Abstract
Atrial fibrillation (AF) is a commonly sustained atrial arrhythmia with associated morbidity and mortality. AF is associated with increased risk of thromboembolism and stroke, requiring use of anticoagulation. Anticoagulation decreases the risk of stroke but is associated with a higher risk of bleeding, necessitating discontinuation in some patients. The left atrial appendage is the likely source of thrombus in the majority of patients with AF. This has led to the development of left atrial appendage occlusion as a means to reduce stroke risk in patients who have a contraindication to long‐term anticoagulation. Multiple implantable devices have surfaced in the last few years, with some promising prospects. The main purpose of this review is to highlight the indications and use of these devices for left atrial appendage occlusion.
Keywords: Atrial Fibrillation, Left Atrial Appendage Occlusion, Stroke Prevention
1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained arrhythmia and currently affects 5 million Americans.1 AF is associated with a > 5‐fold increased risk of stroke.2 The CHA2DS2‐VASc score provides an assessment of stroke risk3 and ranges from 0 to 9, as shown in Table 1.
Table 1.
Stroke (CHA2DS2‐VASc) and bleeding risk (HAS‐BLED) assessment in AF patients
| CHA2DS2‐VASc | Score | HAS‐BLED | Score |
|---|---|---|---|
| CHF | 1 | HTN (SBP >160 mmHg) | 1 |
| HTN | 1 | Abnormal renal and liver function (1 point each) | 1 or 2 |
| Age > 75 y | 2 | Stroke | 1 |
| DM | 1 | Bleeding tendency/predisposition | 1 |
| Stroke/TIA/TE | 2 | Labile INRs (if on warfarin) | 1 |
| Vascular disease (prior MI, PAD, or aortic plaque) | 1 | Elderly (age > 56 y) | 1 |
| Age 65–74 y | 1 | Drugs or alcohol (1 point each) | 1 or 2 |
| Sex category (F) | 1 | ||
| Maximum scorea | 9 | Maximum score | 9 |
Abbreviations: CHF, congestive heart failure; DM, diabetes mellitus; F, female; HTN, hypertension; INR, international normalized ratio; MI, myocardial infarction; PAD, peripheral arterial disease; SBP, systolic blood pressure; TE, thromboembolic event; TIA, transient ischemic attack.
Only 1 age category is counted in the total score, allowing a maximum of 9 points.
The risk of AF‐associated stroke has been shown to be reduced by 64% with anticoagulation, and thus this is the standard of care for thromboembolic prophylaxis in the majority of AF patients.4 Despite the proven efficacy of anticoagulation with both warfarin and recently available novel oral anticoagulants (NOACs), the use, efficacy, and safety of chronic anticoagulation therapy is limited because of bleeding complications. The HAS‐BLED score is currently used for formal assessment of bleeding risk in an AF patient being considered for anticoagulation; its scoring also ranges from 0 to 9 (Table 1).
In nonvalvular AF patients, up to 91% of left atrial (LA) thrombi have been localized to the left atrial appendage (LAA).5, 6 The LAA has a narrow orifice with a trabeculated structure that predisposes to blood stasis and thrombus formation.7 As a result, LAA occlusion with surgical ligation or mechanical occlusion has emerged as a potential alternative to oral anticoagulation (OAC) to decrease the risk of stroke in patients with AF without producing a concomitant increase in bleeding risk.8
2. SURGICAL MANAGEMENT OF LAA
The Left Atrial Appendage Occlusion Study (LAAOS) was the first randomized clinical trial designed to evaluate the feasibility, safety, and efficacy of LAA occlusion for prevention of ischemic stroke in patients undergoing coronary artery bypass grafting surgery.9 A total of 77 patients undergoing the surgery who were deemed suitable for occlusion based on their LAA anatomy were then randomized 2:1 to undergo LAA occlusion or control. Complete occlusion of LAA was achieved in 45% (5/11) of patients using sutures and 72% (24/33) using a stapler (P = 0.14). There were perioperative thromboembolic events in 2.6% of patients in the control group only. After surgery, patients were followed for an average of 13 ± 7 months, during which time no additional patients had a stroke. Similarly, in a retrospective analysis, Kanderian et al10 sought to determine which surgical technique of LAA closure is most successful as assessed by transesophageal echocardiography (TEE). Of 137 patients included, 52 underwent excision and 85 underwent exclusion (73 suture and 12 stapler). Only 55 of 137 (40%) of the closures were successful, more often with excision (73%) than suture exclusion (23%) and stapler exclusion (0%).
