Abstract
Background
There are accumulating studies showing the association between diabetes and all‐cause mortality in peripheral vascular disease. However, the results in these studies are conflicting regarding the impact of diabetes on outcome.
Hypothesis
Diabetes is associated with increased risk of mortality in peripheral artery disease.
Methods
Using MEDLINE and Scopus, we searched for studies published before January 2016. Additionally, studies were identified by manual search of references of original articles or review studies on this topic. Of the 1072 initially identified records, 21 studies with 15,857 patients were included in the final analysis.
Results
Diabetes was associated with a statistically significant increased risk of all‐cause mortality (odds ratio: 1.89, 95% confidence interval: 1.51‐2.35, P < 0.001), without detected publication bias (Egger bias = 0.75, P = 0.631). The stronger effect on outcome was obtained in patients with critical limb ischemia (odds ratio: 2.38, 95% confidence interval: 1.22‐4.63, P < 0.001) as the most severe form of peripheral vascular disease.
Conclusions
Diabetes is associated with an increased risk of mortality in peripheral vascular disease, and the effect is even more pronounced in patients with critical limb ischemia.
Keywords: peripheral artery disease, critical limb ischemia, diabetes, mortality, meta‐analysis
1. INTRODUCTION
Due to the decrease in smoking globally, diabetes is fast becoming the major risk factor for peripheral artery disease (PAD).1 Clustering of cardiovascular risk factors and metabolic changes that often accompany diabetes with subsequent increase in vascular inflammation contribute to the accelerated atherosclerosis and high risk for cardiovascular events.2, 3 Both the duration of diabetes and degree of diabetic control are related to incidence and severity of PAD.4 Peripheral neuropathy, commonly associated with diabetes, is responsible for later clinical presentation of the disease, with more severe manifestations, such as critical limb ischemia (CLI).5 Therefore, patients with diabetes have higher PAD prevalence (range, 20%–30%) and are exposed to premature mortality mostly due to cardiovascular diseases.5 Conflicting data were published regarding the prognostic implication of diabetes on all‐cause mortality in PAD patients.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 We performed a comprehensive systematic review and meta‐analysis of available studies to assess the prognostic significance of diabetes to predict all‐cause mortality in PAD patients. Additionally, we separately analyzed the impact of diabetes on fatal outcome in patients with CLI as the most severe form of peripheral vascular disease, frequently called acute coronary syndrome of the leg.
2. METHODS
During the conduct of this meta‐analysis we followed the Meta‐Analysis of Observational Studies in Epidemiology guidelines. We performed a systematic literature search of MEDLINE and Scopus for all studies published between January 1993 and January 2016, without language restriction, using the following medical subject headings: “diabetes,” “diabetic,” “peripheral artery disease,” “peripheral vascular disease,” “critical limb ischemia,” and “mortality.” Additional studies were identified by manual search of references of original studies or review studies. Studies meeting inclusion criteria were those comparing all‐cause mortality rates in patients with intermittent claudication and/or CLI and a follow‐up period of at least 1 year. Study selection and data extraction were conducted independently by 2 investigators (K.V., M.V.). Any disagreements or differences in the data extraction between the 2 authors were resolved through consensus after rechecking the source data and consultations with additional investigators (A.V.P., D.F., M.M.). The completed database contained the following data: name of the first author, year of publication, country of origin, study design, total number of patients in each study, the number of patients with diabetes and CLI, the definition of PAD, the proportion of patients with coronary artery disease (CAD), the number of patients who died in each group (with and without diabetes), the follow‐up period, conduction of multivariate analysis, and confounding factors. PAD was generally defined according to the ankle–brachial index at <0.9. Five studies included only patients with CLI, and the rest of the studies included those suffering from CLI together with patients with severe claudication (Rutherford stage 3) and indication for either vascular bypass surgery or endovascular treatment. Study quality was assessed using the validated Newcastle‐Ottawa Scale for assessment of nonrandomized and observational studies, and studies were evaluated based on subject selection, comparability of study groups, and assessment of the outcome.
Statistical heterogeneity was assessed using the Cochrane Q test and I 2 statistic. Statistically significant heterogeneity was considered present at P < 0.10 and I2 > 50%. Meta‐analysis of outcome was reported using a random‐effects model, and pooled odds ratio (OR) was reported with 95% confidence interval (CI). Egger's test was used to assess risk for publication bias. Sensitivity analysis was performed by excluding trials 1 at a time to assess the contribution of each study to the pooled estimates. Analyses were conducted using statistical software Stats Direct version 3.0.165 (Stats Direct Ltd., Altrincham, United Kingdom).
