Abstract
Introduction
Dofetilide is a class III antiarrhythmic prescribed to cardiovert persistent atrial fibrillation (AF) to sinus rhythm (SR).
Hypothesis
To determine the clinical predictors of cardioversion and readmission in persistent AF patients on dofetilide.
Methods
We analyzed 160 patients with persistent AF who were started on dofetilide and followed for 1 year. We examined age, sex, race, hypertension, diabetes, smoking, dyslipidemia, CAD, left ventricular ejection fraction (LVEF), creatinine, BMI and concomitant use of calcium channel blockers (CCB), β‐blockers in a multivariable logistic regression model. We also examined the same predictors in Cox regression model for AF‐related readmission within 1 year of follow‐up.
Results
13.5% individuals did not convert to SR on dofetilide. 55.6% converted on the first dose and 83.1% converted by the fourth dose. In multivariable logistic models, dyslipidemia (OR: 2.4, CI: 1.12‐5.16) and LVEF (OR: 3.83,CI: 1.37‐10.8) were associated with failure to convert with the first dose. Female sex and LVEF also were associated with increased risk of failure to convert at all. Concomitant use of CCB associated with decreased risk of failure to convert to SR. In Cox proportional model, female sex, age <63 years and CAD were associated with increased AF readmission within 1 year.
Conclusions
Dyslipidemia and LVEF <40% were associated with failure to cardiovert after first dose, and female sex and LVEF 40% were related to failure to convert at all on dofetilide in persistent AF patients. After 1‐year follow‐up, female sex, known CAD, and age <63 years were associated with increased AF readmissions.
Keywords: Arrhythmia/all, Arrhythmia/all, management
1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, and it is expected to affect up to 12 million people in the United States by the year 2050.1, 2 AF not only leads to adverse quality of life, but is also associated with increased mortality and morbidity.3, 4, 5 Historically, these patients have been treated with rhythm control and/or rate control. Rhythm control is mainly needed in patients with AF who are symptomatic. It has shown to improve the quality of life, decrease disabling symptoms, and increase exercise tolerance, as demonstrated by Sotalol‐Amiodarone Atrial Fibrillation Efficacy Trial (SAFE‐T).6 Since 1999, dofetilide has been approved in the United States for chemical cardioversion of AF to sinus rhythm (SR) and also to maintain SR in symptomatic patients.7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Its safety and efficacy have been studied in several trials.10, 11, 13 However, the real‐life clinical data for the predictors of successful cardioversion and outcomes are lacking. Identifying such factors may help us personalize dofetilide treatment in various clinical subgroups.
The real‐life experience of dofetilide use has not been well‐scrutinized. Moreover, the predictors of its success with cardioversion and its relationship with AF hospitalization rates need to be explored to guide its use in symptomatic AF patients.
To further understand its efficacy and discern the predictors of cardioversion to SR and recurrence of AF, we followed a cohort of patients with persistent AF for 1 year and determined which of our selected predictors were associated with highest conversion failure rates and hospital readmission for AF.
