, 16‐ (Sensation 16, Siemens Medical Systems, Erlangen, Germany), 128‐ (Ingenuity CT; Philips Healthcare, Best, the Netherlands), and 640‐slice CT (Aquilion One; Toshiba Medical Systems Corporation, Otawara, Japan) scanners were used. Figure 1 demonstrates a representative TEE 2‐dimensional image and an MDCT image of an LA thrombus.
Figure 1.
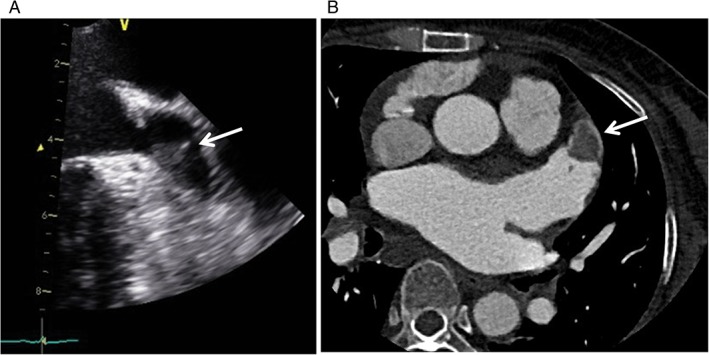
(A) A 51‐year‐old male with an LAA thrombus (arrow) detected by transesophageal echocardiography. (B) A 63‐year‐old female with an LAA thrombus. A thoracic axial MDCT scan shows a well‐demarcated thrombus without enhancement within the tip of the LAA (arrow). Abbreviations: LAA, left atrial appendage; MDCT, multidetector computed tomography
2.5. Outcomes
Clinical follow‐up data were obtained from outpatient medical records or telephone interviews. The primary endpoint analyzed was a composite of ischemic stroke event and incidental LA thrombus.
2.6. Statistical analysis
All values are expressed as the mean ± standard deviation/median (interquartile range [IQR]) or as a number (percentage). The baseline characteristics of the groups were compared using the Student t test for continuous variables and the χ2 statistic for noncontinuous variables.
The primary endpoint‐free survival according to AT‐III activity and MPV was estimated using the Kaplan–Meier method, and the outcomes were compared using the log‐rank test. Cox proportional hazards regression was used for the calculation of independent predictors of the primary endpoint. We entered into a forward stepwise multivariate Cox proportional hazards model aspirin therapy, warfarin therapy, CHA2DS2‐VASc score, AT‐III, and MPV. Receiver operating characteristic (ROC) analysis was performed for determination of sensitivity and specificity with 95% confidence interval (CI) for the MPV at cutoff values. The Statistical Package for the Social Sciences software version 12.0 (IBM, Armonk, NY) was used to perform all statistical analyses, and P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Primary endpoint MPV cutoff value
Median MPV for the overall study population was 7.0 fL (IQR, 6.6–7.6 fL; normal range, 7.2–11.1 fL). The cutoff value for MPV level predictive of the primary endpoint was evaluated using ROC analysis. When the MPV cutoff level was set to 7.0 fL using the ROC curve, the sensitivity was 84.0% (95% CI: 70.9‐92.8) and specificity was 59.5% (95% CI: 53.7‐65.1) for differentiating between the groups with and without the primary endpoint (area under the curve = 0.800, P < 0.001).
3.2. Clinical characteristics
The mean MPV and AT‐III activity for the overall study population were 7.3 ± 1.1 fL (median, 7.0 fL; IQR 6.6–7.6 fL; normal range, 7.2–11.1 fL) and 88.7% ± 17.4% (median, 91.0%; IQR, 78.3–101.0; normal range > 80%), respectively. The baseline clinical characteristics according to the cutoff value for MPV are shown in Table 1. Overall clinical characteristics, except AT‐III activity, were generally comparable between the 2 groups. The CHA2DS2‐VASc score was also similar between the 2 groups.
Table 1.
