Abstract
Background
Heart failure (HF) readmission rates have become an increasingly important quality metric since the advent of the Hospital Readmissions Reduction Program. Despite many well‐intentioned efforts to reduce readmissions, clinicians continue to struggle with the problem of high HF readmission rates.
Hypothesis
HF patients with obstructive sleep apnea (OSA) will have higher burden of rehospitalization and mortality than HF patients without OSA.
Methods
Our study included 344 patient encounters (among 271 unique patients) with a diagnosis of HF from September 2014 through September 2015. Our primary endpoints were all‐cause readmission within 30 and 90 days. Multivariate logistic regression was used to assess the relationship between OSA and readmission when accounting for potential confounders.
Results
The patients’ were 72 ± 10 years old, and predominantly white (76.2%) and male (99.4%). Among the 344 patient encounters, 247 (71.8%) had diagnosed coronary artery disease, 159 (46.2%) had atrial fibrillation, and 99 (28%) had obstructive sleep apnea (OSA). Notably, patients with OSA had an elevated rate of readmission within 30 days (OSA: 30.3% vs no OSA: 19.6%, P = 0.037) and within 90 days (OSA: 57.6% vs no OSA: 36.3P < 0.01). Patients with OSA had increased risk of readmission within 90 days (odds ratio: 2.38, 95% confidence interval: 1.47‐3.83, P < 0.01) even after adjustment for potential confounders of age, race, obesity, diabetes, and chronic obstructive pulmonary disease.
Conclusions
HF patients with OSA have an elevated rate of readmission compared to the general HF population, particularly within the first 90 days after discharge.
Keywords: Heart failure/cardiac transplantation/cardiomyopathy/myocarditis, obstructive sleep apnea, readmissions
1. INTRODUCTION
Over 1 million patients are hospitalized annually for heart failure (HF).1 Rehospitalization following admission for HF has posed a high burden on the healthcare system as it represents the most common nonsurgical cause of readmission.2 Despite nationwide initiatives such as Get With the Guidelines–Heart Failure program, recent readmission data linked to this registry only showed an aggregated decline in 30‐day readmission rate from 20% to 19%.3 Moreover, with increasing evidence that large‐scale telemonitoring for HF patients has not significantly impacted readmission rates, it is imperative to identify and appropriately target patients at high risk of readmission for interventions.4 The Veterans Affairs Pittsburgh Heart Failure Initiative was created to identify risk factors for readmission following hospitalization for HF.
Sleep‐related breathing disorders, particularly obstructive sleep apnea (OSA) and central sleep apnea (CSA) have been shown to have high prevalence in the HF population.5, 6 In a study of 450 consecutive HF patients undergoing polysomnography, 37% were found to have OSA.6 Additionally, OSA is an independent risk factor for development of HF, increasingly so as apnea hypopnea index (AHI) increases.7 Despite the common coexistence between OSA and HF, there are only a few studies quantifying the relationship between comorbid OSA and readmissions in an HF patient population.8, 9 We sought to confirm and extend the prior data by studying whether OSA is associated with increased risk of readmission following hospitalization for HF.
2. METHODS
A total of 344 patient encounters (among 271 unique individual patients) with a primary discharge diagnosis of HF were seen at Veterans Administration Pittsburgh Healthcare System (VAPHS) from September 2014 through September 2015. Discharge diagnoses of HF were verified via International Classification of Diseases, Ninth Revision and Tenth Revision codes. All‐cause readmission served as the primary endpoint. HF readmission served as the secondary endpoint. The incidence of both readmission endpoints is reported at both 30 days and at 90 days. Laboratory data, admission and discharge vital signs, medical comorbidities, and discharge specialty were among the variables collected through chart review of the Veterans Affairs Computerized Patient Record System. HF patients with preserved ejection fraction (HFpEF) (left ventricular ejection fraction [LVEF] ≥50%) and reduced ejection fraction (HFrEF) (LVEF <50%) as measured by echocardiography were included. The diagnoses of OSA were confirmed through review of sleep studies or sleep medicine consult notes. When available through chart review, AHI or explicit mention of OSA severity was also noted. Patients with CSA or CSA predominant sleep‐disordered breathing were not included in the OSA cohort.