2. METHODS
2.1. Study settings and eligibility criteria
After obtaining approval from the institutional review board, 2 authors (HH and JB) retrospectively reviewed 160 consecutive patients admitted in Mount Sinai St. Luke's and Mount Sinai West Hospitals from January 2013 to December 2014 for persistent symptomatic AF. Those who were in SR at baseline, had failed to respond to dofetilide in the past, had discontinuation of dofetilide, or in whom electrical cardioversion was performed prior to the tenth dose were excluded from the study. According to US Food and Drug Administration rules and regulations, dofetilide was initiated with close monitoring of the QT interval in the hospital setting. Dose was decreased if QTc increased by >15% from baseline, as per the FDA algorithm. Electrocardiography (ECG) was performed prior to initial dose and then was repeated after 2 hours and 6 hours of each dose to measure QTc interval, along with daily monitoring of creatinine clearance (CrCl) and electrolytes. Patients were continuously monitored on telemetry during their hospital stay. Any medication that was contraindicated to concomitant use with dofetilide was discontinued. Dosing was based on calculated CrCl by using the Cockcroft‐Gault adjustment formula, according to dosing algorithm.10 Dosing adjustments were also made based on QTc intervals on serial ECGs. At least 6 doses (3 days) were administered in‐hospital, commencing with an initial dose of 500 µg (with CrCl >60 mL/min) twice daily, unless a reduction in dosing was necessary. To avoid increased risk of stroke in the immediate post‐cardioversion time period, therapeutic anticoagulation was achieved prior to starting dofetilide. If anticoagulation was not achieved prior to initiation of therapy, transesophageal echocardiography was done to rule out left atrial thrombus to minimize risk of stroke. Elective direct current cardioversion was used in patients who were hemodynamically unstable during therapy or who did not respond to chemical cardioversion even after 10 doses. All subjects were monitored for ≥24 hours in SR prior to hospital discharge at the final dose of dofetilide for maintenance. The majority of the patient population continued 1‐year follow‐up in an outpatient setting. Dyslipidemia, which was an important predictor of failure to cardiovert, was defined as total cholesterol, low‐density lipoprotein cholesterol, and triglyceride levels >90th percentile and/or high‐density lipoprotein cholesterol <10th percentile in the general population. During follow‐up, patients had regular clinic visits every 6 weeks to check their rhythm. Patients who were treated elsewhere with either cardioversion or AF ablation were excluded from the study. Retrospective data were gathered by 2 authors (HH and JB) and the disagreements were resolved with consensus.
2.2. Dofetilide dosing
Dofetilide was initiated at doses of 500 µg twice daily (87.5%, n = 140), 250 µg twice daily (10%, n = 16), and 125 µg twice daily (2.5%, n = 4), based on baseline CrCl according to the Cockcroft‐Gault equation. A reduction in dosage was required in 40 patients (25%) due to ECG evidence of QTc prolongation by >15%, as per FDA algorithm. All subjects tolerated dofetilide, and therefore it was continued without any subsequent adverse effects for all 6 doses. No patients had to discontinue the drug because of intolerance.
2.3. Outcomes
Primary outcomes measured were the conversion of AF to SR after the first and the fourth dose of dofetilide. Failure to convert on dofetilide was also measured as the primary outcome. The incidence of torsades de pointes (TdP), the need for electrical cardioversion, and hospital readmissions were studied as secondary outcomes.
2.4. Statistical analysis
Baseline patients characteristics were expressed as proportions for categorical variables and mean ± SD for continuous variables. The association of failure to convert to SR on the first dose of dofetilide and inability to convert to SR at all on dofetilide with various clinical risk factors was assessed in multivariate logistic regression models. These models were adjusted for sex, race, dyslipidemia, hypertension, diabetes mellitus, known coronary disease, age, smoking, Cr, left ventricular ejection fraction (LVEF) <40%, concomitant calcium channel blocker and β‐blocker use, serum potassium, and serum magnesium. The association of these predictors with readmission for AF was assessed in multivariate Cox regression model after checking for proportional hazard assumptions. Cumulative incidence of readmission for AF was demonstrated using the Kaplan‐Meier plot. These analyses were performed using SAS version 9.2 software (SAS Institute, Inc., Cary, North Carolina). Statistical significance was considered for a P value of <0.05.
3. RESULTS
From January 2013 to December 2014, 410 patients were examined for potential eligibility for this study. Of those, 194 were confirmed based on strict exclusion criteria, and eventually 160 patients were included in the study and continued follow‐up to 1 year.
3.1. Baseline patient characteristics
Persistent AF was defined according to the Heart Rhythm Society consensus statement as a continuous episode lasting >7 days or an event terminated by electrical/chemical cardioversion after ≥48 hours.17 The mean patient age was 63.3 years, and the majority were males (73.8%); 95 patients were white (60%). Among risk factors, hypertension was present in 90 patients (56%), diabetes mellitus in 27 patients (17%), dyslipidemia in 67 patients (42%), and coronary artery disease (CAD) in 33 patients (20%). Mean LVEF was 54.9% ± 9%. Concomitantly, 109 patients were taking β‐blockers (68%) and 71 patients were taking calcium channel blockers (44%; Table 1).
Table 1.