The 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society Guidelines for the management of AF provide a class IIB/level of evidence C recommendation for surgical excision of the LAA in patients undergoing cardiac surgery.11
2.1. Transvenous device closure of LAA
2.1.1. PLAATO
The first transvascular LAA closure system developed was the Percutaneous Left Atrial Appendage Transcatheter Occlusion device (PLAATO; Ev3 Endovascular, Plymouth, MN), which consisted of a polytetrafluoroethylene membrane on a self‐expanding nitinol cage (Figure 1A).
Figure 1.
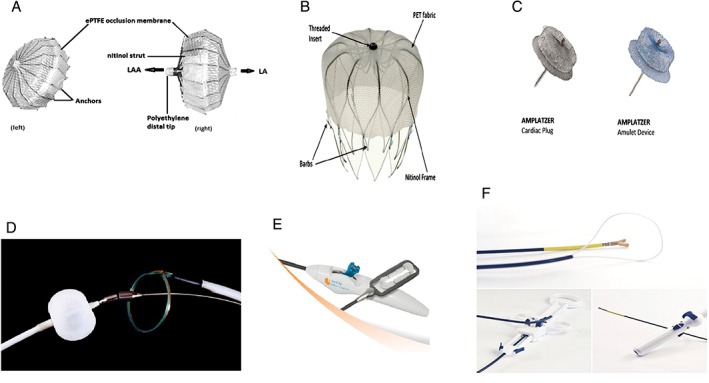
LAA closure devices. (A) PLAATO device illustrating (left) front surface and (right) lateral view, showing nitinol cage structure covered with ePTFE. (B) Watchman device. (C) Amplatzer Cardiac Plug and Amplatzer Amulet device. (D) Lariat suture delivery system. (E) AtriClip delivery system. (F) Aegis Sierra LAA capture and ligation system. Abbreviations: ePTFE, expanded polytetrafluoroethylene; LA, left atrium; LAA, left atrial appendage; PLAATO, percutaneous left atrial appendage transcatheter occlusion
The device was first tested in a canine model in 2002.12 In subsequent clinical testing in patients with nonrheumatic AF,13 percutaneous LAA occlusion was successful in 108 of 111 patients (97.3%; 95% confidence interval: 92.3%‐99.4%). Of the 111 enrolled patients, only 2 (1.8%; 95% confidence interval: 0.2%‐6.4%) experienced a stroke, at 173 and 215 days after the implant procedure, respectively. Despite the success of these studies, the company withdrew the device in 2007 for commercial reasons.
2.1.2. Watchman
The Watchman device (Boston Scientific, Natick, MA), an umbrella‐shaped plug, uses a semispherical nitinol frame partially coated with a 160‐μm‐thick polyethylene terephthalate membrane (Figure 1B). The polyethylene terephthalate faces into the body of the LA and creates a permeable membrane to block embolization of thrombus while providing a scaffold on which re‐endothelialization can occur. The base of the device is anchored into the LAA by fixation barbs (see Supporting Information, video clips 1 and 2, in the online version of this article). The Watchman device is available in 5 sizes, ranging from 21 to 33 mm. The device size needs to be 10% to 20% larger than the diameter of the LAA orifice to ensure sufficient and stable positioning of the device (see Supporting Information, video clip 3, in the online version of this article). Shown in Figure 2 are computed tomography (CT) images of LAA with an implanted Watchman device. The manufacturer recommends follow‐up TEE at 6 weeks. If TEE shows complete closure or a residual leak with a jet width of <5 mm, warfarin is discontinued. Patients are then treated with 4.5 months of clopidogrel and lifelong aspirin.
Figure 2.