2.1. Statistical Methods
Patient characteristics were summarized as mean ± standard deviation for continuous variables and number (%) for categorical variables. Characteristics were compared between OSA and non‐OSA patients using t tests for continuous variables and χ2 tests for categorical variables. Kaplan‐Meier curves are presented to show the readmission rate in the first 90 days after discharge for OSA patients vs non‐OSA patients. The relationship between OSA and risk of readmission were characterized using logistic regression models (outcomes: all‐cause readmission within 30 days, HF readmission within 30 days, all‐cause readmission within 90 days, and HF readmission within 90 days). Multivariable logistic regression analysis was performed to assess the potential for confounding; it was determined that none of the measured variables significantly attenuated the relationship between OSA and readmission, suggesting that OSA is an independent predictor of readmission in HF patients. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
3. RESULTS
There were 344 HF patient discharges from September 2014 to September 2015. Patients were 72 ± 10 years old, predominantly white (76.2%), and male (99.4%). All patient characteristics are summarized in Table 1. Coronary artery disease was present in 71.8% of patients, whereas 46.2% had atrial fibrillation. The mean LVEF was 40.0% ± 16.5%, and 39.2% of patients had HFpEF (LVEF ≥50%).
Table 1.
Descriptive characteristics of study population
| Total Study Population | No OSA | OSA | P Value | |
|---|---|---|---|---|
| No. of patient encounters | 344 | 245 | 99 | |
| Age, y | 72.0 ± 10.2 | 73.2 ± 10.8 | 68.9 ± 7.71 | <0.001 |
| Race | 0.131 | |||
| Black | 82 (23.8%) | 53 (21.6%) | 29 (29.3%) | |
| White | 262 (76.2%) | 192 (78.4%) | 70 (70.7%) | |
| Gender | 0.367 | |||
| Male | 342 (99.4%) | 243 (99.2%) | 99 (100%) | |
| Female | 2 (0.6%) | 2 (0.8%) | 0 (0.0%) | |
| Prior hospitalizations in last 90 days | 0.006 | |||
| 0 | 181 (52.6%) | 139 (56.7%) | 42 (42.4%) | |
| 1 | 97 (28.2%) | 69 (28.2%) | 28 (28.3%) | |
| 2+ | 66 (19.2%) | 37 (15.1%) | 29 (29.3%) | |
| LOS | 5.69 ± 5.14 | 5.49 ± 4.80 | 6.21 ± 5.88 | 0.235 |
| Discharge specialty | 0.066 | |||
| Cardiology | 140 (40.7%) | 98 (40.0%) | 42 (42.4%) | |
| Medicine | 185 (53.8%) | 129 (52.7%) | 56 (56.6%) | |
| ICU | 19 (5.5%) | 18 (7.3%) | 1 (1.0%) | |
| Cardiology consult | 51 (14.8%) | 39 (15.9%) | 12 (12.1%) | 0.218 |
| HFpEF | 135 (39.2%) | 85 (34.7%) | 50 (50.5%) | 0.007 |
| NICM | 72 (20.9%) | 47 (19.2%) | 25 (25.3%) | 0.224 |
| CAD | 247 (71.8%) | 184 (75.1%) | 63 (63.6%) | 0.032 |
| Atrial fibrillation | 159 (46.2%) | 104 (42.4%) | 55 (55.6%) | 0.027 |
| Diabetes | 189 (54.9%) | 136 (55.5%) | 53 (53.5%) | 0.738 |