Baseline characteristics of the study group
| Total population | N = 160 |
|---|---|
| Mean age, y | 63.3 |
| Male sex | 73.8 |
| White race | 60 |
| HTN | 56 |
| Dyslipidemia | 42 |
| DM | 17 |
| CAD | 20 |
| LVEF, mean ± SD | 54.9 ± 9 |
| β‐Blocker | 68 |
| CCB | 44 |
| Dofetilide initial dose | |
| 500 µg BID | 87.5 |
| 250 µg BID | 10 |
| 125 µg BID | 2.5 |
| Chronic warfarin | 63 |
Abbreviations: BID, twice daily; CAD, coronary artery disease; CCB, calcium channel blocker; DM, diabetes mellitus; HTN, hypertension; LVEF, left ventricular ejection fraction; SD, standard deviation.
Data are expressed as percentages unless otherwise noted.
3.2. Outcome data
Out of 160 included patients, 20 (13.5%) individuals did not convert to SR on dofetilide. In total, 89 (55.6%) converted on the first dose and 133 (83.1%) converted by the fourth dose. The pharmacological conversion was considered successful when SR was maintained for a minimum of 24 hours. Torsades de pointes, which is a feared side effect of dofetilide, was observed in 4 patients (0.25%) after initiation of therapy during the hospital stay. 27 patients required direct current cardioversion, due to either nonconversion after 10 doses, recurrence of AF, or hemodynamic instability during treatment (Table 2).
Table 2.
Outcome data
| Outcomes | % of Study Population |
|---|---|
| No conversion at all | 13.5 |
| Follow‐up after 1 year | 73 |
| Conversion after first dose | 55.6 |
| Conversion after fourth dose | 83.1 |
| Electrical cardioversion needed | 16 |
| Torsades de pointes | 0.25 |
| Readmissions within 1 year | 12.5 |
3.3. Predictors of failure to convert on dofetilide
In multivariable logistic models, dyslipidemia (odds ratio [OR]: 2.56, 95% confidence interval [CI]: 1.12‐5.16, P = 0.01) and LVEF <40% were associated with failure to convert with first dose of dofetilide. We also examined the predictors of failure to convert at all to SR. We found female sex (OR: 4.11, 95% CI: 1.06‐15.87, P = 0.04) and LVEF <40% (OR: 5.30, 95% CI: 1.41‐19.95, P = 0.01) were associated with increased risk of failure to convert to SR. Concomitant use of calcium channel blockers (OR: 0.23, 95% CI: 0.06‐0.90, P = 0.03) was associated with decreased risk of failure to convert to SR.
3.4. Predictors of readmission due to AF recurrence
In Cox proportional hazard models, female sex (hazard ratio [HR]: 0.163, 95% CI: 0.041‐0.655, P = 0.01), age <64 years (HR: 0.121, 95% CI: 0.03‐0.49, P = 0.003) and known CAD (HR: 4.16, 95% CI: 1.27‐13.68, P = 0.02) were associated with increased AF readmissions (20 patients) within 1 year (Table 3).