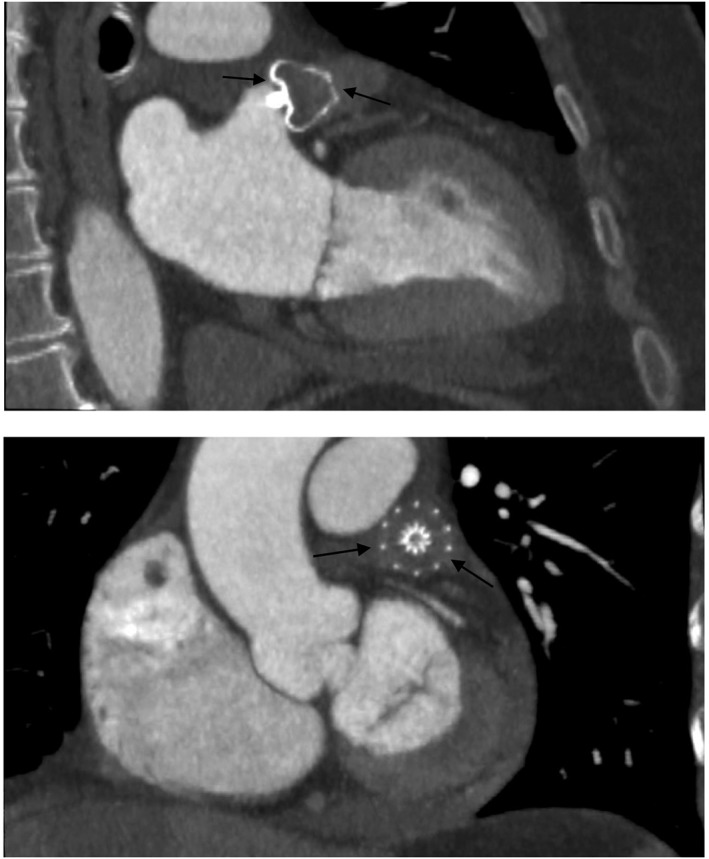
CT images of LAA with implanted Watchman device. Abbreviations: CT, computed tomography; LAA, left atrial appendage
The Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation (PROTECT‐AF) trial was the first trial to examine the efficacy of the Watchman device. The trial included 707 AF patients from 59 centers in the United States and Europe randomized in a 2:1 fashion to device vs warfarin.14 Efficacy was determined as freedom from composite endpoint of stroke, thromboembolic event, or cardiac death. The primary efficacy event rate was 3 per 100 patient‐years in the Watchman group and 4.9 per 100 patient‐years in the control arm after 18 months. Embolic stroke was higher in the intervention group, but hemorrhagic stroke was markedly higher in the warfarin arm. The incidence of safety events was the highest early in the trial, more frequently in the intervention group (7.4 vs 4.4 per 100 patient‐years) and most commonly on the day of procedure (55%), but this improved with increased operator experience. At 3.8 years of follow‐up, the primary efficacy event rate in the Watchman arm had decreased to 2.3 per 100 patient‐years (vs 3.8 per 100 patient‐years with warfarin) and met criteria for superiority.15
The Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients With Atrial Fibrillation vs Long‐Term Warfarin Therapy (PREVAIL) study randomized 407 patients in 2:1 fashion to device vs warfarin.16 PREVAIL did not achieve noninferiority for its primary efficacy endpoint, apparently due to the low rate of stroke in the control arm. Procedural complications decreased to 4.2% in PREVAIL, compared with 8.7% in PROTECT‐AF (P = 0.004). Post‐implantation statistical noninferiority was achieved for events occurring >7 days after device implantation.
A patient‐level meta‐analysis combined the results from PROTECT‐AF, PREVAIL, and the trial registries CAP1 and CAP2 (the Continued Access to PREVAIL).17 When compared with warfarin, Watchman resulted in fewer hemorrhagic strokes (P = 0.004), lower rates of nonprocedural bleeding (P = 0.006), and lower incidence of cardiac or unexplained deaths (P = 0.006). The Watchman group had more ischemic strokes (P = 0.05); however, after excluding procedural ischemic strokes, there was no difference in the rate of ischemic strokes between the 2 groups (P = 0.21).
The Watchman device was approved on the basis of the above data by the US Food and Drug Administration (FDA) in 2015 for nonvalvular AF patients at risk for stroke without contraindication to anticoagulation. The ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology (ASAP) study, a nonrandomized study including 150 patients, was carried out to assess the safety of Watchman in patients with contraindication to anticoagulation. These patients were treated with 6 months of aspirin and clopidogrel, followed by lifelong aspirin. At 14 months of follow‐up, embolism or all‐cause stroke occurred in 4 patients (2.3% per year), less than predicted based on CHADS2 score (7.3%).18
A recent analysis was performed to evaluate acute procedural performance and complication rates for all cases performed in the United States since FDA approval.19 A total of 3822 consecutive patients underwent device implantation between March 2015 and May 2016 by 382 operating physicians at 169 US centers. Seventy‐one percent of implanting physicians were new who performed 50% of the procedures, and the procedure was performed successfully in 95.6% patients. Complication rates were favorable, with pericardial tamponade, procedure‐related stroke, and mortality rates of approximately 1%, 0.08%, and 0.08%, respectively.
