Abstract
Background
Reverse cascade screening is not commonly employed to detect new cases of familial hypercholesterolemia (FH). We aimed to assess the outcome of this screening strategy in families in which the probands were children with severe FH.
Hypothesis
Reverse cascade screening is an effective method to detect new patients with FH.
Methods
Reverse cascade screening was undertaken starting from 47 index children with severe hypercholesterolemia; 39 were homozygous/compound heterozygous FH and 8 were heterozygous FH. Available parents, siblings, and second‐degree relatives were contacted and screened.
Results
From the 39 cases of homozygous/compound heterozygous FH, 80 first‐degree family members were available for screening; 70 were parents and 10 were siblings. All first‐degree relatives screened were genetically diagnosed with FH. None of the parents had been treated with statins at the time of diagnosis, and 10 (12.7%) had premature coronary artery disease. Additionally, 46 second‐degree relatives were screened, of which 41 (89%) were diagnosed with FH. From the 8 heterozygous FH children, 17 first‐ and second‐degree relatives were screened and 12 new cases of FH were also diagnosed. Hence, the overall diagnostic yield of screening was 2.8 new cases of FH per index case.
Conclusions
Reverse cascade screening is a highly effective method for diagnosing new cases of FH in parents, siblings, and second‐degree relatives of index children with severe FH.
Keywords: Reverse Cascade Screening, Severe Familial Hypercholesterolemia
1. INTRODUCTION
Homozygous familial hypercholesterolemia (FH) is a rare inherited condition characterized by an elevated low‐density lipoprotein cholesterol (LDL‐C) level and accelerated coronary artery disease (CAD). Left untreated, most patients develop severe atherosclerosis and do not survive beyond 30 years.1 Recent prevalence figures suggest that there are at least 5000 homozygous and 5.6 million heterozygous FH patients in China. Diagnosis and treatment in childhood is imperative for patients with homozygous FH, as well as their family members who may also have the condition.2
The development of atherosclerosis in childhood in FH mandates an early screening and treatment program.3 The general approaches to screening for FH in early life involve universal and selective strategies. The former has the potential to detect many affected individuals at an early stage; however, its acceptability and cost utility remains to be demonstrated, particularly in a country as populated and diverse as China.3, 4 Although universal screening of children and reverse cascade testing of first‐degree relatives has been recommended in FH,2 and positive experiences have been reported in certain European countries,5, 6 the approach is not recommended by other international organisations.7
Selective screening includes systematic approaches such as classical cascade testing and opportunistic testing based on knowledge of a family history of hypercholesterolemia and/or premature CAD.4, 8 However, the respective effectiveness of these approaches depends on an adequate proportion of index cases detected and the reliability and accuracy of the family history.4, 8 Reverse cascade screening is a form of systematic screening that should be routinely adopted in all families where a child presents with severe or homozygous FH and because of increased likelihood of the diagnosis of FH in first‐degree relatives.4, 9, 10
Pediatric homozygous FH cases are commonly referred to specialist lipid clinics via pediatricians or dermatologists owing to typical physical signs, such as tendon xanthomata. However, the practice of family screening is not commonly undertaken owing to lack of knowledge among physicians of the heritability and transmission of FH. The effectiveness of reverse family screening in China remains unreported. In the present study, we aimed to investigate the outcome of reverse cascade screening in families in which the probands were children with severe FH.
2. METHODS
Between 2003 and 2016, we consecutively recorded 47 pediatric cases of severe FH referred from 16 different provinces to Beijing Anzhen Hospital. After informed consent, reverse cascade screening based on genetic testing was carried out in the parents, siblings, and other relatives as required by sequencing the corresponding fragments from family members (Figure 1).11 Fasting lipid profiles at diagnosis prior to treatment, physical examination, and personal medical history for both index children and family members were recorded. Total cholesterol, triglycerides, LDL‐C, and high‐density lipoprotein cholesterol (HDL‐C) were measured using routine commercial kits (Beckman Coulter, Brea, CA) and a Beckman AU 5400 automated biochemical analyzer (Beckman Coulter). Genomic DNA was extracted from 10 mL of EDTA‐anticoagulated blood. Genetic testing was performed as previously described.11 In brief, to detect mutations in the LDL receptor (LDLR), apolipoprotein B (APOB) and proprotein convertase subtilisin‐kexin type 9 (PCSK9) genes, the promoter and 18 exons of the LDLR gene, a fragment of exon 26 of APOB gene and PCSK9 gene were scanned for mutations in the probands.