Baseline characteristics and medication data on the basis of treatment strategy of atrial fibrillation
| Characteristic | Total (N = 352) | MPV <7 fL (N = 168) | MPV ≥7 fL (N = 184) | P Value |
|---|---|---|---|---|
| Age, y | 68.4 ± 12.1 | 68.3 ± 12.1 | 68.5 ± 12.2 | 0.860 |
| Female sex (%) | 42.6 | 42.3 | 42.9 | 0.899 |
| Hypertension (%) | 50.9 | 52.4 | 49.5 | 0.584 |
| Diabetes (%) | 19.0 | 17.3 | 20.7 | 0.418 |
| Antithrombin III activity (%) | 88.7 ± 17.4 | 90.7 ± 17.0 | 87.0 ± 17.7 | 0.045 |
| Antithrombin III <70% (%) | 13.1 | 11.3 | 14.7 | 0.350 |
| Mean platelet volume (fL) | 7.25 ± 1.00 | 6.53 ± 0.30 | 7.92 ± 0.94 | <0.001 |
| LVEF (%) | 55.4 ± 12.4 | 56.0 ± 11.6 | 54.9 ± 13.0 | 0.391 |
| LVEF <35% (%) | 7.7 | 5.4 | 9.8 | 0.119 |
| Previous stroke or TIA (%) | 4.5 | 5.4 | 3.8 | 0.485 |
| Vascular disease history (%) | 6.5 | 4.8 | 8.2 | 0.199 |
| Any antithrombotic agents (%) | 87.2 | 85.7 | 88.6 | 0.420 |
| Aspirin (%) | 48.9 | 47.0 | 50.5 | 0.509 |
| P2Y12 inhibitor (%) | 10.2 | 9.5 | 10.9 | 0.677 |
| Dual antiplatelet therapy (%) | 9.7 | 8.3 | 10.9 | 0.421 |
| Warfarin (%) | 44.6 | 44.0 | 45.1 | 0.841 |
| CHA2DS2‐VASc score | 2.3 ± 1.6 | 2.3 ± 1.6 | 2.4 ± 1.6 | 0.531 |
| High (score ≥ 2, %) | 63.1 | 60.1 | 65.8 | 0.273 |
| Follow‐up duration (d) | 1063 ± 756 | 1130 ± 757 | 1002 ± 752 | 0.113 |
Abbreviations: CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke, vascular disease, age 65 to 74 years, sex category; LVEF, left ventricular ejection fraction; MPV, mean platelet volume; TIA, transient ischemic attack.
Majority of the patients used >1 antithrombotic agent. Aspirin was the most frequently prescribed antithrombotic agent, followed by warfarin and clopidogrel.
3.3. Correlation between MPV and AT‐III activities
There was a weak and negative correlation between MPV and AT‐III activity (r = −0.135, P = 0.011). The mean AT‐III activity was lower in participants with a high MPV than in those with a low MPV (87.0 vs 90.7%, P = 0.045). The mean MPV tended to be higher in participants with lower AT‐III activity than in those with higher AT‐III activity (7.52 vs 7.21, P = 0.055).
3.4. Primary endpoint‐free survival: AT‐III activity
During a mean follow‐up period of 35.4 months, 50 patients (14.2%) experienced the primary endpoint (32 had ischemic stroke events, 16 had incidental LA thrombus, and 2 had both).
The Kaplan–Meier primary endpoint‐free survival curves of the patients according to AT‐III activity are shown in Figure 2A (<70% [46 patients] and ≥70% [306 patients]. The primary endpoint rates showed a significant increase in the low–AT‐III activity group. Log‐rank analysis showed a significant association between low AT‐III activity and the primary endpoint (23.9% vs 12.7%, log‐rank: P = 0.0287).
Figure 2.
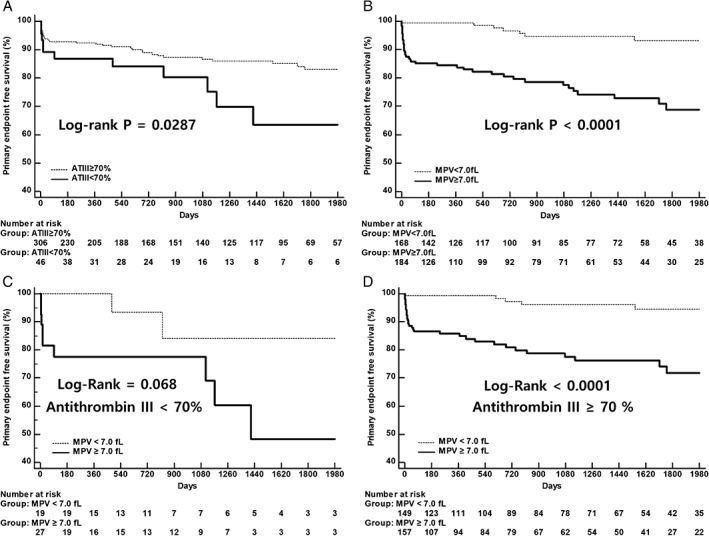
Primary endpoint‐free survival according to (A) ATIII activity and (B) MPV. (C, D) Primary endpoint‐free survival according to MPV based on ATIII activity. Abbreviations: ATIII, antithrombin III; MPV, mean platelet volume
3.5. Primary endpoint‐free survival: MPV
The patients were stratified into 2 groups based on the cutoff values of baseline MPV as <7.0 fL (168 patients) and ≥7.0 fL (184 patients).