| ILD | 14 (4.1%) | 10 (4.1%) | 4 (4.0%) | 0.986 |
| COPD | 131 (38.1%) | 81 (33.1%) | 50 (50.5%) | 0.002 |
| Home oxygen | 77 (22.4%) | 47 (19.2%) | 30 (30.3%) | 0.025 |
| Obesity | 151 (43.9%) | 86 (35.1%) | 65 (65.7%) | <0.001 |
| Depression | 48 (14.0%) | 35 (14.3%) | 13 (13.1%) | 0.779 |
| Current tobacco use | 80 (23.3%) | 53 (21.6%) | 27 (27.3%) | 0.262 |
| ACC/AHA stage | 0.907 | |||
| C | 323 (93.9%) | 230 (93.9%) | 93 (93.9%) | |
| D | 20 (5.8%) | 14 (5.7%) | 6 (6.1%) | |
| Discharge HR | 76.3 ± 14.2 | 76.4 ± 15.1 | 76.2 ± 12.0 | 0.911 |
| Renal dysfunction | 139 (40.4%) | 93 (38.0%) | 46 (46.5%) | 0.145 |
| LVEF | 40.0 ± 16.5 | 37.9 ± 16.4 | 45.1 ± 15.9 | <0.001 |
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; ICU, intensive care unit; ILD, interstitial lung disease; LOS, length of stay; LVEF, left ventricular ejection fraction; NICM, nonischemic cardiomyopathy; OSA, obstructive sleep apnea.
Ninety‐nine of 344 patient encounters (62 individual patients) included a diagnosis of OSA (41 documented in pulmonary or sleep lab consult notes, 51 with documented diagnostic sleep studies, and 7 with positive airway pressure titration studies). An AHI was found in chart review for 70 patient encounters, with a mean of 38 ± 29; 15 had mild OSA (AHI 5–14), 28 had moderate OSA (AHI 15–29), and 27 had severe OSA (AHI >30). Among the 29 OSA patient encounters without documented AHI, the diagnosis of OSA was solely confirmed through explicit documentation in sleep disorder consult notes. Patients with OSA were younger (68.9 ± 7.7 years vs 73.2 ± 10.8 years, P < 0.001), more likely to have been hospitalized in the prior 90 days, had longer length of stay (6.2 ± 5.9 days vs 5.5 ± 4.8 days, P = 0.236), more likely to have preserved ejection fraction (50.5% vs 34.7%, P = 0.007), higher mean LVEF (45.1 ± 15.9 vs 37.9 ± 16.4, P < 0.001), less likely to have coronary artery disease (63.6% vs 75.1%, P = 0.032), more likely to have atrial fibrillation (55.6% vs 42.4%, P = 0.027), more likely to have chronic obstructive pulmonary disease (50.5% vs 33.1%, P = 0.002), and more likely to have obesity (65.7% vs 35.1%, P < 0.0001) as defined by discharge body mass index >30.
All‐cause readmission was 22.7% within 30 days and 42.4% within 90 days; HF‐specific readmission was 9.3% at 30 days and 17.4% within 90 days. Notably, patients with OSA had a higher rate of all‐cause readmission within 30 days (OSA: 30.3% vs no OSA: 19.6%, P = 0.037) and within 90 days (OSA: 57.6% vs no OSA: 36.3%, P < 0.001, Figure 1). Furthermore, all‐cause mortality was similar for OSA patients and non‐OSA patients (Figure 2), indicating that differences in readmission were not affected by survival bias.
Figure 1.