3. RESULTS
A total of 1072 citations were obtained from the electronic search. After reading titles and abstracts, followed by review of potentially relevant studies, 21 studies were included in the final analysis, including a total of 15857 patients (Figure 1). The study characteristics are listed in the Table 1. The mean age of the population was 69.8 years, 68.8% were males, 54.3% had CLI, 44.1% had CAD, and 43.6% patients had diabetes. Ten studies were retrospective and 11 were prospective in nature. Multivariate statistical analysis was performed in 19 studies. Using the Newcastle‐Ottawa scoring system, for included studies the mean score was 7.38, the median was 8.0, with the interquartile range was 7.0–8.0. A total of 28.7% of patients died during median follow‐up period of 2.9 years. Eight studies8, 10, 11, 12, 16, 21, 22, 23 reported the cause of death, and cardiovascular death comprised 54% (range, 33%–75%) and noncardiovascular death 46% (range, 25%–67%). In pooled analysis, 37.3% of PAD patients with diabetes died compared with 22.2% of PAD patients without diabetes (OR: 1.89, 95% CI: 1.51‐2.35) (Figure 2A). The analysis of pooled studies showed a significant heterogeneity (I2 = 84.9%, Cochran Q = 132.5, P < 0.001), but no publication bias was detected (Egger: bias = 0.75, P = 0.631). Sensitivity analysis indicated that none of the studies had a significant influence on the risk of mortality, and similar results to the main findings were obtained. Additional analyses of studies with performed multivariate analysis (OR: 1.89, 95% CI: 1.51‐2.37, I2 = 84.3%) and of prospective studies with performed multivariate analysis that included 7868 patients (OR: 1.82, 95% CI: 1.35‐2.45, I2 = 84%), revealed similar results. Meta‐analysis of 8 prospective studies8, 12, 13, 14, 16, 18, 21, 22 (5628 patients) with performed multivariate analysis and with the exclusion of studies that included only patients with CLI, showed substantially reduced level of heterogeneity (OR: 1.68, 95% CI: 1.37‐2.08, I2 = 49.8%) without publication bias (Egger: bias = 0.38, P = 0.857) (Figure 2B). Five studies11, 19, 20, 24, 26 analyzed exclusively patients with CLI with a total of 3523 patients. Meta‐analysis of those studies showed a stronger impact of diabetes on outcome (OR: 2.38, 95% CI: 1.22‐4.63, I2 = 93%), without detected publication bias (P = 0.778) (Figure 2C). Finally, a meta‐analysis was also conducted including 4 Caucasian studies with CLI, which exhibited an even stronger effect of diabetes on outcome, with a lower level of heterogeneity (OR: 2.96, 95% CI: 1.77‐4.97, I2 = 79.5%) (Figure 2D).
Figure 1.
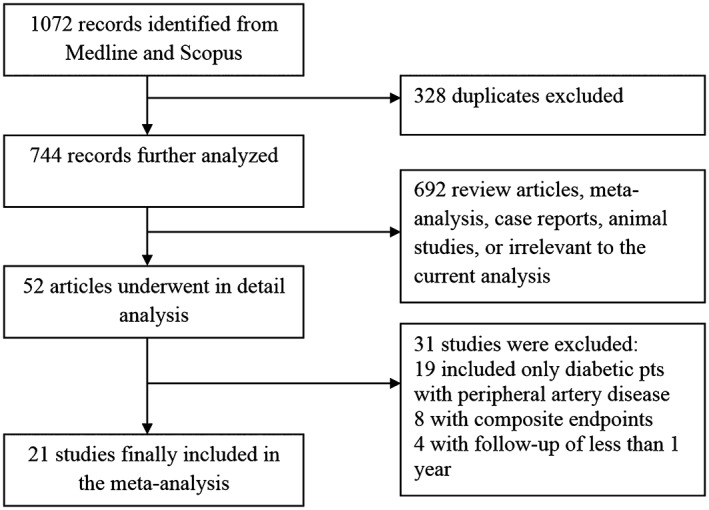
Study flow diagram.
Table 1.