The feasibility and safety of NOACs as an alternative to warfarin in the periprocedural and postprocedural settings after Watchman implantation have been assessed in a retrospective analysis of a series of prospective LAAC registries at 5 participating centers.20 After implantation, 214 patients received NOACs (apixaban 46%, rivaroxaban 46%, dabigatran 7%, and edoxaban 1%). The control group of 212 patients received warfarin. Device‐related thrombosis and bleeding events were comparable between the 2 groups. Thus, the use of NOACs, peri‐ and postprocedurally, was a feasible and safe alternative to warfarin to prevent early thromboembolic complications following Watchman placement without increasing the risk of bleeding.20
2.1.3. Amplatzer Cardiac Plug and Amulet
The Amplatzer Cardiac Plug (ACP; St. Jude Medical, Minneapolis, MN; Figure 1C), a self‐expandable device, is made from nitinol and Dacron and consists of 3 parts: the lobe, the disk, and the middle. The distal lobe and proximal disk are connected by an articulating waist. The ACP comes in 8 sizes to accommodate varying LAA anatomy. The ACP and the second‐generation Amplatzer Amulet device are widely used outside the United States. ACP is the second most commonly used LAAC (after Watchman) in Europe; however, it has not received FDA approval for use in the United States. Most of the data for the ACP devices is derived from small registries maintained at centers outside the United States.21, 22, 23, 24
The largest observational trial included 1047 patients demonstrating both safety and efficacy for stroke prevention using the ACP.25 Major procedural adverse events occurred in 5%. All‐cause mortality at 1 year was 4.2%. On TEE, 4.4% had device thrombus; however, none of these patients developed stroke or transient ischemic attack.
The prospective, multicenter, international Amplatzer Amulet observational study, the largest registry of the 2 devices, enrolled 1073 patients, of which 1060 patients had device implantation with a success rate of 98.8%.26 Major adverse events within 7 days after the procedure occurred in 2.7% of 1073 patients, including ischemic stroke in 0.3%, embolization in 0.1%, bleeding in 0.9%, and pericardial effusion in 0.5%. At 1‐ to 3‐month follow‐up, the majority of patients were on antiplatelet therapy only and TEE showed an LAA closure rate of 99%.
The Amplatzer Amulet LAA Occluder trial is a prospective, randomized, multicenter active control trial to be carried out in 150 countries with estimated enrollment of 1600 patients. The investigational device exemption (IDE) trial is designed to evaluate the safety and effectiveness of the Amplatzer Amulet LAA Occluder (http://www.clinicaltrials.gov NCT 02879448).
2.2. Minimally invasive external occlusion of LAA
2.2.1. Lariat
The Lariat system (SentreHEART, Palo Alto, CA; Figure 1D) consists of 3 components: a balloon catheter, magnet‐tipped guidewires, and an epicardially placed 12‐F suture delivery device.27 The Lariat system requires both epicardial and endocardial approaches to occlude the LAA. Using a transseptal approach, the LA is entered, and the endocardial magnet‐tipped guidewire is placed in the apex of the LAA, over which the balloon catheter is advanced to the neck of the LAA (see Supporting Information, video clips 4 and 5, in the online version of this article). A second magnetic wire is advanced through the pericardial space to the epicardial surface opposing the tip of the LAA (see Supporting Information, video clip 6, in the online version of this article). When the 2 magnetic wires are opposed, a suture can be guided over the rail formed by the 2 magnet‐tipped guidewires and positioned basal to the balloon inflated in the neck of the LAA (see Supporting Information, video clip 7, in the online version of this article). Once adequate placement is confirmed with imaging, the epicardial suture can be secured and the LAA ligated.
Because the Lariat system leaves no hardware inside the LA after the LAA is occluded, there is no clear need for postprocedure anticoagulation. As a result, the Lariat device is being used in clinical practice for LAA occlusion in the United States in patients who have contraindications or failed oral anticoagulation. The Lariat device received 510(k) clearance by the FDA as a suture for tissue approximation, but not specifically for LAA exclusion or stroke prevention. The feasibility and safety of Lariat use have been shown in smaller studies.28, 29 Although approximately 4000 procedures have been performed so far in the United States, the largest safety report included only 154 patients from 8 centers.30 In this multicenter study, major complications occurred in 9.7%, including 14 major bleeds and 16 significant pericardial effusions. Death, MI, or stroke occurred in 4 patients over 3.7 months of follow‐up. Out of the 63 patients who underwent TEE, 13 had residual leak and 3 had LA thrombi.