Table 3.
Predictors of readmission due to AF recurrence
| Predictors | OR for Failure to Convert to SR on First Dose (95% CI) | OR for Failure to Convert to SR After 10 Doses (95% CI) | HR for 1‐Year Readmission Due to AF (95% CI) |
|---|---|---|---|
| Female sex | 0.917 (0.394‐2.131) | 0.243 (0.063‐0.942) | 0.163 (0.041‐0.655) |
| Age >63 y | 0.994 (0.963‐1.026) | 1.026 (0.974‐1.081) | 0.121 (0.030‐0.486) |
| Black race | — | 1.504 (0.421‐5.36) | 1.99 (0.532‐7.46) |
| Dyslipidemia | 2.406 (1.123‐5.158) | 2.67 (0.748‐9.528) | 2.317 (0.618‐8.68) |
| HTN | 0.688 (0.313‐1.510) | 0.616 (0.164‐2.315) | 2.857 (0.724‐11.26) |
| DM | 0.506 (0.177‐1.449) | 2.085 (0.359‐12.12) | 1.38 (0.34‐5.47) |
| CAD | 0.622 (0.255‐1.519) | 0.448 (0.084‐2.399) | 4.164 (1.268‐13.68) |
| Smoking | 1.060 (0.385‐2.915) | 0.403 (0.033‐4.97) | 2.258 (0.491‐10.38) |
| Cr >1.5 mg/dL | 1.078 (0.227‐5.128) | 0.612 (0.047‐8.055) | 0.446 (0.032‐6.171) |
| LVEF <40% | 3.825 (1.373‐10.81) | 5.297 (1.406‐19.94) | 1.22 (0.341‐3.623) |
| CCB use | 1.046 (0.505‐2.164) | 0.233 (0.060‐0.90) | 0.426 (0.127‐1.430) |
| β‐Blocker use | 0.735 (0.340‐1.586) | 2.186 (0.447‐10.69) | 2.696 (0.587‐12.377) |
| Digoxin use | — | — | 1.711 (0.404‐7.25) |
| BMI, kg/m2 | 1.001 (0.995‐1.007) | 0.997 (0.934‐1.065) | 1.015 (0.963‐1.069) |
| Abnormal K level | 1.116 (0.549‐2.269) | 0.579 (0.156‐2.153) | 1.112 (0.341‐3.623) |
| Abnormal Mg level | 1.251 (0.375‐4.17) | 1.116 (0.303‐4.118) | 0.665 (0.091‐4.866) |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; Cr, creatinine; DM, diabetes mellitus; HR, hazard ratio; HTN, hypertension; K, potassium; LVEF, left ventricular ejection fraction; Mg, magnesium; OR, odds ratio; SR, sinus rhythm.
3.5. Sex differences in response to chemical cardioversion of AF to SR
Out of 160 total subjects, 42 (26.2%) were female. Of the 42 females, 9 (21%) did not convert after 10 consecutive doses of dofetilide, compared with 11 (9.3%) males. Out of those who converted, 5 (15%) females compared with 8 (7.5%) males were readmitted with AF during the 1‐year follow‐up. In the multivariable adjusted analysis, females were at higher risk of failure to convert on dofetilide than were males (OR: 3.92, 95% CI: 1.12‐13.7, P = 0.03). Similarly, females were at higher risk of 1‐year AF‐related readmissions than were males (HR: 3.60, 95% CI: 1.11‐11.76, P = 0.03). Males were more likely to achieve SR in the short term as well as stay in SR in the long term on dofetilide Figure 1.
Figure 1.
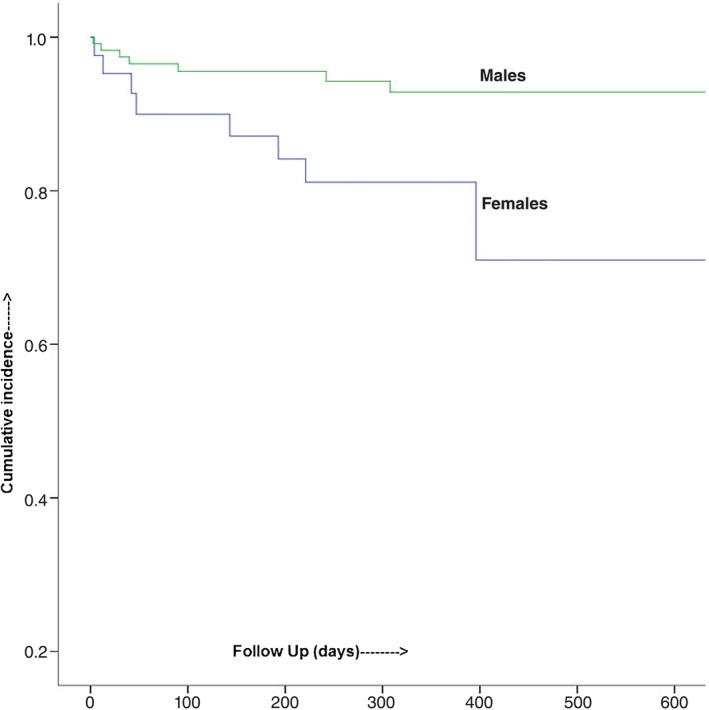
Kaplan‐Meier analysis showing cumulative survival.