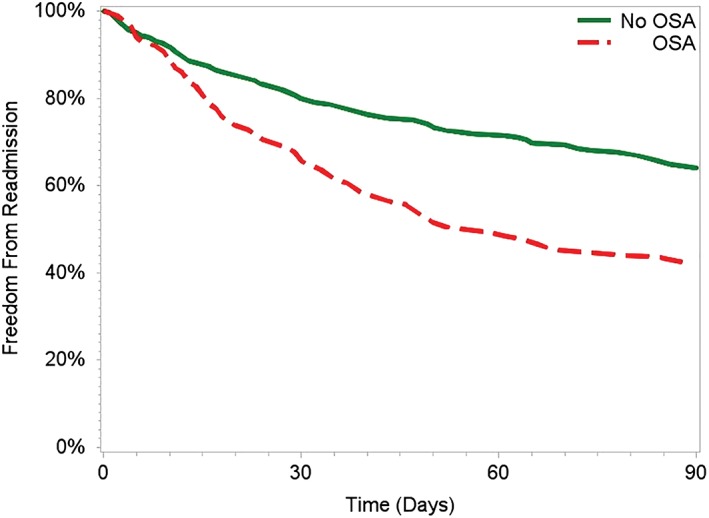
Freedom from all‐cause readmission by OSA status. Abbreviations: OSA, obstructive sleep apnea.
Figure 2.
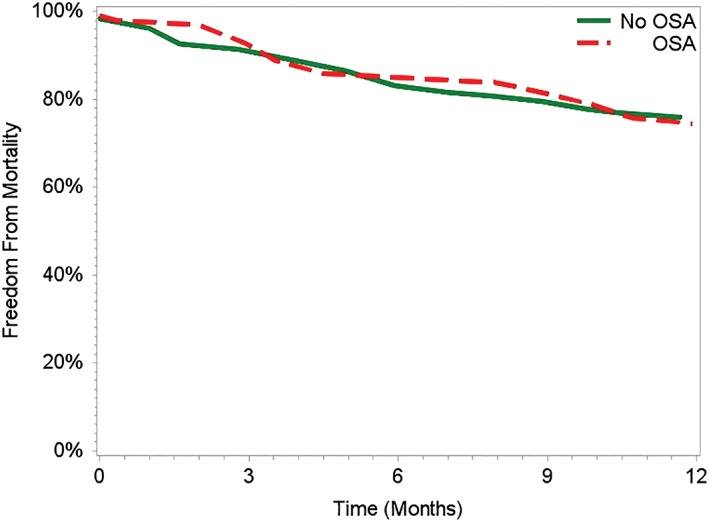
Freedom from mortality by OSA status. Abbreviations: OSA, obstructive sleep apnea.
When performing univariable and multivariable analysis, OSA was the strongest predictor of readmission (Tables 2 and 3). Moreover, when adjusting for potential confounders in multivariable analyses, there was no single factor that significantly attenuated the effect of OSA on readmission risk, suggesting that OSA is independently associated with risk of all‐cause readmission within 30 days and within 90 days (see Supporting Tables 1 and 2 in the online version of this article). When analyzing the endpoint of HF readmission, OSA patients again had increased risk when adjusting for potential confounders, though this relationship was less pronounced for 30‐day readmission than it was for 90‐day readmission (see Supporting Tables 2 and 3 in the online version of this article).
Table 2.