Ligation of the LAA using the Lariat system may also create electrical isolation of the LAA and reduce the recurrence of AF after pulmonary vein isolation (PVI) in patients with persistent AF. A prospective observational study of 138 patients with persistent AF referred for AF ablation was performed. Sixty‐nine patients underwent LAA ligation with the Lariat procedure before undergoing PVI (Lariat group). The primary outcome of freedom from AF at 1 year off antiarrhythmic therapy after 1 ablation procedure was higher in the Lariat group than in those who underwent PVI alone (45 [65%] vs 27 [39%], respectively; P = 0.002).31 A multicenter randomized trial called LAA Ligation Adjunctive to PVI for Persistent or Longstanding Persistent Atrial Fibrillation (the aMAZE Study) has been designed to study this prospectively and is currently enrolling patients (http://www.clinicaltrials.gov NCT02513797). This study is a departure from others in that it will test the hypothesis that LAA closure reduces AF itself rather than stroke or thromboembolism.
2.2.2. AtriClip
AtriClip (AtriCure, West Chester, OH) is a self‐closing external rectangular LAA occluder (Figure 1E). It is available in 4 sizes, from 35 mm to 50 mm, in 5‐mm increments. It consists of 2 nitinol springs joined with 2 titanium members covered with Dacron polyester fabric. The parallel compression planes symmetrically exert a pressure of 2 to 8 psi over the entire contact area, effectively forming a surgically implanted clamp across the base of the LAA. It is applied epicardially via a median sternotomy or video‐assisted thorascopic surgical approach. The FDA 510(k) approval of the device states that it is indicated for LAA occlusion under direct visualization, in conjunction with other open cardiac surgical procedures. The Exclusion of Left Atrial Appendage with AtriClip Exclusion Device in Patients Undergoing Concomitant Cardiac Surgery (EXCLUDE) study, a nonrandomized prospective study conducted at 7 US centers, demonstrated the efficacy of the AtriClip device.32 A total of 71 patients were enrolled, of which 70 had successful placement of the AtriClip device. Although significant adverse events occurred in 34 of 70 patients, no adverse events were related to the device. Of the patients who underwent imaging by CT angiography or TEE, 60 of 61 patients (98.4%) had successful LAA exclusion. The efficacy of the AtriClip as a stroke‐prevention device has not been tested in randomized clinical trials.
2.3. Device deployment and anticoagulation
Deployment techniques and anticoagulation for some of the devices are outlined in Table 2. The Watchman, ACP, Amulet, and LAmbre devices all are deployed endovascularly. The Lariat requires an endo‐epicardial approach. The Watchman is implanted using a catheter‐based delivery system via a transseptal approach, guided by fluoroscopy and TEE to verify positioning and stability. Post‐implantation patients are anticoagulated for 45 days, followed by 6 months of daily aspirin and clopidogrel and then lifelong aspirin.14 The ACP also is deployed endovascularly using a transseptal approach. Anticoagulation is stopped immediately after the procedure, with requirements to take aspirin and clopidogrel for 1 to 6 months.21 The Lariat uses an endo‐epicardial approach with magnet‐tipped guidewires for LAA stabilization, followed by release of pre‐tied suture for LAA ligation. No anticoagulation is used in patients with contraindications.27
Table 2.
LAAO devices, FDA‐approved indication, deployment technique, and anticoagulation recommednation
| LAAO Device | Applicant | FDA Approval Type | Date of Approval | Indication/Equivalent Device | Access/Deployment | Recommended Anticoagulation |
|---|---|---|---|---|---|---|
| Watchman | Atritech | PMA | March 2015 | Stroke prevention | Endovascular | Warfarin for 45 days, then DAPT for 4.5 months |
| Lariat | SentreHEART | 510(k) | May 2006 | Suture for soft‐tissue closure | Endo‐epicardial | None |
| AtriClip | AtriCure | 510(k) | June 2010 | Ligating clip | Epicardial | None |
| ACP/Amulet | St. Jude Medical |
— | — | Endovascular | DAPT | |
| LAmbre | LifeTech Scientific |
— | — | Endovascular | None |
Abbreviations: ACP, Amplatzer Cardiac Plug; DAPT, dual antiplatelet therapy; FDA, US Food and Drug Administration; LAAO, left atrial appendage occlusion; PMA, Premarket Approval.
