Abstract
The role of electrocardiography (ECG) in prognosticating pulmonary embolism (PE) is increasingly recognized. ECG is quickly interpretable, noninvasive, inexpensive, and available in remote areas. We hypothesized that ECG can provide useful information about PE prognostication. We searched MEDLINE, EMBASE, Google Scholar, Web of Science, abstracts, conference proceedings, and reference lists through February 2017. Eligible studies used ECG to prognosticate for the main outcomes of death and clinical deterioration or escalation of therapy. Two authors independently selected studies; disagreement was resolved by consensus. Ad hoc piloted forms were used to extract data and assess risk of bias. We used a random‐effects model to pool relevant data in meta‐analysis with odds ratios (ORs) and 95% confidence intervals (CIs); all other data were synthesized qualitatively. Statistical heterogeneity was assessed using the I 2 value. We included 39 studies (9198 patients) in the systematic review. There was agreement in study selection (κ: 0.91, 95% CI: 0.86‐0.96). Most studies were retrospective; some did not appropriately control for confounders. ECG signs that were good predictors of a negative outcome included S1Q3T3 (OR: 3.38, 95% CI: 2.46‐4.66, P < 0.001), complete right bundle branch block (OR: 3.90, 95% CI: 2.46‐6.20, P < 0.001), T‐wave inversion (OR: 1.62, 95% CI: 1.19‐2.21, P = 0.002), right axis deviation (OR: 3.24, 95% CI: 1.86‐5.64, P < 0.001), and atrial fibrillation (OR: 1.96, 95% CI: 1.45‐2.67, P < 0.001) for in‐hospital mortality. Several ischemic patterns also were significantly predictive. Our conclusion is that ECG is potentially valuable in prognostication of acute PE.
Keywords: Clinical Deterioration, Electrocardiography, Meta‐analysis, Mortality, Prognostication, Pulmonary Embolism
1. INTRODUCTION
Acute pulmonary embolism (PE) can rapidly lead to hemodynamic collapse and death. Current guidelines endorse risk‐stratifying patients, because those at high risk of clinical deterioration or death can be considered for additional treatment beyond anticoagulation, including thrombolysis or thrombectomy.1 Patients at low risk can generally be treated as outpatients.1 Risk‐stratification approaches include hemodynamic status, clinical scores, blood biomarkers, and computed tomographic (CT) or echocardiographic findings. Although the guidelines discuss electrocardiographic (ECG) findings in PE, the use of ECG as a prognostic tool is not reviewed.1 ECG is noninvasive, rapidly interpretable, low cost, and is one of the first tests performed in the emergency department. It is also available in remote areas with a scarcity of modern technological modalities.
Daniel et al. developed an ECG scoring system in 2001 (Daniel score) for the severity of pulmonary hypertension in patients with PE.2 It included tachycardia, right bundle branch block (RBBB), T‐wave inversion (TWI), and S1Q3T3.2 A score was assigned from 0 to 21, with a higher score indicating a worse clinical outcome.2, 3, 4 Since the publication of the Daniel score, several other studies have investigated the use of ECG as a tool for PE prognostication. These studies expanded the use of ECG and included findings not included in the Daniel score, such as ST‐segment depression, ST‐segment elevation (STE), Qr in lead V1, right axis deviation (RAD), and P pulmonale, among others.5, 6, 7, 8, 9, 10 A recent consensus article by the International Society of Electrocardiology, the International Society for Holter and Noninvasive Electrocardiology, and the Iberoamerican Forum of Arrhythmias in the Internet demonstrated the need for a formal and comprehensive evaluation of the evidence for the use of ECG to prognosticate PE.11
We aimed to comprehensively evaluate the data on ECG as a tool to prognosticate PE by performing a systematic review and meta‐analysis of the available evidence. In this article, we focused on clinical deterioration and death as prognostic outcomes.
2. METHODS
We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) and Meta‐Analysis of Observational Studies in Epidemiology (MOOSE) statements for reporting our systematic review and meta‐analysis.
We searched MEDLINE and EMBASE through February 2017 using keywords, MeSH terms, and Emtree headings. In addition, we searched Google Scholar and the Web of Science and examined abstracts, conference proceedings, and reference lists of retrieved articles. Two authors (AQ, GD) independently screened titles and abstracts and retrieved eligible articles if they (1) reported data on the prognostication of acute PE, (2) used ECG in their prognostic model, and (3) diagnosed PE formally by CT pulmonary angiogram, ventilation‐perfusion scan, or autopsy. For this article, we only included studies reporting mortality, or clinical deterioration defined as any of the following: (1) new hemodynamic collapse; (2) treatment upgrading (eg, thrombolysis, surgical thrombectomy); (3) intubation or resuscitation; or (4) systolic blood pressure consistently <100 mm Hg, refractory to volume loading and requiring vasopressors. We excluded studies not written in English.
All disagreements were resolved by consensus and consultation with a senior author (AB). We extracted data in a standardized manner using an ad hoc abstraction form containing study information and quality criteria. We systematically assessed study quality by evaluating the study population, definition of outcomes and ECG findings and their assessment, attrition bias, identification of confounders, and baseline imbalance (see Supporting Information, Table, in the online version of this article).
2.1. Statistical analysis
We analyzed data with the R package (R Foundation for Statistical Computing, http://www.r‐project.org) using the DerSimonian‐Laird random‐effects model. We evaluated between‐study heterogeneity using the I 2 index.12 We reported associations as odds ratios (ORs) and 95% confidence intervals (CIs). We excluded instances in which studies had no events for a particular ECG finding and prognostic outcome, rather than performing a continuity correction in empty cells. We conducted a sensitivity analysis to evaluate whether performing a continuity correction would have changed the association. We used a funnel plot and the Egger test to evaluate the potential for publication bias. Whenever pooling was not possible, qualitative evaluations were made on individual studies.
3. RESULTS
3.1. Article selection
There was agreement between reviewers for study screening (κ: 0.91, 95% CI: 0.86‐0.96). We identified 650 unique records. Seventy studies reported the prognostication of PE using ECG, but only 39 (9198 patients) reported mortality or clinical deterioration data and met inclusion criteria (Figure 1). Most included studies were retrospective cohort in design, and some studies did not appropriately control for confounders (Table 1 and Supporting Information, Table, in the online version of this article).
Figure 1.