Characteristics of studies included in meta‐analysis
| Author, Year | Country | Patients (No.) | Age (Years) | Male (%) | Type of Study | Follow‐up (Years) | DM (%) | CAD (%) | Mortality (%) | Mortality: DM vs Non‐DM (%) | PAD Definition | ABI | CLI (%) | Multivariate Analysis | Confounders |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abularrage, 2010 | USA | 920 | 71.2 | 64.3 | R | 2.9 1 | 49.9 | 61.5 | 46.5 | 49 vs 44 | Stage 3, CLI | — | 41.7 | Yes | Age, sex, CAD, HT, CKD |
| Akbari, 2000 | USA | 962 | 68.4 | 61.9 | P | 5 | 34.5 | 45.8 | 41.8 | 41.8 vs 41.9 | Stage 3, CLI | — | 92 | No | — |
| Cheng, 2000 | China | 665 | 71.1 ± 11.1 | 60.3 | P | 2.3 | 42 | 35.8 | 44.2 | 47 vs 42 | ABI 2 | — | 62.4 | Yes | Age, sex, CAD, HT, CKD, smoking, HLP |
| Collins, 2007 | USA | 796 | 64.7 ± 9.9 | 99 | R | 1.4 | 61.3 | — | 44.5 | 46.9 vs 40.6 | ABI | 0.57 1 | 16.5 | Yes | Age, sex, CAD, HT, CKD, smoking, HLP, medications |
| Dawson, 1993 | Netherlands | 376 | 63.9 ± 11.5 | 83.5 | R | 5.9 | 24.2 | 45.5 | 30.9 | 52.7 vs 23.9 | Stage 3, CLI | — | 58.8 | Yes | Age, sex, CAD, HT, CKD, smoking |
| Dick, 2007 | Switzerland | 400 | 75.5 ± 10.9 | 57.7 | P | 1 | 46.3 | — | 30.5 | 35.1 vs 26.5 | CLI | — | 100 | Yes | Age, sex, HT, CKD, smoking, HLP |
| Garg, 2006 | USA | 460 | 71.9 ± 8.4 | 59.4 | P | 4.8 1 | 31.1 | 35.7 | 29.1 | 40.6 vs 24 | ABI | 0.64 ± 0.15 | 81.2 | Yes | Age, sex, CAD, HT, CKD, smoking, HLP |
| Golledge, 2014 | Australia | 1177 | 71 1 | 74.1 | P | 1.7 1 | 44.4 | 52.2 | 11.5 | 12.6 vs 10.5 | Stage 3, CLI | — | 12.3 | Yes | Age, sex, CAD, HT, CKD, smoking, medications |
| Goodney, 2010 | Lebanon | 2036 | 73 | 67 | P | 1 | 53 | 40 | 10.2 | 13 vs 7 | Stage 3, CLI | — | 75 | Yes | Age, CAD, CKD, smoking, medications |
| Jude, 2001 | United Kingdom | 136 | 64.7 ± 10.8 | 59.6 | R | 4.5 1 | 42.6 | 39 | 36.8 | 51.7 vs 25.6 | Stage 3, CLI | — | 37.5 | Yes | Age, sex, CAD, HT, CKD, smoking |
| Kalman, 1997 | Canada | 358 | 68 ± 10 | 68 | P | 5 | 38 | 47 | 54.2 | 67.6 vs 45.9 | ABI | 0.35 ± 0.21 | 79.1 | Yes | Age, sex, CAD, CKD |
| Karacagil, 1995 | Sweden | 267 | 68.5 ± 12 | 67.9 | R | 3 | 34.5 | — | 22.1 | 38 vs 13.7 | ABI | — | 75.6 | No | — |
| Leibson, 2004 | USA | 335 | 61.2 ± 5.3 | 63.6 | P | 23 | 55.5 | 26 | 78.5 | 81.7 vs 74.5 | ABI | 0.75 ± 0.26 | NM | Yes | Age, sex, CAD, HT, smoking |
| Luther, 1997 | Finland | 188 | 72.5 | 53.2 | R | 5 | 47.3 | — | 43.1 | 58.4 vs 29.3 | CLI | — | 100 | Yes | Age, sex, HT, smoking, HLP |
| Malmstedt, 2008 | Sweden | 1840 | 76.2 ± 9.5 | 53.1 | P | 2.2 1 | 40.3 | 59 | 47 | 65.5 vs 34.4 | CLI | — | 100 | Yes | Age, sex, CAD, HT, CKD, smoking |
| Missouris, 2004 | United Kingdom | 110 | 70.8 ± 10 | 60 | P | 6.1 | 21.8 | — | 55.5 | 58.3 vs 54.7 | ABI | — | 0 | Yes | Age, sex, HT |
| Miura, 2014 | Japan | 2930 | 71.5 ± 8.9 | 78.7 | R | 2.7 | 16.4 | 50.5 | 8.3 | 11.2 vs 7.7 | ABI | 0.6 ± 0.2 | 0 | Yes | Age, sex, CAD, HT, CKD, smoking, medications |
| Mueller, 2014 | Austria | 487 | 70 1 | 69.8 | P | 5 | 34.7 | 31 | 23.4 | 32 vs 18.9 | ABI | 0.62 1 | 19 | Yes | Age, sex, HT, smoking, HLP |