3. NEW LAA OCCLUSION DEVICES IN DEVELOPMENT
New LAA occlusion devices in development include the Sierra Ligation System (Aegis Medical, Vancouver, BC), which is an epicardial electrocardiogram‐guided LAA capture and ligation system. It has been studied in canine models33 (Figure 1F), and a feasibility study is on the way (http://www.clinicaltrials.gov NCT 02583178). LAmbre (LifeTech Scientific Corp., China) is a self‐expanding nitinol device with high success rates in canines.34 WaveCrest (Coherex Medical, Salt Lake City, UT; CE Mark approved) and Occlutech (Occlutech; Jena, Germany) are other LAA occlusion devices currently in development.
4. CURRENT GUIDELINES
The 2016 European Society of Cardiology guidelines recommend that a percutaneous LAA closure device may be considered in patients with high risk of stroke and contraindication for long‐term anticoagulation, class IIb (level of evidence B).35 The 2014 AHA/ACC/HRS guidelines have no recommendations for LAAO devices, as there was no FDA‐approved device at the time of the guidelines. With the recent FDA approval of Watchman, we anticipate recommendations in upcoming guidelines.
5. PATIENT AND PROCEDURE SELECTION FOR LAA OCCLUSION
At our institution, patients with a CHADS2 score ≥ 2 or CHA2DS2‐VASc score ≥ 3 and contraindication to long‐term anticoagulation are screened and evaluated for LAA occlusion procedures. Those who can tolerate short‐term anticoagulation and have a feasible LAA anatomy are candidates for a Watchman device. We use cardiac CT angiography for evaluation of anatomy and LAA measurements before the procedure. For short‐term anticoagulation after implantation of the Watchman device, we prefer warfarin, but we are increasingly making use of NOACs, particularly in those patients not already on warfarin or with a history of labile international normalized ratio on warfarin. The directions for use also suggest administration of aspirin in the early post‐implantation period, although we sometimes avoid the addition of aspirin in patients who have already had a major bleed. Those who cannot tolerate short‐term anticoagulation and have no prior cardiac surgery are candidates for the Lariat procedure if the anatomy is suitable; otherwise, we use the AtriClip device. A schematic outline of our decision tree is presented in Figure 3.
Figure 3.
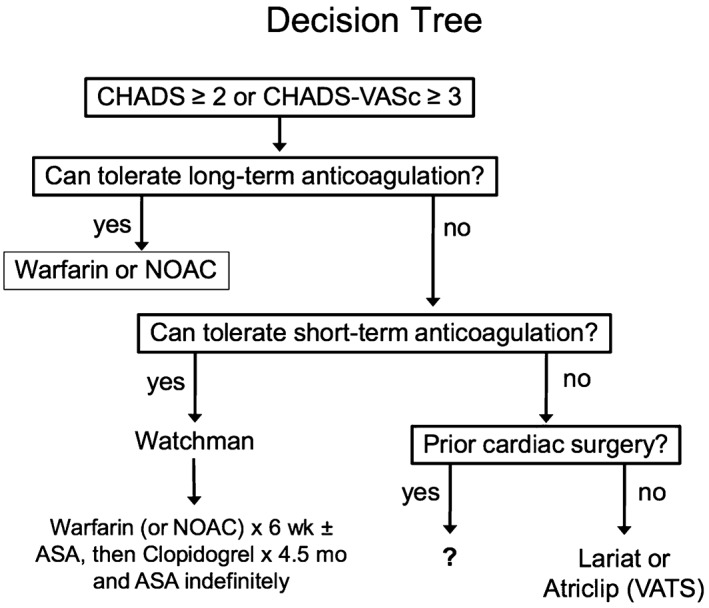
Schematic diagram of decision tree outlining our approach to patient selection for LAA occlusion. Abbreviations: ASA, acetylsalicylic acid (aspirin); LAA, left atrial appendage; NOAC, novel oral anticoagulant; VATS, video‐assisted thoracoscopic surgery
6. CONCLUSION
For the majority of patients with AF and increased risk of stroke, OAC with vitamin K antagonist or NOAC remains the mainstay of therapy. However, roughly 1 in 10 of these patients has a contraindication to anticoagulation due to a prior major bleeding episode or risk of such an event.36 Furthermore, among patients who initially tolerate OAC, subsequent trauma or bleeding events lead to interruption of anticoagulation use. For these patients, LAA occlusion has emerged as an alternative strategy for stroke prevention. Randomized clinical trials are ongoing for several emerging systems. Despite the reduction in stroke and improvement in survival, more studies are required to further determine optimal patient‐selection criteria.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Video clips 1. Watchman deployment
Video clips 2. Fluoroscopy to confirm position of watchman post deployment
Video clips 3. 3D post Watchman Left Atrial Appendage
Video clips 4. Transseptal for Lariat placement
Video clips 5. Lariat magnetic tip guide wire placement
Video clips 6. Lariat second magnetic guide wire placement
Video clips 7. Lariat suture placement
Bajwa RJ, Kovell L, Resar JR, et al. Left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation. Clin Cardiol. 2017;40:825–831. 10.1002/clc.22764
Funding Information
Funding for this research was provided in part by the Edward St. John Foundation for AF Research, the Roz and Marvin H. Weiner and Family Foundation, the Dr. Francis P. Chiaramonte Foundation, the Marilyn and Christian Poindexter Arrhythmia Research Fund, and the Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund.