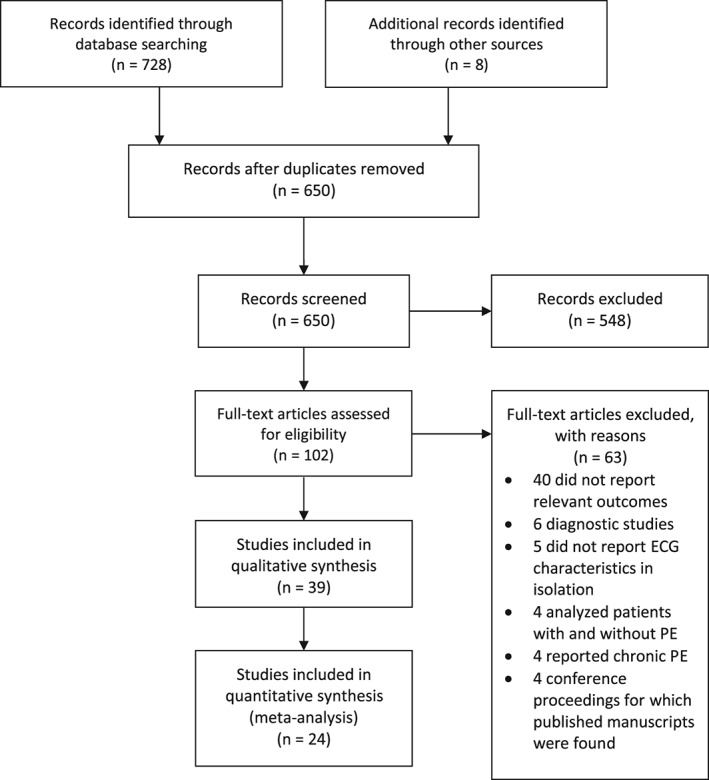
Flow chart of the selection process for inclusion of articles in this systematic review and meta‐analysis. Abbreviations: ECG, electrocardiographic; PE, pulmonary embolism
Table 1.
Characteristics of included studies
| Study | Design | Outcomes | N | Male Sex, N (%) | Mean Age, y (SD) | Included in Meta‐Analysis? | Comments |
|---|---|---|---|---|---|---|---|
| Agrawal 20145 | PC | In‐hosp mortality; clin deterioration | 200 | 123 (61.5) | 43.8 (NR) | Y | — |
| Akgullu 20156 | RC | In‐hosp mortality | 206 | 97 (47.1) | 61.8 (11.8) | Y | Excluded patients with missing lab values and patients with AF |
| Barra 201322 | RC | In‐hosp, 1‐mo, and 6‐mo mortality | 270 | 106 (39.3) | 70.1 (15.8) | Y | — |
| Bouvier 201533 | PC | 30‐d mortality or clin deterioration | 141 | — | — | N (abstract) | — |
| Bulj 201239 | PC | In‐hosp mortality; clin deterioration | 104 | 38 (36.5) | 68.7 (13.4) | N (abstract) | — |
| Buppajarntham 201424 | RC | In‐hosp mortality; clin deterioration | 300 | 122 (40.7) | 60.3 (17.6) | N | — |
| Ermis 201025 | RC | In‐hosp mortality; clin deterioration | 129 | 69 (53.5) | 58.0 (16.5) | Y | Excluded patients with AF at admission |
| Escobar 200731 | PC | 1‐mo mortality; 15‐d mortality due to PE | 644 | 277 (43.0) | — | Y | Only included hemodynamically stable patients at admission |
| Gallotta 200837 | PC | Clin deterioration | 90 | 25 (27.8) | 67.0 (18.0) | Y | Excluded patients with renal failure, recent ACS, and hemodynamically unstable patients at admission |
| Geibel 200530 | PC | 30‐d mortality | 508 | 214 (42.1) | 63.0 (15.0) | Y | — |
| Hariharan 20153 | PC | Adverse clinical eventa | 290 | 147 (51.0) | 59.0 (17.0) | N | Performed subanalysis excluding patients with chronic lung or cardiac disease—did not change results |
| Huang 201127 | RC | 30‐d mortality | 150 | 96 (64) | 71.3 (14.8) | Y | Excluded patients with recent ACS |
| Icli 201529 | RC | 30‐d mortality | 272 | 118 (43.4) | 63.1 (16.8) | N | Excluded patients with missing lab values or echocardiograms |
| Janata 201210 | RC | In‐hosp mortality; clin deterioration for STE‐aVR | 396 | 192 (48.5) | 59.8 (18.5) | Y | — |
| Kayrak 201328 | RC | 30‐d mortality | 359 | 168 (46.8) | 63.6 (15.8) | Y | — |
| Koracevic 200726 | RC | In‐hosp mortality | 125 | 39 (31.2) | 62.5 (—) | N (abstract) | — |
| Kostrubiec 20097 | RC | In‐hosp mortality; clin deterioration | 56 | 22 (39.3) | 64.3 (17.9) | Y | — |
| Kostrubiec 20108 | RC | In‐hosp mortality; clin deterioration | 94 | 42 (45.0) | 63.0 (19.0) | N | — |
| Kosuge 200638 | RC | In‐hosp mortality; clin deterioration | 40 | 15 (37.5) | 63.0 (13.0) | N | No group with normal ECG; TWI in all groups |
| Kucher 200314 | RC | In‐hosp mortality; clin deterioration | 75 | — | — | Y | — |
| Kukla 2011A42 | RC | Clin deterioration | 292 | 109 (37.3) | 65.4 (15.5) | Y | — |
| Kukla 2011B43 | RC | Clin deterioration | 293 | 111 (38.0) | 65.4 (15.5) | Y | — |
| Kukla 2011C15 | RC | In‐hosp mortality | 225 | 88 (39.1) | 66.0 (15.2) | Y | — |
| Kukla 2011D16 | RC | In‐hosp mortality; clin deterioration | 292 | 109 (37.3) | 65.4 (15.5) | Y | — |
| Kukla 2014A36 | RC | Clin deterioration | 500 | 210 (37.3) | 65.4 (15.5) | Y | — |
| Kukla 2014B17 | RC | In‐hosp mortality; clin deterioration | 245 | 103 (42.0) | 66.3 (15.2) | Y | — |
| Kukla 2015A18 | RC | In‐hosp mortality; clin deterioration | 437 | 170 (38.9) | 67.4 (19.0) | Y | TWI presumed secondary to LBBB or LVH |
| Kukla 2015B19 | RC | In‐hosp mortality; clin deterioration | 971 | 408 (42.0) | 66.0 (15.0) | Y | Excluded 35 patients due to missing or poor‐quality ECG |
| Kumasaka 200013 | RC | In‐hosp mortality | 139 | 47 (33.8) | 64.0 (15.0) | Y | — |
| Lee 200235 | PC | Clin deterioration | 65 | 25 (38.5) | 59.4 (15.9) | Y | — |
| Ryu 20104 | RC | In‐hosp mortality | 125 | 56 (44.8) | 62.7 (13.6) | N | Excluded uninterpretable ECGs |
| Stein 199741 | PC | Circulatory collapseb | 123 | — | — | N | Only included patients with no history of cardiac/pulmonary disease |
| Subramaniam 200834 | PC | 12‐mo mortality | 105 | 59 (56.2) | 58 (median) | N | — |
| Tayama 200220 | RC | In‐hosp mortality | 35 | 7 (20.0) | 62 (— ) | Y | — |
| Toosi 20079 | RC | In‐hosp mortality; clin deterioration | 159 | 70 (44.0) | 58.9 (17.7) | Y | Excluded those with no ECG |
| Vanni 200921 | PC | In‐hosp mortality; clin deterioration | 386 | 153 (39.6) | 67.0 (16.0) | N | Excluded hemodynamically unstable and those with cardiac/pulmonary disease |
| Zhan 201440 | RC | Clin deterioration | 20 | 8 (40.0) | 58.0 (10.0) | N | Cardiac/pulmonary disease excluded |
| Zhan 201532 | RC | 1‐mo mortality; clin deterioration | 210 | 92 (43.8) | 57.9 (14.4) | N | Cardiac/pulmonary disease excluded |
| Zorlu 201223 | PC | In‐hosp mortality | 127 | 62 (48.8) | 64.0 (13.0) | N | Patients with no lab values excluded |
Abbreviations: ACS, acute coronary syndrome; AF, atrial fibrillation; clin, clinical; ECG, electrocardiogram; In‐hosp, in‐hospital; lab, laboratory; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; N, no; NR, not reported; PC, prospective cohort; PE, pulmonary embolism; RC, retrospective cohort; SBP, systolic blood pressure; SD, standard deviation; STE, ST‐segment elevation; TWI, T‐wave inversion; Y, yes.