Multivariable analysis of risk factors for readmission within 30 days
| Univariablea | Multivariableb | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |
| All‐cause readmission | ||||||
| OSA | 1.78 | 1.04, 3.04 | 0.03 | 1.83 | 0.99, 3.35 | 0.05 |
| Age | 1.00 | 0.97, 1.02 | 0.76 | 1.00 | 0.96, 1.03 | 0.94 |
| Race (black) | 1.04 | 0.57, 1.87 | 0.90 | 1.05 | 0.53, 2.04 | 0.88 |
| Genderc | NA | NA | NA | NA | NA | NA |
| CAD | 1.18 | 0.66, 2.10 | 0.56 | 1.34 | 0.65, 2.73 | 0.41 |
| AFIB | 1.00 | 0.60, 1.65 | 0.98 | 1.00 | 0.57, 1.75 | 0.99 |
| DM2 | 1.01 | 0.60, 1.68 | 0.97 | 1.16 | 0.64, 2.06 | 0.62 |
| ILD | 0.56 | 0.12, 2.54 | 0.45 | 0.40 | 0.07, 2.23 | 0.29 |
| COPD | 1.65 | 0.90, 2.75 | 0.05 | 1.50 | 0.81, 2.77 | 0.19 |
| Home O2 | 1.64 | 0.92, 2.91 | 0.08 | 1.56 | 0.79, 3.05 | 0.19 |
| Obesity | 0.98 | 0.59, 1.64 | 0.95 | 0.96 | 0.51, 1.77 | 0.89 |
| Depression | 1.02 | 0.49, 2.10 | 0.96 | 1.02 | 0.47, 2.17 | 0.95 |
| Renal dysfunction | 1.27 | 0.76, 2.11 | 0.36 | 1.29 | 0.73, 2.26 | 0.37 |
| LVEF | 0.99 | 0.97, 1.01 | 0.31 | 0.99 | 0.96, 1.01 | 0.21 |
| CHF readmission | ||||||
| OSA | 1.33 | 0.61, 2.88 | 0.46 | 1.52 | 0.60, 3.77 | 0.37 |
| Age | 1.00 | 0.96, 1.03 | 0.93 | 1.00 | 0.95, 1.05 | 0.87 |
| Race (black) | 1.78 | 0.81, 3.86 | 0.14 | 2.38 | 0.94, 5.99 | 0.07 |
| Genderc | NA | NA | NA | NA | NA | NA |
| CAD | 1.45 | 0.60, 3.47 | 0.40 | 1.66 | 0.52, 5.25 | 0.38 |
| AFIB | 1.56 | 0.74, 3.25 | 0.23 | 1.57 | 0.60, 3.55 | 0.27 |
| DM2 | 0.53 | 0.25, 1.11 | 0.09 | 0.75 | 0.32, 1.75 | 0.50 |
| ILDd | NA | NA | NA | NA | NA | NA |
| COPD | 1.12 | 0.53, 2.36 | 0.75 | 0.96 | 0.37, 2.48 | 0.93 |
| Home O2 | 1.66 | 0.75, 3.68 | 0.21 | 2.05 | 0.76, 5.50 | 0.15 |
| Obesity | 0.55 | 0.25, 1.20 | 0.13 | 0.88 | 0.34, 2.20 | 0.77 |
| Depression | 0.39 | 0.08, 1.67 | 0.20 | 0.46 | 0.10, 2.08 | 0.31 |
| Renal dysfunction | 0.87 | 0.41, 1.85 | 0.72 | 0.82 | 0.35, 1.91 | 0.65 |
| LVEF | 0.97 | 0.94, 0.99 | 0.01 | 0.98 | 0.94, 1.01 | 0.11 |
Abbreviations: AFIB, atrial fibrillation; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM2, diabetes mellitus, type 2; ILD, interstitial lung disease; LVEF, left ventricular ejection fraction; NA, not applicable; OSA, obstructive sleep apnea.
Represents 15 separate logistic regression models (1 for each parameter) with outcome of all‐cause readmission (top) and CHF readmission (bottom).
Represents 1 multivariable logistic regression model including all 15 listed parameters with outcome of all‐cause readmission (top) and CHF readmission (bottom).
Odds ratio for gender not estimable because there were no 30‐day readmissions in female patients.
Odds ratio for ILD not estimable because there were no 30‐day CHF readmissions in ILD patients.
Table 3.