4. DISCUSSION
Retrospectively studying a hospital cohort of 160 patients with persistent, symptomatic AF for response to dofetilide therapy, we found that the vast majority (83.1%) converted to SR by the fourth dofetilide dose. Despite stringent QTc‐interval monitoring in our study patients, 4 (0.25%) developed TdP during their admission. Female patients and patients with a reduced LVEF were found to be at a significantly greater risk of failing to convert to SR on dofetilide therapy. Furthermore, female patients, younger patients, and patients with diagnosed CAD were considerably more likely to be readmitted for AF within a 1‐year follow‐up period. Multivariate analysis demonstrated female sex to be an independent predictor of both failure to convert and an increased risk of readmission.
Data on the safety and efficacy of dofetilide and the encouragement for its use in persistent AF are primarily derived from information from 3 randomized controlled trials. The European and Australian Multicenter Evaluative Research on Atrial Fibrillation Dofetilide (EMERALD) study observed conversion to SR in 29% on a dofetilide dose of 500 µg within 72 hours of initiation, with 49% of patients at all doses maintaining SR for 1 year for any dose of the drug.13 In the Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE‐D) trial,10 29.9% of patients on the 500‐µg dose cardioverted acutely, with the vast majority doing so within 36 hours of drug initiation, and 58% of patients on this dose maintained SR at 1‐year follow‐up. In this trial, 0.8% of patients on active drug therapy experienced TdP. The Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) demonstrated higher drug efficacy, with 59% of patients converting to SR and 79% of converted patients maintaining SR at 1 year.11
The novelty of our study lies in exploring the predictors of failure to cardiovert persistent AF patients on dofetilide with the higher rate of acute conversion, with 83.1% of our patients converting to SR within 48 hours of initiating dofetilide therapy and 86.5% converting by the tenth dose. As a surrogate for maintenance of SR at 1 year, only 12.5% patients were readmitted for AF in the 1‐year follow‐up period. Part of this may be accounted for by the eligibility criteria, by excluding patients who had previously failed dofetilide therapy or had to undergo electrical cardioversion prior to the tenth dose. Furthermore, all 3 of those trials were conducted in a consolidated population of AF and atrial flutter patients. Results of these trials suggest that dofetilide may be best suited as a drug for the low‐risk AF patient who has not previously failed therapy with the drug and does not show a tendency toward fast rates or hemodynamic instability. Rates of TdP in our study were low (0.25%), accounted primarily by strict adherence to QTc monitoring.
Dofetilide has proven to be an effective drug in the armamentarium against AF, particularly in patients with structural heart disease or heart failure where long‐term drug therapy is anticipated or amiodarone use is limited by its diverse side‐effect profile. However, it suffers from the major drawback of potentially converting a usually benign, though symptomatically significant, rhythm to a highly fatal one. This adverse effect, combined with the additional cost and discomfort of the required hospital admission, is an important consideration when initiating the drug. Our study provides vital information about the patient profile most likely to benefit from this drug. It begs attention in high‐risk patients, particularly in the case of female patients and patients with reduced LVEF or CAD, toward alternative AF therapies, potentially avoiding the hazards of dofetilide in the face of limited clinical benefit.
4.1. Study limitations
Our study has several limitations. The attempt to identify predictors of cardioversion is noble but faces significant limitations, owing to the retrospective nature of the study. As described above, rigorous exclusion criteria (160 patients were selected out of 410 patients) for patient selection were employed to optimize the study, which may limit the universality of their clinical applicability. To address the patient diversity of the common clinical scenario, including but not restricted to patients with heart failure and patients using other medications concomitantly, further study of the efficacy and safety of dofetilide is warranted. It is known that the probability of converting to and maintaining SR after attempted cardioversion is predicted by some factors, particularly AF burden, duration of AF, and left atrial size. Our study does not select or stratify patients based on such risk factors and may hence be inadequately representative. Also, female sex correlates with the lower rate of cardioversion rests on a subtle sample (73.8% were males) and should be considered with caution.
5. CONCLUSION
The results of our present study concluded that dofetilide is an effective antiarrhythmic drug available to chemically cardiovert AF in the different group of a patient population after in‐hospital initiation and rigorous monitoring of QTc. We found dyslipidemia and low LVEF (<40%) to be the predictors of failure of cardioversion by dofetilide after the first dose. Also, low LVEF (<40%) and female sex were significantly related to an inability to cardiovert at all with dofetilide. Similarly, female sex, known CAD, and comparatively young age were associated with increased 1‐year readmissions due to AF recurrence. This can help us understand the target population for the appropriate response to treatment.