Adverse clinical event was defined as cardiac arrest, new arrhythmia, respiratory support, use of vasopressors, thrombolysis or thrombectomy, major bleeding, recurrent PE, or death from any cause within 5 days.
Circulatory collapse defined as loss of consciousness or SBP <80 mm Hg.
3.2. In‐hospital mortality
Twenty studies (4898 patients) reported data on in‐hospital mortality.4, 5, 6, 7, 9, 10, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Several ECG features were meta‐analyzed for this outcome (Table 2). Figure 2 shows 4 sample forest plots for the association between in‐hospital mortality and each of the following ECG findings: S1Q3T3, any RBBB, TWI in precordial leads, and TWI in precordial or inferior leads. Statistically significant predictors from the meta‐analysis included S1Q3T3, S1Q3T3 variations, complete RBBB, any RBBB, TWI in precordial or inferior leads, ST‐segment depression in leads V4 through V6, ST‐segment depression in any lead, STE‐V1, STE‐III, Qr‐V1, RAD, and atrial fibrillation (AF) at admission. Heterogeneity was generally low. Removing instances in which no events were reported for a particular ECG sign did not have a significant impact on the association compared with performing a continuity correction (data not shown). Some studies could not be pooled, and their findings are summarized in Table 2.
Table 2.
ECG findings as prognosticators of mortality in acute PE
| ECG Sign | No. of Studies (No. of Patients) | OR | 95% CI | P Value | I 2, % | References |
|---|---|---|---|---|---|---|
| In‐hospital mortality | ||||||
| Sinus tachycardia | 5 (737) | 1.93 | 0.96‐3.89 | 0.067 | 42.6 | 5, 6, 9, 13, 14 |
| S1Q3T3 | 9 (2155) | 3.38 | 2.46‐4.66 | <0.001 | 0 | 6, 7, 9, 10, 13, 15, 16, 17, 18 |
| S wave in lead I | 3 (250) | 1.16 | 0.33‐4.06 | 0.819 | 0 | 7, 9, 20 |
| Q wave in lead III | 3 (250) | 0.47 | 0.13‐1.71 | 0.251 | 0 | 7, 9, 20 |
| TWI in lead III | 2 (215) | 1.58 | 0.45‐5.54 | 0.473 | 38.1 | 7, 9 |
| S1Q3T3 variationsa | 3 (629) | 1.94 | 1.02‐3.72 | 0.0447 | 0 | 5, 10, 14 |
| Complete RBBB | 5 (1173) | 3.90 | 2.46‐6.20 | <0.001 | 0 | 6, 7, 10, 15, 16 |
| Incomplete RBBB | 4 (833) | 2.38 | 0.94‐6.01 | 0.0672 | 35.9 | 6, 9, 10, 14 |
| Any RBBB | 9 (1982) | 3.02 | 2.10‐4.34 | <0.001 | 5.52 | 6, 7, 9, 10, 13, 14, 15, 16, 18 |
| TWI in precordial leads | 9 (1535) | 1.40 | 0.99‐1.98 | 0.057 | 1.20 | 5, 7, 9, 10, 13, 14, 15, 16, 20 |
| TWI in precordial/inferior leads | 11 (2197) | 1.62 | 1.19‐2.21 | 0.002 | 7.22 | 5, 7, 9, 10, 13, 14, 15, 16, 18, 20 |
| ST‐segment depression in V4 through V6 | 2 (517) | 2.50 | 1.43‐4.36 | 0.0013 | 0 | 15, 16 |
| ST depression, any | 5 (1258) | 2.26 | 1.54‐3.32 | <0.001 | 0 | 6, 10, 13, 15, 16 |
| STE in lead aVR | 3 (913) | 1.68 | 0.79‐3.56 | 0.176 | 81.8 | 10, 15, 16 |
| STE in lead V1 | 4 (985) | 4.27 | 2.73‐6.66 | <0.001 | 0 | 10, 14, 15, 16 |
| STE in lead III | 2 (517) | 3.08 | 1.63‐5.81 | <0.001 | 0 | 15, 16 |
| Qr sign in V1 | 3 (589) | 4.72 | 2.54‐8.78 | <0.001 | 0 | 14, 15, 16 |
| RAD | 4 (798) | 3.24 | 1.86‐5.64 | <0.001 | 11.4 | 5, 6, 13, 16 |
| LAD | 2 (498) | 1.52 | 0.49‐4.70 | 0.468 | 68.5 | 6, 16 |
| Low‐voltage limb leads | 2 (517) | 1.59 | 0.63‐4.03 | 0.323 | 0 | 15, 16 |
| Low‐voltage limb/precordial | 3 (723) | 1.69 | 0.75‐3.79 | 0.203 | 0 | 6, 15, 16 |
| Clockwise rotation | 3 (760) | 1.44 | 0.83‐2.51 | 0.190 | 0 | 10, 14, 16 |
| P pulmonale | 4 (1019) | 1.71 | 0.76‐3.85 | 0.196 | 35.7 | 6, 10, 15, 16 |
| AF at admission | 4 (1733) | 1.96 | 1.45‐2.67 | <0.001 | 0 | 15, 16, 19, 22 |
| AF | 1 (125) | NR | NR | 0.736 | — | 26 (abstract) |
| RV strain patternb | 1 (386) | 4.13 | 1.22‐14.0 | 0.023 | — | 21 |
| Adjusted in‐hospital mortality | ||||||
| STE in V1 | 2 (688) | 3.23 | 1.71‐6.11 | <0.001 | 19.2 | 10, 16 |
| Complete RBBB | 1 (396) | 5.790 | 2.47‐13.6 | <0.001 | — | 10 |
| Sum of TWI | 1 (292) | 0.81 | 0.69‐0.95 | 0.0098 | — | 16 |
| No. of leads with TWI | 1 (292) | 1.68 | 1.68‐2.26 | 0.00068 | — | 16 |
| AF at admission | 1 (971) | 1.4 | 0.8‐2.3 | 0.2 | — | 19 |
| Prolonged QTc | 1 (300) | 1.3 | 0.3‐6.6 | — | 24 | |
| AF at admissionc | 1 (127) | 0.449 | 0.17‐1.22 | 0.115 | — | 23 |
| 30‐day mortality | ||||||
| Sinus tachycardia | 3 (1302) | 1.66 | 1.20‐2.31 | 0.0025 | 0 | 27, 30, 31 |
| S1Q3T3 | 4 (1661) | 0.99 | 0.69‐1.41 | 0.949 | 0 | 27, 28, 30, 31 |
| Any RBBB | 3 (1302) | 1.14 | 0.74‐1.77 | 0.555 | 17.1 | 27, 30, 31 |
| TWI – precordial leads | 2 (658) | 1.36 | 0.92‐2.02 | 0.123 | 0 | 27, 30 |
| AF at admission | 2 (395) | 2.47 | 1.18‐5.17 | 0.0167 | 25.6 | 22, 27 |
| Low‐voltage peripheral leads | 1 (508) | 1.94 | 1.24‐3.04 | 0.0039 | — | 30 |