| Suzuki, 2013 | Japan | 884 | 71.4 ± 10.2 | 69.2 | R | 1.5 | 70.9 | 51 | 42 | 41.9 vs 42 | CLI | — | 100 | Yes | Age, sex, CAD, HLP, medications |
| Vrsalovic, 2016 | Croatia | 319 | 71 1 | 66.5 | R | 2 1 | 53.9 | 41 | 11.9 | 16.3 vs 6.8 | ABI | 0.58 ± 0.14 | 42 | Yes | Age, sex, CAD, HT, CKD, smoking, HLP, medications |
| Wölfle, 2003 | Germany | 211 | 69 1 | 65.4 | R | 1 | 44.5 | — | 12.8 | 22.3 vs 5.1 | CLI | — | 100 | Yes | Age, sex, CAD, HT, smoking, HLP |
Abbreviations: ABI, ankle‐brachial index; CAD, coronary artery disease; CKD, chronic kidney disease; CLI, critical limb ischemia; DM, diabetes mellitus; HLP, hyperlipidemia; HT, hypertension; NM, not mentioned; P, prospective; PAD, peripheral artery disease; R, retrospective.
Median.
ABI < 0.9.
Figure 2.
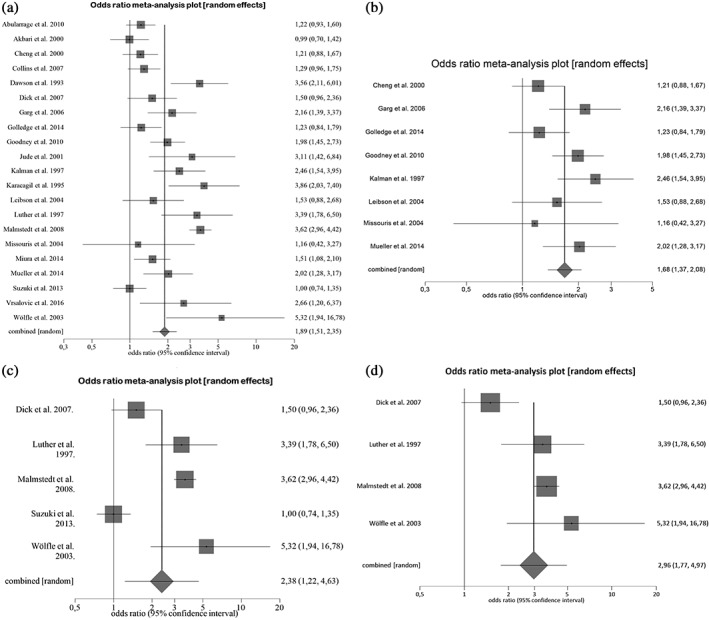
Meta‐analysis (random effects model) testing the association between diabetes and all‐cause mortality in (a) all studies with peripheral artery disease, (b) prospective studies with performed multivariate analysis without critical limb ischemia, (c) all studies with critical limb ischemia, and (d) Caucasian studies with critical limb ischemia.
4. DISCUSSION
The prognostic role of diabetes, although well established in ischemic heart disease and cerebrovascular disease, was not extensively studied in PAD patients.27, 28 According to our meta‐analysis, the coexistence of PAD and diabetes identified patients at high risk of mortality, and patients with diabetes and CLI had even worse prognoses.
PAD is a marker of advanced atherosclerosis, and polyvascular involvement is often present in PAD patients.29, 30 Diabetes accelerates atherosclerosis by mechanisms that include increased platelet activity, increased levels of coagulation factors, and inflammatory biomarkers.31 In this way, patients with diabetes and PAD harbor a proinflammatory and prothrombotic milieu associated with unfavorable cardiovascular outcomes. In addition, due to peripheral neuropathy, PAD is usually asymptomatic at earlier stages, and patients usually lack the typical symptoms of angina when concomitant ischemic heart disease is present.5 Therefore, sudden death may be the first clinical presentation of the associated CAD in PAD patients with diabetes. In our meta‐analysis, the high mortality rates in CLI that exceed other patients with PAD most probably reflect the systemic atherosclerotic burden associated with CLI (ie, frequently associated CAD).1 Our group has recently reported that the coexistence of CLI and diabetes synergistically increased the risk of all‐cause mortality in patients with symptomatic PAD and preserved left ventricular ejection fraction.25
Because PAD is a marker of generalized atherosclerosis, affecting multiple vascular beds and due to the absence of typical angina symptoms in diabetes (silent myocardial ischemia), comprehensive diagnostic evaluation should be considered in this subpopulation that is at a very high risk for fatal outcome. Special attention should be paid to patients with CLI, where an even stronger effect of diabetes on worse clinical outcome was obtained.