Figure 1.
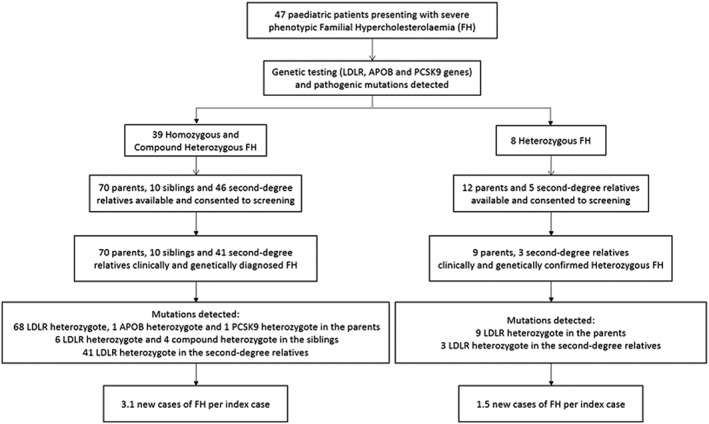
Schematic describing reverse cascade screening using genetic testing. Abbreviations: APOB, apolipoprotein B; FH, familial hypercholesterolemia; LDLR, low‐density lipoprotein receptor; PCSK9, proprotein convertase subtilisin/kexin type 9
Smoking was defined as current cigarette smoking. Obesity was defined in children as a body mass index ≥95th percentile for age and sex,12 and in adults as ≥28 kg/m2.13 Hypertension was defined in children as either systolic and/or diastolic blood pressure ≥ 95th percentile measured on ≥3 separate occasions,14 and in adults a systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg.15 Diabetes mellitus for both children and adults was defined as one of the following: (1) fasting plasma glucose concentration ≥ 7.0 mmol/L, (2) a 2‐h plasma glucose values concentration ≥ 11.1 mmol/L during an oral glucose tolerance test, or (3) glycated hemoglobin ≥6.5%.16 CAD was defined by stress test, coronary computed tomography angiography, or coronary angiography.
3. RESULTS
Of the 47 children, 39 were homozygous (true homozygous and compound heterozygous) FH and 8 were heterozygous FH. All children presented with cutaneous/tendon xanthomata. Table 1 shows the clinical characteristics of the 39 pediatric patients with homozygous FH. 34 mutations were detected, including nonsense, missense, frameshift, and in‐frame deletions in the LDLR gene (located in exons 2, 3, 4, 6, 9, 10, 12, 13, 14 and intron 3, 5, 8). In addition, one mutation in the APOB gene and one mutation in the PCSK9 gene were also detected. All mutations were assessed as pathogenic by previous functional studies17, 18, 19 and by reference to online databases (MutationTaster,20 ClinVar21). At diagnosis, the mean age of the index children was 9.1 ± 4.8 years, and the mean untreated LDL‐C level was 15.1 ± 3.8 mmol/L; 54% had CAD, 95% had carotid atherosclerosis and peripheral vascular disease, 23% had hypertension, and none had diabetes mellitus. None of the children were on lipid‐lowering treatment at diagnosis.
Table 1.