| STE in I, II, or V4 through V6 | 1 (508) | 2.03 | 1.06‐3.90 | 0.034 | — | 30 |
| ST depression in I, II, or V4 through V6 | 1 (508) | 2.52 | 1.30‐4.90 | 0.0063 | — | 30 |
| Q in III, aVF not II | 1 (508) | 1.58 | 1.04‐2.39 | 0.032 | — | 30 |
| Tpeak‐Tend interval | 1 (272) | 12.9 | 3.05‐54.7 | 0.001 | — | 29 |
| AF§ | 1 (141) | 6.3 | 1.05‐37.7 | NR | — | 33 (abstract) |
| TWId | 1 (141) | 6.1 | 1.3‐29.1 | NR | — | 33 (abstract) |
| Adjusted 30‐day mortality | ||||||
| Sinus tachycardia | 1 (644) | 2.4 | 1.30‐4.20 | 0.003 | — | 31 |
| Any abnormalitye | 1 (508) | 2.56 | 1.49‐4.57 | <0.001 | — | 30 |
| AF/flutter | 1 (210) | 1.36 | 0.39‐4.80 | NS | — | 32 |
| S1Q3T3 | 1 (210) | 3.16 | 0.99‐10.2 | 0.052 | — | 32 |
| LAD | 1 (210) | 1.26 | 0.44‐3.68 | NS | — | 32 |
| RAD | 1 (210) | 1.02 | 0.34‐3.07 | NS | — | 32 |
| Low QRS voltage | 1 (210) | 1.63 | 0.43‐6.22 | NS | — | 32 |
| Clockwise rotation | 1 (210) | 0.28 | 0.05‐1.58 | NS | — | 32 |
| Notched S in V1 | 1 (210) | 1.22 | 0.40‐3.78 | NS | — | 32 |
| RBBB in V1 | 1 (210) | 0.64 | 0.16‐2.55 | NS | — | 32 |
| Qr sign in V1 | 1 (210) | 0.77 | 0.24‐2.53 | NS | — | 32 |
| No. of leads with TWI | 1 (210) | 1.18 | 0.96‐1.45 | NS | — | 32 |
| LV subendocardial ischemic pattern | 1 (210) | 3.711 | 0.78‐17.6 | NS | — | 32 |
| RV transmural ischemic pattern | 1 (210) | 4.22 | 1.14‐15.6 | 0.031 | — | 32 |
| LV subendocardial + RV transmural ischemia | 1 (210) | 4.02 | 1.13‐14.3 | 0.032 | — | 32 |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; ECG, electrocardiographic; HR, hazard ratio; LAD, left axis deviation; LV, left ventricular; NS, not significant; OR, odds ratio; PE, pulmonary embolism; QTc, corrected QT interval; RAD, right axis deviation; RBBB, right bundle branch block; RV, right ventricular; ST, ST segment; STE, ST‐segment elevation; TWI, T‐wave inversion.
Includes S1Q3T3/S1Q3, S1S2S3, S1Q3/S1rSr3′/S1S2S3.
Included ≥1 of: complete or incomplete RBBB, S waves in lead I combined with Q waves in lead III with or without T inversion in lead III (S1Q3T3), or inverted T waves in precordial leads V1, V2, and V3.
This study specifically looked at mortality related to PE and reported an HR, not an OR.
Included 30‐day mortality or clinical deterioration as an outcome.
Any 1 of: atrial arrhythmia; complete RBBB; peripheral low voltage; Q in leads III and aVF, but not in II; STE in leads I, II, and V4 through V6; and ST depression in leads I, II, and V4 through V6.
Figure 2.
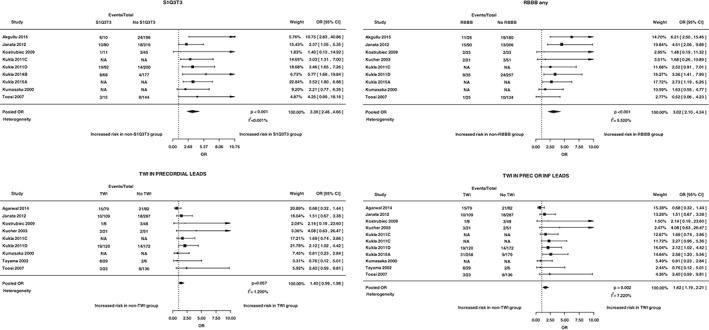
Sample forest plots. These 4 graphs show sample forest plots for the association between in‐hospital mortality and each of the following ECG findings: S1Q3T3, any RBBB, TWI in precordial leads, and TWI in precordial or inferior leads. Abbreviations: CI, confidence interval; ECG, electrocardiographic; inf, inferior; OR, odds ratio; prec, precordial; RBBB, right bundle branch block; TWI, T‐wave inversion
Some studies reported adjusted in‐hospital mortality data. Only STE‐V1 could be pooled and was found to be significantly predictive (Table 2). All other adjusted in‐hospital mortality data could not be pooled. Single studies identified complete RBBB and the number of leads with TWI to be significantly predictive (Table 2).
Some studies reported continuous ECG measures as predictors of in‐hospital mortality. Akgullu et al6 looked at QT‐interval dispersion and P‐wave dispersion and found that patients who died had a longer dispersion for both measures (median and interquartile range [IQR]: 104 [97–119] vs 78 [68–84], and 73 [54–79] vs 48 [35–55], respectively; P < 0.001 for both). Ermis and colleagues25 investigated QT‐interval dispersion and also found a longer mean (SD) dispersion in the group that died (89 [46] vs 65 [23]; P = 0.001). They also reported that a QT‐interval dispersion of 71.5 ms had a sensitivity of 71%, a specificity of 73%, and an area under the curve (AUC) of 0.73 (SE: 0.54; P = 0.001).
Kostrubiec et al7 reported the median (IQR) for the Daniel score and found it to be nonsignificantly higher in patients who died: 3.5 (0–15) vs 3 (0–18). The AUC (95% CI) for a score ≥ 3 in this study was 0.52 (0.37‐0.64). Toosi et al9 also found a nonsignificantly higher mean (SD) Daniel score in patients who died: 7.5 (SD not reported) vs 4.4 (5.6). The AUC (SE) with a score ≥ 3 for this study was 0.63 (0.078). Ermis et al25 reported median (IQR) for the Daniel score and found a significantly higher Daniel score for patients who died: 6.0 (7.0) vs 4.0 (4.0), P = 0.007. Ryu et al4 used a Daniel score cutoff of 12 and found that 3/38 (8%) of those in the high‐ECG score group died, whereas 12/87 (14%) of those in the low‐ECG group died (P = 0.55).