REFERENCES
- 1. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991:22:983–988. [DOI] [PubMed] [Google Scholar]
- 3. Lane DA, Lip GY. Use of the CHA2DS2‐VASc and HAS‐BLED scores to aid decision‐making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126:860–865. [DOI] [PubMed] [Google Scholar]
- 4. Hart RG, Pearce LA, Aguilar MI, et al. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 5. Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. [DOI] [PubMed] [Google Scholar]
- 6. Manning WJ, Silverman DI, Keighley CS, et al. Transesophageal echocardiographically facilitated early cardioversion from atrial fibrillation using short‐term anticoagulation: final results of a prospective 4.5‐year study. J Am Coll Cardiol. 1995;25:1354–1361. [DOI] [PubMed] [Google Scholar]
- 7. Al‐Saady NM, Obel OA, Camm AJ, et al. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waks JW, Manning WJ. Left atrial appendage closure to reduce the risk of thromboembolic complications in atrial fibrillation: pay now and possibly pay later? J Am Coll Cardiol. 2015;65:2624–2627. [DOI] [PubMed] [Google Scholar]
- 9. Crystal E, Lamy A, Connolly SJ, et al; Left Atrial Appendage Occlusion Study . Left Atrial Appendage Occlusion Study (LAAOS): a randomized clinical trial of left atrial appendage occlusion during routine coronary artery bypass graft surgery for long‐term stroke prevention. Am Heart J. 2003;145:174–178. [DOI] [PubMed] [Google Scholar]
- 10. Kanderian AS, Gillinov AM, Pettersson GB, et al. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008;52:924–929. [DOI] [PubMed] [Google Scholar]
- 11. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society [published correction appears in Circulation. 2014;130:e270–e271]. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 12. Nakai T, Lesh MD, Gerstenfeld EP, et al. Percutaneous left atrial appendage occlusion (PLAATO) for preventing cardioembolism: first experience in canine model. Circulation. 2002;105:2217–2222. [DOI] [PubMed] [Google Scholar]
- 13. Ostermayer SH, Reisman M, Kramer PH, et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high‐risk patients with non‐rheumatic atrial fibrillation: results from the international multicenter feasibility trials. J Am Coll Cardiol. 2005;46:9–14. [DOI] [PubMed] [Google Scholar]
- 14. Holmes DR, Reddy VY, Turi ZG, et al; PROTECT‐AF Investigators . Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non‐inferiority trial [published correction appears in Lancet. 2009;374:1596]. Lancet. 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 15. Reddy VY, Sievert H, Halperin J, et al; PROTECT‐AF Steering Committee and Investigators . Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial [published correction appears in JAMA. 2015;313:1061]. JAMA. 2014;312:1988–1998. [DOI] [PubMed] [Google Scholar]
- 16. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long‐term warfarin therapy: the PREVAIL trial [published correction appears in J Am Coll Cardiol. 2014;64:1186]. J Am Coll Cardiol. 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 17. Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient‐level meta‐analysis. J Am Coll Cardiol. 2015;65:2614–2623. [DOI] [PubMed] [Google Scholar]
- 18. Reddy VY, Möbius‐Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013;61:2551–2556. [DOI] [PubMed] [Google Scholar]
- 19. Reddy VY, Gibson DN, Kar S, et al. Post‐approval U.S. experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2017;69:253–261. [DOI] [PubMed] [Google Scholar]
- 20. Enomoto Y, Gadiyaram VK, Gianni C, et al. Use of non‐warfarin oral anticoagulants instead of warfarin during left atrial appendage closure with the Watchman device. Heart Rhythm. 2017;14:19–24. [DOI] [PubMed] [Google Scholar]