The Kaplan–Meier primary endpoint‐free survival curves of the patients according to MPV are shown in Figure 2B. The primary endpoint rates showed a significant increase in the high‐MPV group. Log‐rank analysis showed a significant association between MPV and the primary endpoint (23.4% vs 4.2%, log‐rank: P < 0.0001). This value was more useful in patients with a high AT‐III level (Figure 2C,D).
3.6. Independent predictors of primary endpoint
In multivariable analysis, after adjustment for aspirin therapy, warfarin therapy, CHA2DS2‐VASc score, AT‐III, and MPV, MPV was an independent risk factor of the primary endpoint (hazard ratio: 6.41, 95% CI: 2.87‐14.29, P < 0.0001) (Table 2). Even though low AT‐III activity was not a significant independent risk factor of the primary endpoint, it showed a trend toward an increased risk for the primary endpoint (hazard ratio: 1.84, 95% CI: 0.93‐3.65, P = 0.078) (Table 2).
Table 2.
Multivariate Cox proportional hazard analyses determining the significant and independent predictors for the primary endpoint
| Factor | HR (95% CI) | P Value |
|---|---|---|
| Aspirin therapy | 0.95 (0.46‐1.96) | 0.880 |
| Warfarin therapy | 1.04 (0.50‐2.16) | 0.910 |
| CHA2DS2‐VASc score ≥ 2 | 1.11 (0.61‐2.03) | 0.725 |
| Antithrombin III <70% | 1.84 (0.93‐3.65) | 0.078 |
| Mean platelet volume ≥ 7.0 fL | 6.41 (2.87‐14.29) | <0.001 |
Abbreviations: CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke, vascular disease, age 65 to 74 years, sex category; CI, confidence interval; HR, hazard ratio.
3.7. Combined effect of AT‐III and MPV
A combined analysis of primary endpoint according to both AT‐III and MPV using the Kaplan–Meier event‐free survival curve is shown in Figure 3. It demonstrated an exaggerated primary endpoint risk in the combined low–AT‐III activity and high‐MPV group.
Figure 3.
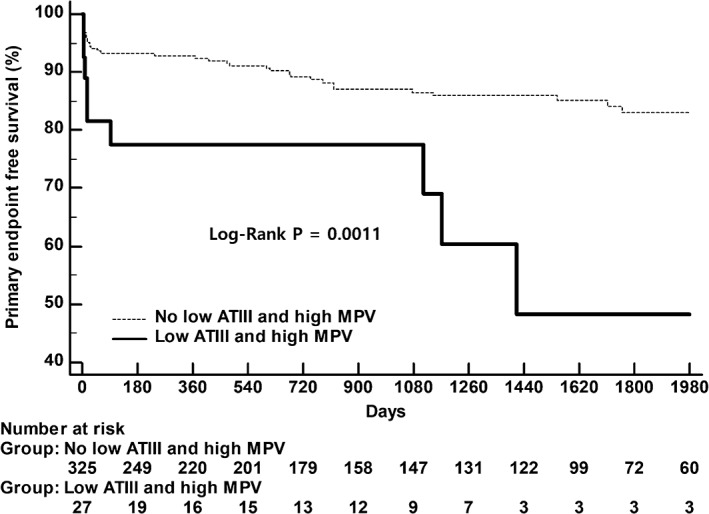
Primary endpoint‐free survival according to a combination of ATIII activity and MPV. Abbreviations: ATIII, antithrombin III; MPV, mean platelet volume
4. DISCUSSION
The main findings of the current observational study showed that high MPV and AT‐III deficiency were predictive markers for stroke or LA thrombus in patients with AF, and their predictive power for these events was independent of antiplatelet treatment, anticoagulation therapy, and high CHA2DS2‐VASc score. Although AT‐III deficiency is a modest predictor of stroke or LA thrombus, it might help to stratify the risk of these events in patients with AF. In our analysis, this relationship between MPV and the primary endpoint appeared to be more obvious in patients of the high–AT‐III group. Furthermore, stroke or LA thrombus risk was exaggerated in AF patients with an MPV of ≥7.0 fL and AT‐III deficiency compared to those with a low‐MPV and high–AT‐III level.