3.3. Thirty‐day mortality
Eight studies (2354 patients) reported data on 30‐day mortality.22, 27, 28, 29, 30, 31, 32, 33 Statistically significant features in the meta‐analysis included sinus tachycardia and AF at admission (Table 2). The I 2 value was generally low. Individual studies also reported adjusted 30‐day mortality data and found sinus tachycardia and right ventricular (RV) transmural ischemic pattern to be significantly predictive (Table 2). Numerous other unique ECG features were reported, and these are summarized in Table 2.
3.4. Longer‐term mortality
Barra et al22 investigated the association between AF at admission and 6‐month mortality and found an OR (95% CI) of 3.93 (1.95‐7.94; P < 0.001). An adjusted model with history of AF had an OR (95% CI) of 2.49 (1.14‐5.44; P = 0.023). Subramaniam et al34 examined the association between the Daniel score and 12‐month mortality and found no significant difference between groups: mean (SD) of 2.03 (2.34) in patients who died vs 2.40 (2.91) in those alive at 12 months (P = 0.65).
3.5. Clinical deterioration
Twenty‐one studies (4105 patients) had clinical deterioration as an outcome.3, 7, 8, 9, 14, 16, 18, 21, 24, 25, 27, 32, 35, 36, 37, 38, 39, 40, 41, 42, 43 Statistically significant features from the meta‐analysis included sinus tachycardia, S1Q3T3, TWI, complete RBBB, any RBBB, ST‐segment depression in V4 through V6, STE‐aVR, STE‐V1, STE‐III, Qr‐V1, and AF at admission (Table 3). Findings from studies that could not be pooled are summarized in Table 3. Removing instances in which no events were reported for a particular ECG sign did not have a significant impact on the association compared with performing a continuity correction (data not shown).
Table 3.
ECG findings as prognosticators of clinical deterioration in acute PE
| ECG Sign | No. of Studies (No. of Patients) | OR | 95% CI | P Value | I 2, % | References |
|---|---|---|---|---|---|---|
| Clinical deterioration | ||||||
| Sinus tachycardia | 6 (631) | 4.61 | 2.46‐8.65 | <0.001 | 40.8 | 7, 9, 14, 25, 27, 35 |
| S1Q3T3 | 8 (1822) | 3.89 | 2.50‐6.05 | <0.001 | 59.5 | 9, 16, 18, 25, 27, 35, 36, 37 |
| S wave in lead I | 2 (215) | 1.36 | 0.59‐3.11 | 0.470 | 0 | 7, 9 |
| Q wave in lead III | 4 (409) | 1.23 | 0.58‐2.59 | 0.582 | 27.5 | 7, 9, 25, 35 |
| TWI in lead III | 3 (280) | 2.30 | 0.67‐7.86 | 0.185 | 68.5 | 7, 9, 35 |
| S1Q3T3 variantsa | 2 (204) | 1.46 | 0.62‐3.46 | 0.384 | 0 | 14, 25 |
| Complete RBBB | 4 (977) | 2.47 | 1.61‐3.80 | <0.001 | 0 | 7, 16, 25, 36 |
| Incomplete RBBB | 3 (363) | 2.07 | 0.99‐4.33 | 0.052 | 0 | 9, 14, 25 |
| Any RBBB | 10 (1953) | 2.04 | 1.51‐2.75 | <0.001 | 7.7 | 7, 9, 14, 16, 18, 25, 27, 35, 36, 37 |
| RV strainb | 1 (386) | 5.82 | 1.82‐18.7 | 0.003 | — | 21 |
| TWI in precordial leads | 10 (1805) | 2.46 | 1.89‐3.21 | <0.001 | 9.7 | 7, 9, 14, 16, 25, 27, 35, 36, 37, 42 |
| TWI in precordial/inferior leads | 11 (2092) | 2.45 | 1.82‐3.28 | <0.001 | 35.8 | 7, 9, 14, 16, 18, 25, 27, 35, 36, 37, 42 |
| ST‐segment depression V4 through V6 | 3 (1084) | 2.71 | 2.01‐3.67 | <0.001 | 0 | 16, 36, 42 |
| STE in lead V1 | 4 (1159) | 5.14 | 3.80‐6.95 | <0.001 | 0 | 14, 16, 36, 42 |
| STE‐III | 3 (1084) | 3.06 | 2.07‐4.53 | <0.001 | 9.0 | 16, 36, 42 |
| STE‐aVR | 5 (1773) | 3.29 | 2.14‐5.07 | <0.001 | 0 | 10, 16, 36, 42, 43 |
| STE in contiguous leads | 1 (94) | 3.04 | 0.47‐19.7 | 0.243 | — | 8 |
| QR in V1 | 3 (864) | 4.65 | 2.05‐10.6 | <0.001 | 63.7 | 14, 16, 36 |
| Low QRS voltage | 1 (292) | 0.86 | 0.40‐1.84 | 0.706 | — | 16 |
| P pulmonale | 1 (292) | 2.06 | 0.85‐4.98 | 0.109 | — | 16 |
| Clockwise rotation | 2 (367) | 1.42 | 0.55‐3.69 | 0.469 | 62.9 | 14, 16 |
| AF at admission | 5 (1978) | 1.78 | 1.35‐2.36 | <0.001 | 13.4 | 16, 19, 27, 35, 36 |
| Adjusted clinical deterioration | ||||||
| S1Q3T3 | 1 (210) | 1.79 | 0.79‐4.08 | NS | — | 32 |
| Complete RBBB | 1 (292) | 2.87 | 1.15‐7.19 | 0.02 | — | 16 |
| Complete RBBB | 1 (40) | NR | NR | 0.50 | — | 38 |
| Complete RBBB | 1 (500) | 2.95 | 1.47‐5.91 | 0.002 | — | 36 |
| RBBB | 1 (104) | 111 | 12.7‐973 | <0.001 | — | 39 (abstract) |
| RBBB in V1 | 1 (210) | 0.91 | 0.33‐2.55 | NS | — | 32 |
| RV strainb | 1 (386) | 2.58 | 1.05‐6.36 | 0.038 | — | 21 |
| Sum of TWI | 1 (292) | 0.88 | 0.78‐0.98 | 0.022 | — | 16 |
| No. of leads with TWI | 1 (292) | 1.46 | 1.16‐1.85 | 0.001 | — | 16 |
| No. of leads with TWI | 1 (210) | 1.09 | 0.93‐1.30 | NS | — | 32 |
| 7+ leads with TWI | 1 (40) | 16.8 | 1.17–213 | 0.037 | — | 38 |
| ST‐segment depression V4 through V6 | 1 (500) | 2.24 | 1.27‐3.96 | 0.006 | — | 36 |
| STE in lead V1 | 1 (292) | 3.99 | 1.96‐8.18 | <0.001 | — | 16 |
| STE in lead V1 | 1 (500) | 7.62 | 4.50‐12.9 | <0.001 | — | 36 |
| STE in aVR | 1 (500) | 2.49 | 1.41‐4.39 | 0.002 | — | 36 |
| Qr sign in V1 | 1 (75) | 8.7 | 1.4–56.7 | 0.02 | — | 14 |
| Qr sign in V1 | 1 (210) | 1.03 | 0.41‐2.60 | NS | — | 32 |
| Qr sign in V1 | 1 (500) | 2.66 | 1.38‐5.10 | 0.003 | — | 36 |
| Fragmented QRS V1 | 1 (500) | 3.00 | 1.48‐6.05 | 0.002 | — | 36 |
| LAD | 1 (210) | 0.56 | 0.23‐1.37 | NS | — | 32 |
| RAD | 1 (210) | 1.06 | 0.44‐2.55 | NS | — | 32 |
| Low QRS voltage | 1 (500) | 3.44 | 1.57‐7.56 | 0.002 | — | 36 |
| Low QRS voltage | 1 (210) | 1.00 | 0.36‐3.09 | NS | — | 32 |
| Prolonged QTc | 1 (300) | 4.3 | 1.3‐14.3 | <0.001 | — | 24 |
| Clockwise rotation | 1 (210) | 0.53 | 0.16‐1.75 | NS | — | 32 |
| Notched S in V1 | 1 (210) | 1.53 | 0.60‐3.94 | NS | — | 32 |
| AF at admission | 1 (292) | 0.95 | 0.85‐1.05 | 0.3 | — | 16 |
| AF/flutter | 1 (210) | 1.02 | 0.35‐2.95 | NS | — | 32 |
| LV subendocardial ischemic pattern | 1 (210) | 4.96 | 1.67‐14.8 | 0.004 | — | 32 |
| RV transmural ischemic pattern | 1 (210) | 3.12 | 1.19‐8.23 | 0.021 | — | 32 |
| LV subendocardial plus RV transmural ischemic pattern | 1 (210) | 3.03 | 1.22‐7.56 | 0.017 | — | 32 |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; ECG, electrocardiographic; LAD, left axis deviation; LV, left ventricular; NR, not reported; NS, not significant; OR, odds ratio; PE, pulmonary embolism; QTc, corrected QT interval; RAD, right axis deviation; RBBB, right bundle branch block; RV, right ventricular; STE, ST‐segment elevation; TWI, T‐wave inversion.