Clinical and molecular characteristics of the children with homozygous and compound heterozygous FH and their family members with FH
| Index Cases | Family Members With FH | |||
|---|---|---|---|---|
| Homozygous and Compound Heterozygous FH Children, N = 39 | Parents, N = 70 | Siblings, N = 10 | Second‐Degree Relatives, N = 41 | |
| Sex F/M, n | 18/21 | 34/36 | 4/6 | 19/22 |
| Age, y | 9.1 ± 4.8 | 37.5 ± 6.7 | 11.4 ± 7.7 | 41.5 ± 17.7 |
| Smoking | 0 (0) | 21 (30.0) | 1 (10) | 14 (34.1) |
| Obesity | 2 (5.1) | 3 (4.3) | 0 (0) | 6 (14.6) |
| HTN | 11 (23.4) | 23 (32.9) | 2 (20) | 18 (43.9) |
| DM | 0 (0) | 2 (2.9) | 0 (0) | 1 (2.4) |
| Tendon xanthomata | 39 (100) | 12 (17.1) | 4 (40) | 0 (0) |
| Arcus cornealis | 31 (79.5) | 4 (5.7) | 3 (30) | 3 (7.3) |
| Total cholesterol, mmol/L | 17.8 ± 4.3 | 7.6 ± 1.4 | 10.5 ± 5.3 | 7.7 ± 1.3 |
| LDL‐C, mmol/L | 15.1 ± 3.8 | 5.7 ± 1.3 | 8.5 ± 4.8 | 5.5 ± 1.4 |
| HDL‐C, mmol/L | 1.2 ± 0.8 | 1.4 ± 0.4 | 1.3 ± 0.3 | 1.5 ± 0.4 |
| TG, mmol/L | 1.3 ± 0.8 | 1.1 ± 0.4 | 1.0 ± 0.4 | 1.3 ± 0.6 |
| CAD | 21 (53.8) | 10 (12.7) | 3 (30) | 5 (12.2) |
| Lipid‐lowering therapy | 0 (0) | 0 (0) | 0 (0) | 2 (4.9) |
| Mutations | HoFH LDLR (n = 17) | HeFH LDLR (n = 68) | HeFH LDLR (n = 6) | HeFH LDLR (n = 41) |
| HzFH LDLR (n = 20) | HeFH APOB (n = 1) | HzFH LDLR (n = 4) | ||
| LDLR + APOB (n = 1) | HeFH PCSK9 (n = 1) | |||
| LDLR + PCSK9 (n = 1) | ||||
Abbreviations: APOB, apolipoprotein B; CAD, coronary artery disease; DM, diabetes mellitus; F, female; FH, familial hypercholesterolemia; HDL‐C, high‐density lipoprotein cholesterol; HeFH, heterozygous FH; HoFH, homozygous FH; HTN, hypertension; HzFH, compound heterozygous FH; LDL‐C, low‐density lipoprotein cholesterol; LDLR, low‐density lipoprotein receptor; M, male; PCSK9, proprotein convertase subtilisin/kexin type 9; SD, standard deviation; TG, triglycerides.
Data are expressed as n (%) or mean ± SD unless otherwise noted.
The majority (94.9%) of homozygous FH children were subsequently treated with statins, ezetimibe, and probucol, as monotherapy or combination therapy. Compliance with lipid‐lowering therapy and lifestyle advice was emphasized for reducing CAD risk. Despite significant reductions in LDL‐C (data not shown), none achieved guideline‐recommended cholesterol targets; apheresis and novel drugs such as the PCSK9 inhibitors, lomitapide and mipomersen were not utilized as these treatments are very expensive and not available in China. Initiatives to expand the access of these therapies to our high‐risk patients will be imperative to improve their care.
From these children, 80 first‐degree family members (70 parents and 10 siblings) were available for screening (Table 1). All first‐degree family members screened were found to have the same mutations as the index child (100% yield). The average age at diagnosis of the parents was 37.5 ± 6.7 years, and none of them were on treatment; mean LDL‐C level was 5.7 ± 1.3 mmol/L. Twelve (17.1%) parents presented with tendon xanthomata and 4 (5.7%) had arcus cornealis. Ten (12.7%) parents also had premature cardiovascular disease, and 2 (2.9%) had premature cerebral or peripheral vascular disease. Among the 10 siblings, 4 were genetically diagnosed with compound heterozygous FH at 13.25 ± 7.1 years and 6 with heterozygous FH at 10.17 ± 8.5 years; the mean untreated plasma LDL‐C concentrations were 13.1 ± 4.1 mmol/L and 5.4 ± 1.6 mmol/L, respectively. Additionally, 41 out of 46 second‐degree relatives (aunts, uncles, cousins) screened were diagnosed with heterozygous FH (mean untreated LDL‐C was 5.5 ± 1.4 mmol/L), of whom 5 were children. From the 8 heterozygous FH children, 17 first‐ and second‐degree relatives were screened and 12 new cases of FH were diagnosed. The overall diagnostic yield of reverse cascade screening was 2.8 new cases of FH per index case.