Multivariable analysis of risk factors for readmission within 90 days
| Univariablea | Multivariableb | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |
| All‐cause readmission | ||||||
| OSA | 2.38 | 1.47, 3.83 | <0.01 | 2.67 | 1.54, 4.60 | <0.01 |
| Age | 0.99 | 0.97, 1.01 | 0.43 | 0.99 | 0.90, 1.02 | 0.51 |
| Race (black) | 1.15 | 0.70, 1.90 | 0.57 | 1.04 | 0.58, 1.86 | 0.88 |
| Genderc | NA | NA | NA | NA | NA | NA |
| CAD | 1.01 | 0.62, 1.62 | 0.96 | 1.39 | 0.76, 2.53 | 0.28 |
| AFIB | 1.13 | 0.73, 1.73 | 0.58 | 1.09 | 0.67, 1.75 | 0.73 |
| DM2 | 1.09 | 0.70, 1.68 | 0.69 | 1.27 | 0.77, 2.08 | 0.34 |
| ILD | 0.53 | 0.10, 1.72 | 0.29 | 0.34 | 0.08, 1.40 | 0.13 |
| COPD | 1.25 | 0.80, 1.94 | 0.32 | 1.02 | 0.59, 1.75 | 0.93 |
| Home O2 | 1.53 | 0.92, 2.55 | 0.09 | 1.64 | 0.80, 3.05 | 0.11 |
| Obesity | 0.86 | 0.55, 1.33 | 0.49 | 0.64 | 0.37, 1.10 | 0.10 |
| Depression | 0.87 | 0.46, 1.62 | 0.66 | 0.86 | 0.44, 1.68 | 0.66 |
| Renal dysfunction | 1.28 | 0.82, 1.98 | 0.26 | 1.23 | 0.75, 2.00 | 0.39 |
| LVEF | 1.00 | 0.98, 1.01 | 0.52 | 0.99 | 0.97, 1.01 | 0.21 |
| CHF readmission | ||||||
| OSA | 2.41 | 1.35, 4.28 | <0.01 | 3.21 | 1.58, 6.51 | <0.01 |
| Age | 0.99 | 0.96, 1.02 | 0.41 | 1.01 | 0.97, 1.04 | 0.77 |
| Race (black) | 2.16 | 1.18, 3.93 | 0.01 | 2.57 | 1.23, 5.36 | 0.01 |
| Genderc | NA | NA | NA | NA | NA | NA |
| CAD | 1.10 | 0.58, 2.06 | 0.77 | 1.83 | 0.81, 4.11 | 0.14 |
| AFIB | 1.53 | 0.80, 2.68 | 0.13 | 1.48 | 0.78, 2.78 | 0.22 |
| DM2 | 0.62 | 0.35, 1.08 | 0.09 | 0.74 | 0.38, 1.43 | 0.36 |
| ILD | 0.78 | 0.17, 3.59 | 0.75 | 0.57 | 0.10, 3.18 | 0.52 |
| COPD | 0.85 | 0.47, 1.52 | 0.58 | 0.57 | 0.26, 1.20 | 0.13 |
| Home O2 | 1.48 | 0.78, 2.77 | 0.22 | 1.80 | 0.80, 4.05 | 0.15 |
| Obesity | 0.70 | 0.39, 1.24 | 0.21 | 0.67 | 0.30, 1.39 | 0.27 |
| Depression | 0.78 | 0.33, 1.84 | 0.57 | 0.93 | 0.37, 2.31 | 0.88 |
| Renal dysfunction | 0.76 | 0.42, 1.35 | 0.34 | 0.55 | 0.28, 1.08 | 0.08 |
| LVEF | 0.98 | 0.96, 1.00 | 0.07 | 0.98 | 0.95, 1.00 | 0.11 |
Abbreviations: AFIB, atrial fibrillation; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM2, diabetes mellitus, type 2; ILD, interstitial lung disease; LVEF, left ventricular ejection fraction; NA, not applicable; OSA, obstructive sleep apnea.
Represents 15 separate logistic regression models (1 for each parameter) with outcome of all‐cause readmission (top) and CHF readmission (bottom).
Represents 1 multivariable logistic regression model including all 15 listed parameters with outcome of all‐cause readmission (top) and CHF readmission (bottom).
Odds ratio for gender not estimable because there were no 30‐day CHF readmissions in female patients.