Includes S1Q3/S1rSr3′/S1S2S3/Q3T3.
Included ≥1 of: complete or incomplete RBBB, S waves in lead I combined with Q waves in lead III with or without T inversion in lead III (S1Q3T3), or inverted T waves in precordial leads V1, V2, and V3.
Some studies reported an adjusted clinical deterioration outcome. None of these data could be pooled. Statistically significant predictors identified in individual studies included complete RBBB, RV strain, TWI, ST‐segment depression in V4 through V6, STE‐V1, STE‐aVR, Qr‐V1, fragmented QRS in V1, low QRS voltage, prolonged QTc, left ventricular subendocardial ischemic pattern, and RV transmural ischemic pattern (Table 3).
Kostrubiec et al7 reported the median (IQR) for the Daniel score and found the score to be significantly higher in patients who had clinical deterioration: 8 (1–17) vs 3 (0–18), P = 0.04. The AUC (95% CI) for a score ≥ 3 in this study was 0.73 (0.59‐0.84). Toosi et al9 also found a significantly higher mean (SD) Daniel score in patients that clinically deteriorated: 6.5 (6.1) vs 4.2 (4.3), P = 0.036. The AUC (SE) with a score ≥ 3 for his study was 0.64 (0.067).
3.6. Other adverse clinical outcomes
Stein et al. used ECG to prognosticate which patients with PE would likely have circulatory collapse, defined as loss of consciousness or a systolic blood pressure < 80 mm Hg.41 Of the ECG findings they investigated, complete RBBB was most predictive, being present in 2 of 5 patients with circulatory collapse and 5 of 118 patients with no circulatory collapse (P = 0.0257). Zhan et al. included hemodynamically stable patients at admission and assessed which patients would become hemodynamically unstable.40 They found S1Q3, abnormal QRS morphology in V1, STE‐V1, STE‐V2, STE‐III, STE‐aVR, ST‐segment depression in V4 through V6, and ST‐segment depression in lead I to be significantly associated with the development of hemodynamic instability.40
Hariharan and colleagues performed a prospective study in which they used ECG to predict patients that were more likely to have an adverse clinical course within 5 days, defined as any of the following: cardiac arrest, new arrhythmia, respiratory support, use of vasopressors, thrombolysis or thrombectomy, major bleeding, recurrent PE, or death from any cause.3 Multiple ECG findings were significantly predictive of this outcome. They then performed a multivariate analysis and found TWI in V1 through V3, S wave in lead I, and sinus tachycardia to have OR (95% CI) of 4.76 (1.71‐13.28), 2.04 (1.17‐3.54), and 2.58 (1.37‐4.85), respectively.3 They developed the “TwiST” score, with 5 points for TWI in V1 through V3, 2 points for S wave in lead I, and 3 points for sinus tachycardia.3 They found a TwiST score ≤ 2 to have a 76% sensitivity and 59% specificity, whereas a TwiST score ≥ 5 had 52% sensitivity and 87% specificity.3 They also computed test characteristics for the utility of the Daniel score in predicting the adverse clinical outcome and found a score ≤ 2 to have a 57% sensitivity and 74% specificity, whereas a Daniel score ≥ 7 had 44% sensitivity and 87% specificity.
3.7. Risk of publication bias
Publication bias was not detected by the funnel plot, as all studies had data points falling within the 95% CI bounds (see Supporting Information, Figure, in the online version of this article).
4. DISCUSSION
This systematic review and meta‐analysis of 39 studies (9198 patients) found that ECG features predict a negative outcome in patients with acute PE, including clinical deterioration, in‐hospital mortality, and 30‐day mortality. Specific features most predictive of in‐hospital death included S1Q3T3, complete RBBB, TWI, ST‐segment depression in V4 through V6, STE‐V1, STE‐III, Qr‐V1, RAD, AF, and RV transmural ischemic pattern. Similar findings were predictive of clinical deterioration, although other findings included sinus tachycardia and STE‐aVR. Adjusted analyses were generally consistent with these findings. The cause of clinical deterioration and death in patients with PE is usually due to RV failure, so it is expected that ECG features suggesting RV failure would predict a negative outcome.21 As for 30‐day mortality, adjusted analyses from individual studies demonstrated sinus tachycardia and RV transmural ischemic pattern to be significantly predictive. Sensitivity analyses demonstrated that excluding studies with no reported events had minimal impact on the association as compared with applying a continuity correction.