Many studies have reported regarding the association between MPV levels and stroke6, 13, 14, 15 or LA stasis,16 especially in patients with AF.7, 17, 18, 19 Moreover, some investigations have demonstrated the controversial relationship between AT‐III and stroke.20, 21 However, the association of AT‐III deficiency and MPV with the development of ischemic stroke event and incidental LA thrombus in patients with AF has not been investigated. To the best of our knowledge, this is the first study to investigate the long‐term (mean follow‐up period of 3 years, with a maximum of 5.9 years) impact of AT‐III deficiency and MPV level on the occurrence of stroke or LA thrombus in patients with AF.
Although the prevalence of AT‐III deficiency is 0.02% to 0.17% in a healthy population and 1.1% in patients with venous thromboembolism,22, 23 AT‐III deficiency has been recognized as an important thrombophilic condition and is an established risk factor mainly for venous thromboembolism.24, 25, 26, 27 In fact, there were no precise data about the prevalence of AT‐III deficiency in patients with AF. In this cohort, however, the prevalence of AT‐III deficiency in patients with AF was estimated to be somewhat high (13.1%). This finding needs to be validated by further studies.
Our data have some significant clinical implications in several important aspects. Systemic thromboembolism is a serious problem in patients with AF.28 In the preceding years, numerous forms of risk stratification methods, such as the CHADS2 and CHA2DS2‐VASc scores, have been used for predicting the risk of embolic events in AF patients.3, 29 However, in several studies, the CHADS2 and CHA2DS2‐VASc scores showed just moderate discrimination ability in predicting thromboembolic complications.2, 30, 31 In this study, the CHA2DS2‐VASc score was not an independent risk predictor of ischemic stroke or LA thrombus. In addition, 16 patients with low CHA2DS2‐VASc scores had ischemic stroke or incidental LA thrombus during follow‐up in this study. The risk of ischemic stroke and LA thrombus in patients with low CHA2DS2‐VASc scores should not be underestimated. In our study, MPV and AT‐III activity were independently predictive of the development of ischemic stroke or incidental LA thrombus in multivariate analysis after adjusting for the CHA2DS2‐VASc scores. It seemed that high‐MPV level and AT‐III deficiency could potentially improve the CHA2DS2‐VASc scores in predicting ischemic stroke or LA thrombus.
The gold and conventional standard for ruling out an LA thrombus is TEE; however, this is a somewhat invasive study, and there are several contraindications for this technique, such as esophageal bleeding or recent surgery.32 Hence, an alternative examination procedure for detecting LA thrombus is required. MDCT is a potential alternative for the assessment of LA thrombus because appropriate temporal and spatial resolution can be attained with this method.33, 34, 35, 36, 37 In addition, MDCT is a noninvasive modality, and can be applied to the head area for diagnosing stroke with an extended scan range. In most studies evaluating LA thrombus, TEE has been used for detecting LA thrombus.16, 38, 39, 40 In this cohort, however, MDCT was used for detecting LA thrombus in around 60% of the patients. This was another unique aspect of our investigation.
4.1. Study limitations
The current investigation has some limitations, commonly stemming from its relatively small sample size. In addition, this study was not a prospective study, and the results and conclusions are subject to the limitations inherent in these forms of investigations. Due to our original inclusion criteria, which only included patients with AF who were evaluated for MPV and AT‐III activity, selection bias was possible. This cohort had a small number of patients with AT‐III deficiency. However, the prevalence of AT‐III deficiency is very low. Thus, a relatively high proportion of patients with AT‐III deficiency have been included in this study population.
5. CONCLUSION
The results of this study demonstrated that high MPV and AT‐III deficiency were predictive markers for stroke or LA thrombus. Their predictive power for stroke was independent of antiplatelet treatment, anticoagulation therapy, and high CHA2DS2‐VASc score in patients with AF. It seemed that high MPV level and AT‐III deficiency could potentially improve the ability of CHA2DS2‐VASc scores in predicting ischemic stroke or LA thrombus.
Author contributions
Seo‐Won Choi, MD, and Bo‐Bae Kim, MD, contributed equally to the study. All authors had access to the data and played a role in writing this article.