The results of our systematic review and meta‐analysis, which included high‐quality studies, for the first time clearly showed the increased risk of all‐cause mortality associated with diabetes in PAD. These findings are in line with previously published meta‐analysis on the prognostic role of diabetes in ischemic heart disease and cerebrovascular disease.27, 28
Further prospective studies are needed to evaluate the prognostic role of diabetes in the whole spectrum of symptomatic and asymptomatic patients with peripheral vascular disease.
4.1. Study limitations
Only diabetic patients who had symptomatic PAD of the lower extremities were included; therefore, asymptomatic diabetics and those with PAD of other arteries than lower limbs were not evaluated in this systematic review. Due to the study designs, cardiovascular death was rarely reported, and all‐cause mortality was the most common outcome. Only 1 study10 reported the relative risk rate for cardiovascular cause of death in diabetic patients. Although the prevalence of CAD was reported in most of the studies, a subset of patients having both CAD and PAD with or without associated diabetes were not separately analyzed. We acknowledge moderate heterogeneity noted in the analysis. The level of heterogeneity could be attributed to the differences in the study population—mean age, diabetes duration, severity of PAD (ie, proportion of patients with CLI), prevalence of CAD, and length of follow‐up period.
5. CONCLUSION
This meta‐analysis showed that diabetes is associated with an increased risk of mortality in PAD, with a more pronounced effect in patients with CLI as the most serious manifestation of the disease.
5.1. Conflict of interests
The authors declare no potential conflict of interests.
Vrsalovic M, Vucur K, Presecki AV, Fabijanic D and Milosevic M. Impact of diabetes on mortality in peripheral artery disease: a meta‐analysis. Clin Cardiol. 2017;40:287–291. 10.1002/clc.22657
REFERENCES
- 1. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. [DOI] [PubMed] [Google Scholar]
- 2. Creager MA, Lüscher TF, Cosentino F, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. [DOI] [PubMed] [Google Scholar]
- 3. Vrsalović M, Vučur K. Diabetes, renal dysfunction, inflammation, and anemia: the deadly quartet in peripheral artery disease. Endocr Oncol Metab. 2016;2:82–87. [Google Scholar]
- 4. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921–929. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association . Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341. [DOI] [PubMed] [Google Scholar]
- 6. Abularrage CJ, Conrad MF, Hackney LA, et al. Long‐term outcomes of diabetic patients undergoing endovascular infrainguinal interventions. J Vasc Surg. 2010;52:314–322. [DOI] [PubMed] [Google Scholar]
- 7. Akbari CM, Pomposelli FB Jr, Gibbons GW, et al. Lower extremity revascularization in diabetes: late observations. Arch Surg. 2000;135:452–456. [DOI] [PubMed] [Google Scholar]
- 8. Cheng SW, Ting AC, Lau H, et al. Survival in patients with chronic lower extremity ischemia: a risk factor analysis. Ann Vasc Surg. 2000;14:158–165. [DOI] [PubMed] [Google Scholar]
- 9. Collins TC, Beyth RJ, Nelson DB, et al. Process of care and outcomes in patients with peripheral arterial disease. J Gen Intern Med. 2007;22:942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dawson I, van Bockel JH, Brand R. Late nonfatal and fatal cardiac events after infrainguinal bypass for femoropopliteal occlusive disease during a thirty‐one‐year period. J Vasc Surg. 1993;18:249–260. [PubMed] [Google Scholar]
- 11. Dick F, Diehm N, Galimanis A, et al. Surgical or endovascular revascularization in patients with critical limb ischemia: influence of diabetes mellitus on clinical outcome. J Vasc Surg. 2007;45:751–761. [DOI] [PubMed] [Google Scholar]