4. DISCUSSION
In our study, 22.8% of patients admitted with a primary diagnosis of HF had comorbid OSA. This is similar to prior prevalence studies, confirming that OSA is a frequently occurring comorbidity among hospitalized HF patients.5, 6 Furthermore, HF patients with OSA have an increased rate of readmission following hospitalization for HF, both within 30 and 90 days of discharge, even after accounting for a host of potential confounders. These findings build on other related studies that showed increased risk of cardiac readmissions in patients with sleep‐disordered breathing.8, 9 Our study expands upon these prior studies by finding that OSA is associated with increased all‐cause and HF readmission in patients with both HFrEF and HFpEF.
Both short‐term and long‐term improvements in cardiac function have been well documented in HF patients treated for comorbid OSA. The treatment of OSA among systolic HF patients has been shown to improve LVEF, daytime systolic blood pressure, and heart rate.10, 11 Moreover, improvements in diastolic parameters on transthoracic echocardiography (TTE) (E/A ratio, isovolumetric relaxation time, and mitral deceleration time) have been noted after a mere 12 weeks of nasal continuous positive airway pressure (CPAP).12 Improvements in left ventricular mass and cardiac remodeling have also been demonstrated on cardiac magnetic resonance imaging and TTE in a cohort of OSA patients treated with CPAP over a 1‐year follow‐up period. Specifically, treatment with CPAP resulted in reductions in left ventricular mass index and reductions in left atrial volume index.13
With the therapeutic benefits of positive airway pressure well established, adherence with CPAP is critical to improved outcomes. Admittedly, a weakness of our study is the limited amount of CPAP adherence data available for the patients we identified as having OSA. It is well known that nonadherence to CPAP is prevalent (30%–60%).14 Based upon chart review, frequent mention is made of nonadherence in our patient population as well. We can assume that nonadherence in our population falls into or near the range documented in these prior studies. Two prior small studies have shown that adherence with CPAP on hospital discharge is associated with decreased readmissions and emergency department visits in HF patients with sleep‐disordered breathing.8, 15 The large majority of patients included in these studies had OSA. Combining these findings with our data, OSA not only appears to be a risk factor for readmission, but adherence with CPAP represents a potential therapeutic intervention to reduce readmissions.
The increased risk of readmission for HF is likely accounted for by the adverse hemodynamic and neurohormonal effects of OSA as outlined above.16 However, it is worth noting that the majority of readmissions in our cohort were not for HF, a measure not assessed in prior studies. Comorbidities likely have a significant impact on the high all‐cause readmission rates among patients hospitalized with HF. However, even after adjusting for potential confounding comorbidities, OSA is an independent risk factor for all‐cause readmission following hospitalization for HF. Comprehensive medical care and multidisciplinary co‐management is essential to the delivery of high‐quality care of the HF patient. In patients with comorbid OSA, further patient education of sleep apnea, follow‐up with sleep medicine providers, and system‐wide efforts to reduce barriers to CPAP adherence are potential interventions to reduce readmissions.
Although OSA is associated with increased readmission in HF patients, the mortality impact of comorbid OSA remains inconclusive. In both this study and in a study published by Roebuck et al, OSA was not associated with increased mortality in HF patients.17 However, both of these studies were unable to make distinctions in regard to treatment and adherence with CPAP. In contrast, other studies with adherence data have demonstrated that untreated OSA is associated with increased death in patients with systolic HF.18, 19
Admittedly, our study has several key limitations, including its retrospective nature, which limited our ability to collect comprehensive data on CPAP adherence and OSA diagnosis. Nevertheless, the findings are plausible and build on prior work showing a relationship between OSA status and hospital readmission in the HF population. As OSA is well known to be underdiagnosed, it is plausible that some patients in the no‐OSA group had undiagnosed OSA. Given the known data from prior studies discussed above, it is more likely that this misclassification would have biased the results toward the null. However, despite this possibility we still see a significant effect of OSA on readmission rates. These data, although not definitive, further implicate OSA as a treatable target in reducing the rate of hospital readmissions. Prospective studies with systematic assessment of OSA and treatment adherence will be needed to further explore this relationship.