Conflicts of interest
The authors declare no potential conflicts of interest.
Choi S.‐W., Kim B.‐B., Choi D.‐H., et al. Stroke or left atrial thrombus prediction using antithrombin III and mean platelet volume in patients with nonvalvular atrial fibrillation. Clin Cardiol. 2017;40:1013–1019. 10.1002/clc.22759
Funding information This work was support Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning, Grant/Award number: 2016R1A2B4011905; research fund from Chosun University, 2016; Ministry of Education, Science and Technology, Grant/Award number: 2016R1D1A1B03932488.
REFERENCES
- 1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Fang MC, Go AS, Chang Y, et al. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2008;51:810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 4. Miller VT, Rothrock JF, Pearce LA, Feinberg WM, Hart RG, Anderson DC. Ischemic stroke in patients with atrial fibrillation: effect of aspirin according to stroke mechanism. Stroke Prevention in Atrial Fibrillation Investigators. Neurology. 1993;43:32–36. [DOI] [PubMed] [Google Scholar]
- 5. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17:47–58. [DOI] [PubMed] [Google Scholar]
- 6. Bath P, Algert C, Chapman N, Neal B. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 2004;35:622–626. [DOI] [PubMed] [Google Scholar]
- 7. Ha SI, Choi DH, Ki YJ, et al. Stroke prediction using mean platelet volume in patients with atrial fibrillation. Platelets. 2011;22:408–414. [DOI] [PubMed] [Google Scholar]
- 8. Choi DH, Kang SH, Song H. Mean platelet volume: a potential biomarker of the risk and prognosis of heart disease. Korean J Intern Med. 2016;31:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mammen EF. Antithrombin: its physiological importance and role in DIC. Semin Thromb Hemost. 1998;24:19–25. [DOI] [PubMed] [Google Scholar]
- 10. Stehling F, Weber R, Ozcelik A, et al. Acute changes of coagulation and fibrinolysis parameters after experimental thromboembolic stroke and thrombolytic therapy. Neurosci Lett. 2008;441:39–43. [DOI] [PubMed] [Google Scholar]
- 11. Maclean PS, Tait RC. Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis and treatment options. Drugs. 2007;67:1429–1440. [DOI] [PubMed] [Google Scholar]
- 12. Lane DA, Bayston T, Olds RJ, et al. Antithrombin mutation database: 2nd (1997) update. For the Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 1997;77:197–211. [PubMed] [Google Scholar]
- 13. Butterworth RJ, Bath PM. The relationship between mean platelet volume, stroke subtype and clinical outcome. Platelets. 1998;9:359–364. [DOI] [PubMed] [Google Scholar]
- 14. D'Erasmo E, Aliberti G, Celi FS, Romagnoli E, Vecci E, Mazzuoli GF. Platelet count, mean platelet volume and their relation to prognosis in cerebral infarction. J Intern Med. 1990;227:11–14. [DOI] [PubMed] [Google Scholar]
- 15. Tohgi H, Suzuki H, Tamura K, Kimura B. Platelet volume, aggregation, and adenosine triphosphate release in cerebral thrombosis. Stroke. 1991;22:17–21. [DOI] [PubMed] [Google Scholar]
- 16. Providencia R, Faustino A, Paiva L, et al. Mean platelet volume is associated with the presence of left atrial stasis in patients with non‐valvular atrial fibrillation. BMC Cardiovasc Disord. 2013;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong SP, Choi DH, Kim HW, et al. Stroke prevention in patients with non‐valvular atrial fibrillation: new insight in selection of rhythm or rate control therapy and impact of mean platelet volume. Curr Pharm Des. 2013;19:5824–5829. [DOI] [PubMed] [Google Scholar]
- 18. Tekin G, Tekin YK, Sivri N, Yetkin E. Mean platelet volume in patients with nonvalvular atrial fibrillation. Blood Coagul Fibrinolysis. 2013;24:537–539. [DOI] [PubMed] [Google Scholar]
- 19. Bayar N, Arslan S, Cagirci G, et al. Usefulness of mean platelet volume for predicting stroke risk in paroxysmal atrial fibrillation patients. Blood Coagul Fibrinolysis. 2015;26:669–672. [DOI] [PubMed] [Google Scholar]
- 20. Meng R, Li ZY, Ji X, Ding Y, Meng S, Wang X. Antithrombin III associated with fibrinogen predicts the risk of cerebral ischemic stroke. Clin Neurol Neurosurg. 2011;113:380–386. [DOI] [PubMed] [Google Scholar]