In 2001, Daniel et al. developed a 21‐point ECG score for the severity of pulmonary hypertension in patients with PE.2 Subsequent studies showed that the Daniel score was significantly higher in patients with clinical deterioration, but not significantly higher for in‐hospital mortality.4, 7, 9, 25 Furthermore, the predictive capacity of the Daniel score as measured by the AUC was found to be only modest. Hariharan et al. developed the TwiST score in 2015 with 5 points for TWI in V1 through V3, 2 points for S wave in lead I, and 3 points for sinus tachycardia.3 They found the TwiST score to have a slightly higher sensitivity and specificity than the Daniel score for predicting an adverse clinical outcome.3
A recent meta‐analysis by Shopp et al. reviewed the use of 12‐lead ECG to predict circulatory shock in patients with PE.44 However, they only used ECG findings on the Daniel score (namely, tachycardia, RBBB, TWI in V1 through V4, S1Q3T3), in addition to STE‐aVR and AF.44 Our meta‐analysis adds updated evidence for the use of ECG and includes features on the Daniel score and newly studied ECG findings since the Daniel score's publication. We found several ECG components to be predictive of clinical deterioration for in‐hospital mortality. These findings may be pragmatically useful, as ECG is one of the first tests performed in the emergency department and is noninvasive, rapidly interpretable, and low cost. Additionally, it is available in remote areas with a scarcity of modern technologies. Hence, clinicians may be able to use ECG to appropriately select higher‐risk patients requiring more intensive care or monitoring, even if they are deemed low‐risk patients by other clinical criteria. This may include normotensive patients with a high risk of RV failure. These patients have been shown to benefit from more intensive care services, including systemic or catheter‐directed fibrinolysis and pulmonary selective vasodilation.45, 46, 47, 48
4.1. Study limitations
Despite rigorous methodology, our review had some limitations. First, the assessment of publication bias was limited, as only a handful of ECG finding and outcome associations had ≥10 studies, the minimum number recommended for testing funnel‐plot symmetry.49 Second, most studies were retrospective in design, and some did not control for confounders. As such, higher‐quality studies, such as prospective cohort studies with appropriate controlling for confounders, are needed to more definitively assess which ECG findings can offer prognostic information in addition to currently used risk‐stratification tools. Finally, some studies did not independently adjudicate ECG features and outcomes, potentially leading to misclassification bias.
5. CONCLUSION
Acute PE can rapidly lead to hemodynamic collapse and death, and risk‐stratifying patients is imperative to determine those requiring more intensive treatment or monitoring. This meta‐analysis suggests that ECG can be a valuable tool in the prognostication of PE, especially when modern technology is not accessible. Nonetheless, most studies were retrospective, and some studies did not appropriately control for confounders. Hence, more prospective cohort studies with appropriate controlling for confounders would more definitively evaluate which ECG findings can offer prognostic information in addition to currently used risk‐stratification tools. These findings can aid in developing a new ECG scoring system to assist clinicians in risk‐stratifying patients with PE.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Supplemental Figure Funnel plot. This figure shows a funnel plot for the association between S1Q3T3 and in‐hospital mortality. The white, light gray, and dark gray areas represent the 90%, 95%, and 99% confidence bounds, respectively.
Supplemental Table Quality of included studies.
Qaddoura A, Digby GC, Kabali C, Kukla P, Zhan ZQ, Baranchuk AM. The value of electrocardiography in prognosticating clinical deterioration and mortality in acute pulmonary embolism: A systematic review and meta‐analysis. Clin Cardiol. 2017;40:814–824. 10.1002/clc.22742
References
- 1. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline. Chest. 2016;149:315–352. [DOI] [PubMed] [Google Scholar]
- 2. Daniel KR, Courtney DM, Kline JA. Assessment of cardiac stress from massive pulmonary embolism with 12‐lead ECG. Chest. 2001;120:474–481. [DOI] [PubMed] [Google Scholar]
- 3. Hariharan P, Dudzinski DM, Okechukwu I, et al. Association between electrocardiographic findings, right heart strain, and short‐term adverse clinical events in patients with acute pulmonary embolism. Clin Cardiol. 2015;38:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryu HM, Lee JH, Kwon YS, et al. Electrocardiography patterns and the role of the electrocardiography score for risk stratification in acute pulmonary embolism. Korean Circ J. 2010;40:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agrawal N, Ramegowda RT, Patra S, et al. Predictors of inhospital prognosis in acute pulmonary embolism: keeping it simple and effective! Blood Coagul Fibrinolysis. 2014;25:492–500. [DOI] [PubMed] [Google Scholar]
- 6. Akgullu C, Omurulu K, Eryilmaz U, et al. Predictors of early death in patients with acute pulmonary embolism. Am J Emerg Med. 2015;33:214–221. [DOI] [PubMed] [Google Scholar]
- 7. Kostrubiec M, Hrynkiewicz A, Pedowska‐Wioszek J, et al. Is it possible to use standard electrocardiography for risk assessment of patients with pulmonary embolism? Kardiol Pol. 2009;67:744–750. [PubMed] [Google Scholar]
- 8. Kostrubiec M, Jankowski K, Pedowska‐Wloszek J, et al. Signs of myocardial ischemia on electrocardiogram correlate with elevated plasma cardiac troponin and right ventricular systolic dysfunction in acute pulmonary embolism. Cardiol J. 2010;17:157–162. [PubMed] [Google Scholar]
- 9. Toosi MS, Merlino JD, Leeper KV. Electrocardiographic score and short‐term outcomes of acute pulmonary embolism. Am J Cardiol. 2007;100:1172–1176. [DOI] [PubMed] [Google Scholar]
- 10. Janata K, Hochtl T, Wenzel C, et al. The role of ST‐segment elevation in lead aVR in the risk assessment of patients with acute pulmonary embolism. Clin Res Cardiol. 2012;101:329–337. [DOI] [PubMed] [Google Scholar]
- 11. Digby GC, Kukla P, Zhan Z, et al. The value of electrocardiographic abnormalities in the prognosis of pulmonary embolism: a consensus paper. Ann Noninvasive Electrocardiol. 2015;20:207–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huedo‐Medina TB, Sánchez‐Meca J, Marín‐Martínez F, et al. Assessing heterogeneity in meta‐analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. [DOI] [PubMed] [Google Scholar]
- 13. Kumasaka N, Sakuma M, Shirato K. Clinical features and predictors of in‐hospital mortality in patients with acute and chronic pulmonary thromboembolism. Intern Med. 2000;39:1038–1043. [DOI] [PubMed] [Google Scholar]
- 14. Kucher N, Walpoth N, Wustmann K, et al. QR in V1 – an ECG sign associated with right ventricular strain and adverse clinical outcome in pulmonary embolism. Eur Heart J. 2003;24:1113–1119. [DOI] [PubMed] [Google Scholar]
- 15. Kukla P, Długopolski R, Krupa E, et al. The value of ECG parameters in estimating myocardial injury and establishing prognosis in patients with acute pulmonary embolism. Kardiol Pol. 2011C;69:933–938. [PubMed] [Google Scholar]