4. DISCUSSION
We demonstrate for the first time that reverse cascade screening based on genetic testing is an effective strategy for detecting FH in Chinese families. The particular value of reverse cascade screening from children carried out in a highly motivated environment allows the earlier detection of young parents and siblings who warrant early treatment to arrest the development of atherosclerotic cardiovascular disease.2, 3, 4, 9 With an index case with pediatric homozygous FH, the chance of detecting parents with FH is 100%, and the chance of detecting a sibling with FH is 50%.1 This probability is a priori significantly higher than if reverse cascade screening was undertaken from index children with heterozygous FH, emphasizing the greater yield of reverse cascade screening in the present setting.
Several international studies have emphasized that cascade screening in adults is highly cost‐effective.22, 23, 24 However, owing to China's one‐child policy (and, recently, two‐child policy), few children have siblings, so the yield of cascade screening may be restricted. Adjusting for pedigree size, yield of detection of FH in the strategy presented was approximately 3‐fold higher than that found in cascade screening programs in heterozygous FH adults.25, 26, 27, 28, 29, 30 The yield of any cascade testing program, and hence its potential to identify a greater proportion of affected individuals in the community, is dependent on an effective detection of index cases.10
The real challenge is identifying sufficient index cases in the community from whom cascade testing can begin.31 It has been proposed that universal screening at childhood immunization or at school entry may offer a solution.2, 3, 4 A recent study from the United Kingdom also showed that child–parent screening is highly feasible for identifying parents when their child was diagnosed during child immunization visits.32 However, this screening strategy needs to be adapted to individual countries.4, 33 Introducing universal screening in China would offer a solution to the general problem, but the worth of this approach needs to be demonstrated and supported by government policy. We concede that the cost‐effectiveness of universal screening coupled with reverse cascade screening in China requires further evaluation,34 particularly with respect to the existing population planning policy and a vastly disparate healthcare system.
We also demonstrate that heterozygous FH parents of the homozygous children have a milder phenotype compared with adults with FH in Western populations.35 Despite having a pathogenic FH mutation, 79% and 86% of the parents did not meet the diagnosis of FH according to the Dutch Lipid Clinic Network (DLCN) and Simon Broome criteria, respectively. This accords with previous studies35, 36 and may explain the underdiagnosis of adults with heterozygous FH in the Chinese population using the less sensitive Western criteria.37 A modified set of criteria based on a Chinese community population has been published but requires validation.38 Using these modified criteria, 59% of the parents in the present study would not have been diagnosed with FH. Premarital or prenatal screening with genetic testing is likely to be a more effective method of FH detection in China, but this is highly contentious.39, 40
Given China's large population, the emerging economy and the projected increase in cardiovascular mortality, FH is a public‐health problem that requires much attention. Implementation of guidelines and extensive work on education and awareness programs are imperative to improve the care of FH in China.
5. CONCLUSION
Reverse cascade screening is an effective method of diagnosing new cases of FH in parents, siblings, and second‐degree relatives of index children with severe FH. Furthermore, the outcomes of screening these high‐risk families clearly show that FH remains markedly under‐recognized and undertreated in China.
Conflicts of interest
The authors declare no potential conflicts of interest.