- 21. Alonso A, Tang W, Agarwal SK, Soliman EZ, Chamberlain AM, Folsom AR. Hemostatic markers are associated with the risk and prognosis of atrial fibrillation: the ARIC study. Int J Cardiol. 2012;155:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tait RC, Walker ID, Perry DJ, et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol. 1994;87:106–112. [DOI] [PubMed] [Google Scholar]
- 23. De Stefano V, Finazzi G, Mannucci PM. Inherited thrombophilia: pathogenesis, clinical syndromes, and management. Blood. 1996;87:3531–3544. [PubMed] [Google Scholar]
- 24. Dentali F, Gianni M. VTE recurrence in patients with inherited deficiencies of natural anticoagulants. Thromb Haemost. 2009;101:5–6. [PubMed] [Google Scholar]
- 25. Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166:729–736. [DOI] [PubMed] [Google Scholar]
- 26. Simioni P, Sanson BJ, Prandoni P, et al. Incidence of venous thromboembolism in families with inherited thrombophilia. Thromb Haemost. 1999;81:198–202. [PubMed] [Google Scholar]
- 27. Sanson BJ, Simioni P, Tormene D, et al. The incidence of venous thromboembolism in asymptomatic carriers of a deficiency of antithrombin, protein C, or protein S: a prospective cohort study. Blood. 1999;94:3702–3706. [PubMed] [Google Scholar]
- 28. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 29. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 30. Van Staa TP, Setakis E, Di Tanna GL, Lane DA, Lip GY. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J Thromb Haemost. 2011;9:39–48. [DOI] [PubMed] [Google Scholar]
- 31. Chao TF, Liu CJ, Wang KL, et al. Using the CHA2DS2‐VASc score for refining stroke risk stratification in 'low‐risk' Asian patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:1658–1665. [DOI] [PubMed] [Google Scholar]
- 32. Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D'Ambra MN, Eltzschig HK. Safety of transesophageal echocardiography. J Am Soc Echocardiogr. 2010;23:1115–1127; quiz 1220–1221. [DOI] [PubMed] [Google Scholar]
- 33. Homsi R, Nath B, Luetkens JA, Schwab JO, Schild HH, Naehle CP. Can contrast‐enhanced multi‐detector computed tomography replace transesophageal echocardiography for the detection of thrombogenic milieu and thrombi in the left atrial appendage: a prospective study with 124 patients. Rofo. 2016;188:45–52. [DOI] [PubMed] [Google Scholar]
- 34. Hur J, Kim YJ, Lee HJ, et al. Left atrial appendage thrombi in stroke patients: detection with two‐phase cardiac CT angiography versus transesophageal echocardiography. Radiology. 2009;251:683–690. [DOI] [PubMed] [Google Scholar]
- 35. Kim YY, Klein AL, Halliburton SS, et al. Left atrial appendage filling defects identified by multidetector computed tomography in patients undergoing radiofrequency pulmonary vein antral isolation: a comparison with transesophageal echocardiography. Am Heart J. 2007;154:1199–1205. [DOI] [PubMed] [Google Scholar]
- 36. Martinez MW, Kirsch J, Williamson EE, et al. Utility of nongated multidetector computed tomography for detection of left atrial thrombus in patients undergoing catheter ablation of atrial fibrillation. JACC Cardiovasc Imaging. 2009;2:69–76. [DOI] [PubMed] [Google Scholar]
- 37. Wu X, Wang C, Zhang C, Zhang Y, Ding F, Yan J. Computed tomography for detecting left atrial thrombus: a meta‐analysis. Arch Med Sci. 2012;8:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pant R, Patel M, Garcia‐Sayan E, et al. Impact of B‐type natriuretic peptide level on the risk of left atrial appendage thrombus in patients with nonvalvular atrial fibrillation: a prospective study. Cardiovasc Ultrasound. 2016;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang RB, Dong JZ, Yan XL, et al. Serum uric Acid and risk of left atrial thrombus in patients with nonvalvular atrial fibrillation. Can J Cardiol. 2014;30:1415–1421. [DOI] [PubMed] [Google Scholar]
- 40. Santangeli P, Sestito A. Acute left atrial thrombosis during anticoagulant therapy in a patient with antithrombin deficiency. Acta Cardiol. 2008;63:635–637. [DOI] [PubMed] [Google Scholar]