- 12. Garg PK, Tian L, Criqui MH, et al. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golledge J, Quigley F, Velu R, et al. Association of impaired fasting glucose, diabetes and their management with the presentation and outcome of peripheral artery disease: a cohort study. Cardiovasc Diabetol. 2014;13:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodney PP, Nolan BW, Schanzer A, et al. Factors associated with death 1 year after lower extremity bypass in Northern New England. J Vasc Surg. 2010;51:71–78. [DOI] [PubMed] [Google Scholar]
- 15. Jude EB, Oyibo SO, Chalmers N, et al. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24:1433–1437. [DOI] [PubMed] [Google Scholar]
- 16. Kalman PG, Johnston KW. Predictors of long‐term patient survival after in situ vein leg bypass. J Vasc Surg. 1997;25:899–904. [DOI] [PubMed] [Google Scholar]
- 17. Karacagil S, Almgren B, Bowald S, et al. Comparative analysis of patency, limb salvage and survival in diabetic and non‐diabetic patients undergoing infrainguinal bypass surgery. Diabet Med. 1995;12:537–541. [DOI] [PubMed] [Google Scholar]
- 18. Leibson CL, Ransom JE, Olson W, et al. Peripheral arterial disease, diabetes, and mortality. Diabetes Care. 2004;27:2843–2849. [DOI] [PubMed] [Google Scholar]
- 19. Luther M, Lepäntalo M. Femorotibial reconstructions for chronic critical leg ischemia: influence on outcome by diabetes, gender and age. Eur J Vasc Endovasc Surg 1997;13:569–577. [DOI] [PubMed] [Google Scholar]
- 20. Malmstedt J, Leander K, Wahlberg E, et al. Outcome after leg bypass surgery for critical limb ischemia is poor in patients with diabetes: a population‐based cohort study. Diabetes Care. 2008;31:887–892. [DOI] [PubMed] [Google Scholar]
- 21. Mueller T, Hinterreiter F, Luft C, et al. Mortality rates and mortality predictors in patients with symptomatic peripheral artery disease stratified according to age and diabetes. J Vasc Surg. 2014;59:1291–1299. [DOI] [PubMed] [Google Scholar]
- 22. Missouris CG, Kalaitzidis RG, Kerry SM, et al. Predictors of mortality in patients with peripheral vascular disease: a prospective follow‐up study. Br J Diabetes Vasc Dis. 2004;4:196–200. [Google Scholar]
- 23. Miura T, Soga Y, Miyashita Y, et al. Five‐year prognosis after endovascular therapy in claudicant patients with iliofemoral artery disease. J Endovasc Ther. 2014;21:381–388. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki K, Iida O, Yamauchi Y, et al. Impact of diabetes mellitus on critical limb ischemia with below the knee disease: Japan below‐the‐knee artery treatment subanalysis [published online August 21, 2013]. Angiology. doi: 10.1177/0003319713499606. [DOI] [PubMed]
- 25. Vrsalović M, Vučur K. Diabetes and critical limb ischemia: the deadly duo in patients with symptomatic peripheral artery disease. Acta Clin Croat. 2016;55:240–246. [DOI] [PubMed] [Google Scholar]
- 26. Wölfle KD, Bruijnen H, Loeprecht H, et al. Graft patency and clinical outcome of femorodistal arterial reconstruction in diabetic and non‐diabetic patients: results of a multicenter comparative analysis. Eur J Vasc Endovasc Surg. 2003;25:229–234. [DOI] [PubMed] [Google Scholar]
- 27. Lee WL, Cheung AM, Cape D, et al. Impact of diabetes on coronary artery disease in women and men: a meta‐analysis of prospective studies. Diabetes Care. 2000;23:962–968. [DOI] [PubMed] [Google Scholar]
- 28. Shou J, Zhou L, Zhu S, et al. Diabetes is an independent risk factor for stroke recurrence in stroke patients: a meta‐analysis. J Stroke Cerebrovasc Dis. 2015;24:1961–1968. [DOI] [PubMed] [Google Scholar]
- 29. Vrsalović M, Vučur K, Car B, et al. C‐reactive protein, renal function, and cardiovascular outcome in patients with symptomatic peripheral artery disease and preserved left ventricular systolic function. Croat Med J. 2015;56:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vrsalovic M, Vucur K, Jelakovic B. Atrial fibrillation predicts cardiovascular outcome in hypertensive patients with symptomatic peripheral artery disease and preserved ejection fraction. J Clin Hypertens (Greenwich). 2016;18:953–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beckman JA, Paneni F, Cosentino F, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J. 2013;34:2444–2452. [DOI] [PubMed] [Google Scholar]