Wu X, Pang J, Wang X, et al. Reverse cascade screening for familial hypercholesterolemia in high‐risk Chinese families. Clin Cardiol. 2017;40:1169–1173. 10.1002/clc.22809
Funding information This work was supported by grants from the National Natural Science Foundation of China (Nos. 81170793 and 81370443)
REFERENCES
- 1. Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiegman A, Gidding SS, Watts GF, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36:2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin AC, Gidding SS, Wiegman A, et al. Known and unknowns in the care of paediatric familial hypercholesterolaemia. J Lipid Res. 2017;58:1765–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin AC, Bell DA, Brett T, et al. Beyond cascade screening: detection of familial hypercholesterolaemia at childhood immunization and other strategies. Curr Opin Lipidol. 2017;28:321–327. [DOI] [PubMed] [Google Scholar]
- 5. Klančar G, Grošelj U, Kovač J, et al. Universal screening for familial hypercholesterolemia in children. J Am Coll Cardiol. 2015;66:1250–1257. [DOI] [PubMed] [Google Scholar]
- 6. Ibarretxe D, Feliu A, Ferré R, et al. Heterozygous familial hypercholesterolemia detection in children: the DECOPIN project. Atherosclerosis. 2015;241:e113. [DOI] [PubMed] [Google Scholar]
- 7. Bibbins‐Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for lipid disorders in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:625–633. [DOI] [PubMed] [Google Scholar]
- 8. Watts GF, Gidding S, Wierzbicki AS, et al. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int J Cardiol. 2014;171:309–325. [DOI] [PubMed] [Google Scholar]
- 9. Wald DS, Bestwick JP, Wald NJ. Child‐parent screening for familial hypercholesterolaemia: screening strategy based on a meta‐analysis. Br Med J. 2007;335:599‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin AC, Allen C, Pang J, et al. Detecting familial hypercholesterolemia: the Jack and the Beanstalk principle. J Clin Lipidol. 2017;11:575–578. [DOI] [PubMed] [Google Scholar]
- 11. Lin J, Wang LY, Liu S, et al. Functional analysis of low‐density lipoprotein receptor in homozygous familial hypercholesterolemia patients with novel 1439 C > T mutation of low‐density lipoprotein receptor gene. Chin Med J (Engl). 2008;121:776–781. [PubMed] [Google Scholar]
- 12. Baker S, Barlow S, Cochran W, et al. Overweight children and adolescents: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:533–543. [DOI] [PubMed] [Google Scholar]
- 13. WGOC Group . The guidelines for prevention and control of overweight and obesity in Chinese adults. Acta Nutrimenta Sinica. 2004;26:1–4. [PubMed] [Google Scholar]
- 14. Flynn JT, Falkner BE. New clinical practice guideline for the management of high blood pressure in children and adolescents. Hypertension. 2017;70:683–686. [DOI] [PubMed] [Google Scholar]
- 15. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report [published correction appears on JAMA. 2003;290:197]. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 16. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology: clinical practice guidelines for developing a diabetes mellitus comprehensive care plan–2015. Endocrine Pract. 2015;21(s1):1–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su P, Wang L, Lin J, et al. A novel mutation of the LDL receptor gene leading to familial hypercholesterolemia. Eur J Lipid Sci Technol. 2009;111:646–651. [Google Scholar]
- 18. Wang L, Lin J, Liu S, et al. Mutations in the LDL receptor gene in four Chinese homozygous familial hypercholesterolemia phenotype patients. Nutr Metab Cardiovasc Dis. 2009;19:391–400. [DOI] [PubMed] [Google Scholar]
- 19. Lin J, Wang LY, Liu S, et al. A novel mutation in proprotein convertase subtilisin/kexin type 9 gene leads to familial hypercholesterolemia in a Chinese family. Chin Med J (Engl). 2010;123:1133–1138. [PubMed] [Google Scholar]
- 20. Schwarz JM, Rödelsperger C, Schuelke M, et al. MutationTaster evaluates disease‐causing potential of sequence alterations. Nat Methods. 2010;7:575–576. [DOI] [PubMed] [Google Scholar]