- 21. Gloekler S, Shakir S, Doblies J, et al. Early results of first‐ versus second‐generation Amplatzer occluders for left atrial appendage closure in patients with atrial fibrillation. Clin Res Cardiol. 2015;104:656–665. [DOI] [PubMed] [Google Scholar]
- 22. Nietlispach F, Gloekler S, Krause R, et al. Amplatzer left atrial appendage occlusion: single‐center 10‐year experience. Catheter Cardiovasc Interv. 2013;82:283–289. [DOI] [PubMed] [Google Scholar]
- 23. Santoro G, Meucci F, Stolcova M, et al. Percutaneous left atrial appendage occlusion in patients with non‐valvular atrial fibrillation: implantation and up to four years' follow‐up of the Amplatzer Cardiac Plug. EuroIntervention. 2016;11:1188–1194. [DOI] [PubMed] [Google Scholar]
- 24. Park JW, Bethencourt A, Sievert H, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Cardiovasc Interv. 2011;77:700–706. [DOI] [PubMed] [Google Scholar]
- 25. Tzikas A, Shakir S, Gafoor S, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the Amplatzer Cardiac Plug. EuroIntervention. 2016;11:1170–1179. [DOI] [PubMed] [Google Scholar]
- 26. Hildick‐Smith D. Amulet Observational Study: multicenter, prospective registry results with a left atrial appendage closure device for stroke prevention in patients with atrial fibrillation. Presented at: Transcatheter Cardiovascular Therapeutics Meeting; November 2, 2016; Washington, DC. [Google Scholar]
- 27. Bartus K, Bednarek J, Myc J, et al. Feasibility of closed‐chest ligation of the left atrial appendage in humans. Heart Rhythm. 2011;8:188–193. [DOI] [PubMed] [Google Scholar]
- 28. Bartus K, Han FT, Bednarek, J , et al. Percutaneous left atrial appendage suture ligation using the Lariat device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. 2013;62:108–118. [DOI] [PubMed] [Google Scholar]
- 29. Gafoor S, Franke J, Bertog S, et al. Left atrial appendage occlusion in octogenarians: short‐term and 1‐year follow‐up. Catheter Cardiovasc Interv. 2014;83:805–810. [DOI] [PubMed] [Google Scholar]
- 30. Price MJ, Gibson DN, Yakubov SJ, et al. Early safety and efficacy of percutaneous left atrial appendage suture ligation: results from the U.S. Transcatheter LAA Ligation Consortium. J Am Coll Cardiol. 2014;64:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lakkireddy D, Mahankali AS, Kanmanthareddy A, et al. Left Atrial Appendage Ligation and Ablation for Persistent Atrial Fibrillation: the LAALA‐AF Registry. JACC Clin Electrophysiol. 2015;1:153–160. [DOI] [PubMed] [Google Scholar]
- 32. Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142:1002–1009. [DOI] [PubMed] [Google Scholar]
- 33. Friedman PA, Asirvatham SJ, Dalegrave C, et al. Percutaneous epicardial left atrial appendage closure: preliminary results of an electrogram‐guided approach. J Cardiovasc Electrophysiol. 2009;20:908–915. [DOI] [PubMed] [Google Scholar]
- 34. Lam YY, Yan BP, Doshi SK, et al. Preclinical evaluation of a new left atrial appendage occluder (LifeTech LAmbre device) in a canine model. Int J Cardiol. 2013;168:3996–4001. [DOI] [PubMed] [Google Scholar]
- 35. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 36. O'Brien EC, Holmes DN, Ansell JE, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) registry. Am Heart J. 2014;167:601.e1–609.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video clips 1. Watchman deployment
Video clips 2. Fluoroscopy to confirm position of watchman post deployment
Video clips 3. 3D post Watchman Left Atrial Appendage
Video clips 4. Transseptal for Lariat placement
Video clips 5. Lariat magnetic tip guide wire placement
Video clips 6. Lariat second magnetic guide wire placement
Video clips 7. Lariat suture placement