- 16. Kukla P, Długopolski R, Krupa E, et al. Electrocardiography and prognosis of patients with acute pulmonary embolism. Cardiol J. 2011D;18:648–653. [DOI] [PubMed] [Google Scholar]
- 17. Kukla P, McIntyre W, Fijorek K, et al. Use of ischemic ECG patterns for risk stratification in intermediate‐risk patients with acute PE. Am J Emerg Med. 2014B;32:1248–1252. [DOI] [PubMed] [Google Scholar]
- 18. Kukla P, McIntyre W, Fijorek K, et al. T‐wave inversion in patients with acute pulmonary embolism: prognostic value. Heart Lung. 2015A;44:68–71. [DOI] [PubMed] [Google Scholar]
- 19. Kukla P, McIntyre W, Koracevic G, et al. Relation of Atrial fibrillation and right‐sided cardiac thrombus to outcomes in patients with acute pulmonary embolism. Am J Cardiol. 2015B;115:825–830. [DOI] [PubMed] [Google Scholar]
- 20. Tayama E, Ouchida M, Teshima H, et al. Treatment of acute massive/submassive pulmonary embolism. Circ J. 2002;66:479–483. [DOI] [PubMed] [Google Scholar]
- 21. Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med. 2009;122:257–264. [DOI] [PubMed] [Google Scholar]
- 22. Barra SN, Paiva LV, Providência R, et al. Atrial fibrillation in acute pulmonary embolism: prognostic considerations. Emerg Med J. 2014;31:308–312. [DOI] [PubMed] [Google Scholar]
- 23. Zorlu A, Yucel H, Bektasoglu G, et al. Increased γ‐glutamyl transferase levels predict early mortality in patients with acute pulmonary embolism. Am J Emerg Med. 2012;30:908–915. [DOI] [PubMed] [Google Scholar]
- 24. Buppajarantham S, Seetha Rammohan HR, Junpaparp P, et al. Prognostic value of prolonged QTc interval in patients with acute pulmonary embolism. Acta Cardiol. 2014;69:550–555. [DOI] [PubMed] [Google Scholar]
- 25. Ermis N, Ermis H, Sen N, et al. QT dispersion in patients with pulmonary embolism. Wien Klin Wochenschr. 2010;122:691–697. [DOI] [PubMed] [Google Scholar]
- 26. Koracevic G, Atanaskkovic V, Salinger S, et al. Atrial fibrillation is not a predictor of in‐hospital mortality in pulmonary embolism [abstract]. Eur Respir J. 2007;617 http://www.ers‐education.org/events/international‐congress/stockholm‐2007.aspx?idParent=708.17400874 [Google Scholar]
- 27. Huang C, Lin Y, Lin Y, et al. Risk stratification and clinical outcomes in patients with acute pulmonary embolism. Clin Biochem. 2011;44:1110–1115. [DOI] [PubMed] [Google Scholar]
- 28. Kayrak M, Erdogan HI, Solak Y, et al. Prognostic value of neutrophil to lymphocyte ratio in patients with acute pulmonary embolism: a restrospective study. Heart Lung Circ. 2014;23:56–62. [DOI] [PubMed] [Google Scholar]
- 29. Icli A, Kayrak M, Akilli H, et al. Prognostic value of Tpeak‐Tend interval in patients with acute pulmonary embolism. BMC Cardiovasc Disord. 2015;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geibel A, Zehender M, Kasper W, et al. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J. 2005;25:843–848. [DOI] [PubMed] [Google Scholar]
- 31. Escobar C, Jiménez D, Martí D, et al. Prognostic value of electrocardiographic findings in hemodynamically stable patients with acute symptomatic pulmonary embolism. Rev Esp Cardiol. 2008;61:244–250. [PubMed] [Google Scholar]
- 32. Zhan Z, Wang C, Wang Z, et al. Significance of ST‐segment deviation in patients with acute pulmonary embolism and negative T waves. Cardiol J. 2015;22:583–589. [DOI] [PubMed] [Google Scholar]
- 33. Bouvier P, Chiche O, Moceri P, et al. Prognostic value of reflux of contrast into the inferior vena cava or hepatic veins in pulmonary embolism [abstract]. Arch Cardiovasc Dis Supp. 2015;7:109–110. 10.1016/S1878-6480(15)71800-8. [DOI] [Google Scholar]
- 34. Subramaniam RM, Mandrekar J, Chang C, et al. Pulmonary embolism outcome: a prospective evaluation of CT pulmonary angiographic clot burden score and ECG score. AJR Am J Roentgenol. 2008;190:1599–1604. [DOI] [PubMed] [Google Scholar]
- 35. Lee PY, Yeh HI, Hou CJY, et al. Comparison of clinical features and outcome in massive and non‐massive pulmonary embolism. Acta Cardiol Sin. 2002;18:173–180. [Google Scholar]
- 36. Kukla P, McIntyre W, Fijorek K, et al. Electrocardiographic abnormalities in patients with acute pulmonary embolism complicated by cardiogenic shock. Am J Emerg Med. 2014A;32:507–510. [DOI] [PubMed] [Google Scholar]
- 37. Gallotta G, Palmieri V, Piedimonte V, et al. Increased troponin I predicts in‐hospital occurrence of hemodynamic instability in patients with sub‐massive or non‐massive pulmonary embolism independent to clinical, echocardiographic and laboratory information. Int J Cardiol. 2008;124:351–357. [DOI] [PubMed] [Google Scholar]
- 38. Kosuge M, Kimura K, Ishikawa T, et al. Prognostic significance of inverted T waves in patients with acute pulmonary embolism. Circ J. 2006;70:750–755. [DOI] [PubMed] [Google Scholar]
- 39. Bulj N, Degoricija V, Sharma M, et al. Clinical and electrocardiography markers of right ventricle strain and early adverse outcome in patients with pulmonary embolism: results of single centre prospective study [abstract]. Intensive Care Med Supp. 2012;NA:318. http://poster‐consultation.esicm.org/ModuleConsultationPoster/posterDeta il.aspx?intIdPoster=3504. [Google Scholar]
- 40. Zhan Z, Wang C, Nikus KC, et al. Electrocardiogram patterns during hemodynamic instability in patients with acute pulmonary embolism. Ann Noninvasive Electrocardiol. 2014;19:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stein PD, Henry JW. Acute pulmonary embolism stratified according to their presenting syndromes. Chest. 1997;112:974–979. [DOI] [PubMed] [Google Scholar]
- 42. Kukla P, Długopolski R, Krupa E, et al. How often pulmonary embolism mimics acute coronary syndrome? Kardiol Pol. 2011B;69:235–240. [PubMed] [Google Scholar]
- 43. Kukla P, Długopolski R, Krupa E, et al. The prognostic value of ST‐segment elevation in the lead aVR in patients with acute pulmonary embolism. Kardiol Pol. 2011A;69:649–654. [PubMed] [Google Scholar]
- 44. Shopp JD, Stewart LK, Emmett TW, et al. Findings from 12‐lead electrocardiography that predict circulatory shock from pulmonary embolism: systematic review and meta‐analysis. Acad Emerg Med. 2015;22:1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Riera‐Mestre A, Becattini C, Giustozzi M, et al. Thrombolysis in hemodynamically stable patients with acute pulmonary embolism: a meta‐analysis. Thromb Res. 2014;134:1265–1271. [DOI] [PubMed] [Google Scholar]
- 46. Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all‐cause mortality, major bleeding, and intracranial hemorrhage: a meta‐analysis. JAMA. 2014;311:2414–2421. [DOI] [PubMed] [Google Scholar]
- 47. Nakamura S, Takano H, Kubota Y, et al. Impact of the efficacy of thrombolytic therapy on the mortality of patients with acute submassive pulmonary embolism: a meta‐analysis. J Thromb Haemost. 2014;12:1086–1095. [DOI] [PubMed] [Google Scholar]
- 48. Kline JA, Hernandez J, Garrett JS, et al. Pilot study of a protocol to administer inhaled nitric oxide to treat severe acute submassive pulmonary embolism. Emerg Med J. 2014;31:459–462. [DOI] [PubMed] [Google Scholar]
- 49. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure Funnel plot. This figure shows a funnel plot for the association between S1Q3T3 and in‐hospital mortality. The white, light gray, and dark gray areas represent the 90%, 95%, and 99% confidence bounds, respectively.
Supplemental Table Quality of included studies.