- 21. Landrum MJ, Lee JM, Riley GR, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2013;42:D980–D985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ademi Z, Watts GF, Pang J, et al. Cascade screening based on genetic testing is cost‐effective: evidence for the implementation of models of care for familial hypercholesterolaemia. J Clin Lipidol. 2014;8:390–400. [DOI] [PubMed] [Google Scholar]
- 23. Lázaro P, Pérez de Isla L, Watts GF, et al. Cost‐effectiveness of a cascade screening program for the early detection of familial hypercholesterolemia. J Clin Lipidol. 2017;11:260–271. [DOI] [PubMed] [Google Scholar]
- 24. Kerr M, Pears R, Miedzybrodzka Z, et al. Cost‐effectiveness of cascade testing for familial hypercholesterolaemia, based on data from familial hypercholesterolaemia services in the UK. Eur Heart J. 2017;38:1832–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bourbon M, Alves AC, Medeiros AM, et al; Portuguese FH Study Investigators. Familial hypercholesterolaemia in Portugal. Atherosclerosis. 2008;196:633–642. [DOI] [PubMed] [Google Scholar]
- 26. Muir LA, George PM, Laurie AD, et al. Preventing cardiovascular disease: a review of the effectiveness of identifying the people with familial hypercholesterolaemia in New Zealand. N Z Med J. 2010;123:97–102. [PubMed] [Google Scholar]
- 27. Umans‐Eckenhausen MA, Defesche JC, Sijbrands EJ. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet. 2001;357:165–168. [DOI] [PubMed] [Google Scholar]
- 28. Bell DA, Pang J, Burrows S, et al. Effectiveness of genetic cascade screening for familial hypercholesterolaemia using a centrally coordinated clinical service: an Australian experience. Atherosclerosis. 2015;239:93–100. [DOI] [PubMed] [Google Scholar]
- 29. Jannes CE, Santos RD, de Souza Silva PR, et al. Familial hypercholesterolemia in Brazil: cascade screening program, clinical and genetic aspects. Atherosclerosis. 2015;238:101–107. [DOI] [PubMed] [Google Scholar]
- 30. Leren TP. Cascade genetic screening for familial hypercholesterolemia. Clin Genet. 2004;66:483–487. [DOI] [PubMed] [Google Scholar]
- 31. Morris JK, Wald DS, Wald NJ. The evaluation of cascade testing for familial hypercholesterolemia. Am J Med Genet A. 2012;158A:78–84. [DOI] [PubMed] [Google Scholar]
- 32. Wald DS, Bestwick JP, Morris JK, et al. Child‐parent familial hypercholesterolemia screening in primary care. N Engl J Med. 2016;375:1628–1637. [DOI] [PubMed] [Google Scholar]
- 33. Martin AC, Bell DA, Brett T, et al. Beyond cascade screening: detection of familial hypercholesterolaemia at childhood immunization and other strategies. Curr Opin Lipidol. 2017;28:321–327. [DOI] [PubMed] [Google Scholar]
- 34. Norman R, Watts GF, Weintraub W, et al. Challenges in the health economics of familial hypercholesterolemia. Curr Opin Lipidol. 2016;27:563–569. [DOI] [PubMed] [Google Scholar]
- 35. Pimstone SN, Sun XM, du Souich C, et al. Phenotypic variation in heterozygous familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1998;18:309–315. [DOI] [PubMed] [Google Scholar]
- 36. Sun XM, Patel DD, Webb JC, et al. Familial hypercholesterolemia in China: identification of mutations in the LDL‐receptor gene that result in a receptor‐negative phenotype. Arterioscler Thromb Vasc Biol. 1994;14:85–94. [DOI] [PubMed] [Google Scholar]
- 37. Zhou M, Zhao D. Familial hypercholesterolemia in Asian populations. J Atheroscler Thromb. 2016;23:539–549. [DOI] [PubMed] [Google Scholar]
- 38. Shi Z, Yuan B, Zhao D, et al. Familial hypercholesterolemia in China: prevalence and evidence of underdetection and undertreatment in a community population. Int J Cardiol. 2014;174:834–836. [DOI] [PubMed] [Google Scholar]
- 39. Mao X. Chinese geneticists' views of ethical issues in genetic testing and screening: evidence for eugenics in China. Am J Hum Genet. 1998;63:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hesketh T. Getting married in China: pass the medical first. BMJ. 2003;326:277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]