Conflicts of interest
The authors declare no potential conflicts of interest.
Hassan Virk HU, Qureshi WT, Makkar N, Bastawrose J, Souvaliotis N, Aziz J and Aziz E. Short‐ and long‐term clinical predictors of pharmacological cardioversion of persistent atrial fibrillation by dofetilide: A retrospective cohort study of 160 patients, Clin Cardiol, 2017;40:474–479. 10.1002/clc.22680
REFERENCES
- 1. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence [published correction appears in Circulation. 2006;114:e498]. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 2. Andersson T, Magnuson A, Bryngelsson IL, et al. All‐cause mortality in 272 186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long‐term case–control study. Eur Heart J. 2013;34:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romero JR, Wolf PA. Epidemiology of stroke: legacy of the Framingham Heart Study. Glob Heart. 2013;8:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wattigney WA, Mensah GA, Croft JB. Increased atrial fibrillation mortality: United States, 1980–1998. Am J Epidemiol. 2002;155:819–826. [DOI] [PubMed] [Google Scholar]
- 5. Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. [DOI] [PubMed] [Google Scholar]
- 6. Singh SN, Tang XC, Singh BN, et al; for the SAFE‐T Investigators . Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation: a Veterans Affairs Cooperative Studies Program substudy. J Am Coll Cardiol . 2006;48:721–730. [DOI] [PubMed] [Google Scholar]
- 7. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to revise the 2001 Guidelines for the management of patients with atrial fibrillation). J Am Coll Cardiol. 2006;48:e149–e246. [DOI] [PubMed] [Google Scholar]
- 8. Wann LS, Curtis AB, January CT, et al. ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:223–242. [DOI] [PubMed] [Google Scholar]
- 9. Center for Drug Evaluation and Research, US Food and Drug Administration . Tikosyn (dofetilide) NDA approval letter. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20-931_Tikosyn_Approv.pdf. Dated October 1, 1999. Accessed April 26, 2012.
- 10. Singh S, Zoble RG, Yellen L, et al. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE‐D) study. Circulation. 2000;102:2385–2390. [DOI] [PubMed] [Google Scholar]
- 11. Pedersen OD, Brendorp B, Elming H, et al. Does conversion and prevention of atrial fibrillation enhance survival in patients with left ventricular dysfunction? Evidence from the Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) study. Card Electrophysiol Rev. 2003;7:220–224. [DOI] [PubMed] [Google Scholar]
- 12. Bhatia GS, Lip GY. Atrial fibrillation post–myocardial infarction: frequency, consequences, and management. Curr Heart Fail Rep. 2004;1:149–155. [DOI] [PubMed] [Google Scholar]
- 13. Greenbaum RA, Campbell TJ, Channer KS, et al. Conversion of atrial fibrillation and maintenance of sinus rhythm by dofetilide: the EMERALD (European and Australian Multicenter Evaluative Research on Atrial Fibrillation Dofetilide) study. Circulation. 1998;98:1633–1640. [Google Scholar]
- 14. Torp‐Pedersen C, MØller M, Bloch‐Thomsen PE, et al; Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group . Dofetilide in patients with congestive heart failure and left ventricular dysfunction. N Engl J Med . 1999;341:857–865. [DOI] [PubMed] [Google Scholar]
- 15. KØber L, Bloch‐Thomsen PE, MØller M, et al. Effect of dofetilide inpatients with recent myocardial infarction and left‐ventricular dysfunction: a randomised trial. Lancet. 2000;356:2052–2058. [DOI] [PubMed] [Google Scholar]
- 16. Abraham JM, Saliba WI, Vekstein C. Safety of oral dofetilide for rhythm control of atrial fibrillation and atrial flutter. Circ Arrhythm Electrophysiol. 2015;8:772–776. [DOI] [PubMed] [Google Scholar]
- 17. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS) . Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm . 2012;9:632.e21–696.e21. [DOI] [PubMed] [Google Scholar]