5. CONCLUSION
High readmission rates among the HF population continue to remain a challenge for providers. Ongoing identification of risk factors, high‐risk patients, and therapeutic targets are vital in efforts to deliver high value, cost‐conscious care. We propose that OSA patients are a high‐risk cohort meriting close outpatient follow‐up, surveillance, and ongoing multidisciplinary management.
Supporting information
Table S1. Relative Risk of Readmission Within 30 Days associated with OSA
Table S2. Relative Risk of Readmission Within 90 Days associated with OSA
ACKNOWLEDGMENTS
The authors thank the Quality and Patient Safety Department at VAPHS, as well as the Cardiology Department at VAPHS.
Conflicts of interest
The authors declare no potential conflicts of interest.
Sommerfeld A, Althouse AD, Prince J, Atwood CW, Mulukutla SR, Hickey GW. Obstructive sleep apnea is associated with increased readmission in heart failure patients. Clin Cardiol. 2017;40:873–878. 10.1002/clc.22738
REFERENCES
- 1. Desai AK, Stevenson LW. Rehospitalization for heat failure: predict of prevent? Circulation. 2012;126:501–506. [DOI] [PubMed] [Google Scholar]
- 2. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 3. Bergethon KE, Ju C, DeVore AD, et al. Trends in 30‐Day readmission rates for patients hospitalized with heart failure: findings from Get with the Guidelines‐Heart Failure Registry. Circ Heart Fail. 2016;9:e002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition–Heart Failure (BEAT‐HF) randomized clinical trial. JAMA Intern Med. 2016;176:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Javaheri S1, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure: types and their prevalences, consequences, and presentations. Circulation . 1998;97:2154–2159. [DOI] [PubMed] [Google Scholar]
- 6. Sin DD, Fitzgerald F, Parker JD, Newton, G , Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. [DOI] [PubMed] [Google Scholar]
- 7. Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure. the Sleep Heart Health Study. Circulation . 2010;122:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kauta SR, Keenan BT, Goldberg L, Schwab RJ. Diagnosis and treatment of sleep disordered breathing in hospitalized cardiac patients: a reduction in 30‐day hospital readmission rates. J Clin Sleep Med. 2014;10:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail. 2012;18:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–1241. [DOI] [PubMed] [Google Scholar]
- 11. Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–366. [DOI] [PubMed] [Google Scholar]
- 12. Arias MA, Garcia‐Rio F, Alonso‐Fernandez A, Mediano O, Martinez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112:375–383. [DOI] [PubMed] [Google Scholar]
- 13. Colish J, Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141:674–681. [DOI] [PubMed] [Google Scholar]
- 14. Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnea: implications for future interventions. Indian J Med Res. 2010;131:245–258. [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma S, Mather P, Gupta A, et al. Effect of early intervention with positive airway pressure therapy for sleep disordered breathing on six‐month readmission rates in hospitalized patients with heart failure. Am J Cardiol. 2016;117:940–945. [DOI] [PubMed] [Google Scholar]
- 16. Pearse S, Cowie M. Sleep‐disordered breathing in heart failure. Eur J Heart Fail. 2016;18:353–361. [DOI] [PubMed] [Google Scholar]
- 17. Roebuck T, Solin P, Kaye DM, et al. Increased long‐term mortality in heart failure due to sleep apnea is not yet proven. Eur Respir J. 2004;23:735–740. [DOI] [PubMed] [Google Scholar]
- 18. Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. Am J Cardiol. 2006;49:1625–1631. [DOI] [PubMed] [Google Scholar]
- 19. Kasai T, Narui K, Dohi T, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. 2008;133:690–696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Relative Risk of Readmission Within 30 Days associated with OSA
Table S2. Relative Risk of Readmission Within 90 Days associated with OSA


