Abstract
Background
Epicardial adipose tissue is associated with coronary artery disease (CAD). Circulating endothelial progenitor cell (EPC) level represents a marker of endothelial dysfunction and vascular health. However, the relationship between epicardial fat and circulating EPC remains unknown. This study aimed to investigate association between echocardiographic epicardial fat thickness (EFT) and circulating EPC level.
Hypothesis
Epicardial fat causes inflammation and contributes to progression of CAD.
Methods
We enrolled 213 consecutive patients with stable angina, and EFT was determined by echocardiography. Quantification of EPC markers (defined as CD34 +, CD34 + KDR +, CD34 + KDR + CD133 + cells) in peripheral blood samples was used to measure circulating EPCs. All patients were divided into 3 tertiles according to EFT levels: group 1, low tertile of EFT; group 2, middle tertile of EFT; and group 3, high tertile of EFT.
Results
Among the 3 groups, CAD disease severity determined by SXscore was negatively correlated with EFT, but the difference did not reach statistical significance (P = 0.066). Additionally, patients in the high and middle tertiles of EFT had higher circulating EPC levels than did those in the low tertile of EFT (P = 0.001 and P < 0.001, respectively). In multivariate analysis, EPC level was significantly associated with echocardiographic EFT (standardized β = −0.233, P = 0.001), independent of multiple covariates.
Conclusions
Epicardial adipose tissue is associated with circulating EPC levels. There was a trend between epicardial fat and severity of CAD, though analysis did not reach statistical significance, and this may be attributed to the interaction between several risk factors of CAD.
Keywords: Epicardial Fat, Endothelial Progenitor Cell, Atherosclerosis
1. INTRODUCTION
Visceral fat is adipose tissue surrounding the internal organs and has been shown to correlate to an unfavorable cardiovascular risk profile. The epicardial adipose tissue is composed of visceral fat, which is deposited between the myocardium and visceral pericardium. With the progression of imaging modalities, echocardiography, multidetector computed tomography (MDCT), and cardiac magnetic resonance imaging (MRI) are all capable of measuring the epicardial adipose tissue.1, 2, 3 Echocardiographic epicardial fat thickness (EFT) measurement is a simpler and less expensive method than MDCT or MRI and can provide more information on echocardiographic parameters. Owing to the endocrine and paracrine properties of secreting pro‐inflammatory and anti‐inflammatory cytokines and chemokines, accumulating evidence has indicated that epicardial adipose tissue is related to coronary atherosclerosis.4, 5, 6 Clinical studies have shown that epicardial adipose tissue is associated with obesity,7 dyslipidemia,7 coronary atherosclerosis,8, 9, 10 coronary artery disease (CAD),2, 11, 12, 13 enhanced coronary events,14, 15 and subclinical target organ damage, such as carotid intima‐media thickness,16 arterial stiffness,17, 18 and endothelial dysfunction.19, 20, 21 These findings imply that epicardial adipose tissue is a quantifiable risk marker in CAD and also plays an important role in coronary artery plaque development. However, the mechanism underlying the link between epicardial fat tissue and coronary atherosclerosis remains unclear.
Convincing evidence has indicated that endothelial dysfunction and injured endothelial lining can be restored by bone marrow–derived endothelial progenitor cells (EPCs). From the bone marrow, the circulating EPCs are mobilized into peripheral blood, home to sites of damaged endothelium, and differentiate into endothelial cells.22 Circulating EPCs were shown to play a pivotal role in vascular homeostasis and help to maintain endothelial integrity.23 Previous reports have demonstrated that a reduced number of EPCs is associated with enhanced risk for cardiovascular events in patients with CAD.24, 25 However, the association between extent of epicardial adipose tissue and level of circulating EPCs has been poorly evaluated in previous research. This study was designed to investigate whether echocardiographic EFT is associated with circulating EPCs, independent of other risk factors.
2. METHODS
2.1. Study population
We initially screened a total of 225 consecutive patients who were admitted to Taipei Veterans General Hospital between January 2013 and February 2014 to undergo elective coronary angiography because of suspected CAD. Subjects were excluded from the study on the basis of the following criteria: (1) cancer, (2) hematologic disease, (3) concomitant infection, and (4) trauma or surgical procedures within the last 90 days. On the basis of these screening criteria, 7 patients were excluded; another 5 eligible patients did not give informed consent. For the total of 213 patients who were enrolled, the following information was obtained during personal interviews and from medical files: medical history, including information about conventional cardiovascular risk factors (smoking, hypertension, diabetes mellitus, hyperlipidemia, CAD, and chronic kidney disease), previous cardiovascular events (myocardial infarction, heart failure, arrhythmia, and cerebrovascular disease), and current medications.
This study was approved by the Taipei Veterans General Hospital research ethics committee. All patients gave written informed consent, and research was conducted according to the principles expressed in the Declaration of Helsinki.
2.2. SYNTAX score calculation
The SYNTAX score (SXscore) is an angiographic grading tool to determine the complexity of CAD. SXscore is the sum of the points assigned to each individual lesion identified in the coronary tree with >50% diameter narrowing in vessels >1.5 mm in diameter. A detailed description of the score calculation was reported elsewhere.26 The SXscore of patients with CAD was computed by 2 of 5 experienced cardiologists who were blinded to clinical information and laboratory data. To decrease interobserver variation, the scores calculated by individual angiographers were reviewed by a senior angiographer. The opinion of the third observer was obtained in case of divergence, and the final conclusion was made by consensus.
2.3. Laboratory investigations
Blood samples were drawn in the morning after overnight fasting. Plasma biochemical measurements, including assessments of fasting blood glucose, uric acid, creatinine, total cholesterol, low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol, and triglyceride (TG) levels, were performed by standard laboratory procedures.
2.4. Measurement of EFT
Images were obtained by 2‐dimensional transthoracic echocardiography (iE33; Philips Medical Systems, Best, Netherlands) and reviewed by a single echocardiologist who was blinded to the clinical data. According to the method provided by the study group of Iacobellis,1 the maximum EFT was measured at end‐systole on the free wall of the right ventricle, at the point perpendicular to the aortic annulus in parasternal long‐axis view, and at the point perpendicular to the interventricular septum at the level of midchordal and tip of the papillary muscle in parasternal short‐axis view. The relatively echo‐free space between the visceral layer of the pericardium and the outer wall of the myocardium was defined as EFT, as shown in Figure 1. The average value of 3 cardiac cycles from each echocardiographic view was determined as the EFT. The intraobserver and interobserver correlation coefficients were 0.97 and 0.93, indicating good reproducibility and reliability. Thirty‐three patients enrolled in our study have received cardiac CT, and we also found good correlation between CT epicardial fat volume and echocardiographic EFT (r = 0.433, P = 0.012; see Supporting Information, Figure 1, in the online version of this article). The CT epicardial fat was defined as pixels within a window of −195 to −45 HU within the region of interest. (Fat volume was measured by the Image Laboratory of Professor Shu‐Mei Guo, Department of Computer Science and Information Engineering, National Cheng Kung University, Tainan, Taiwan.)
Figure 1.
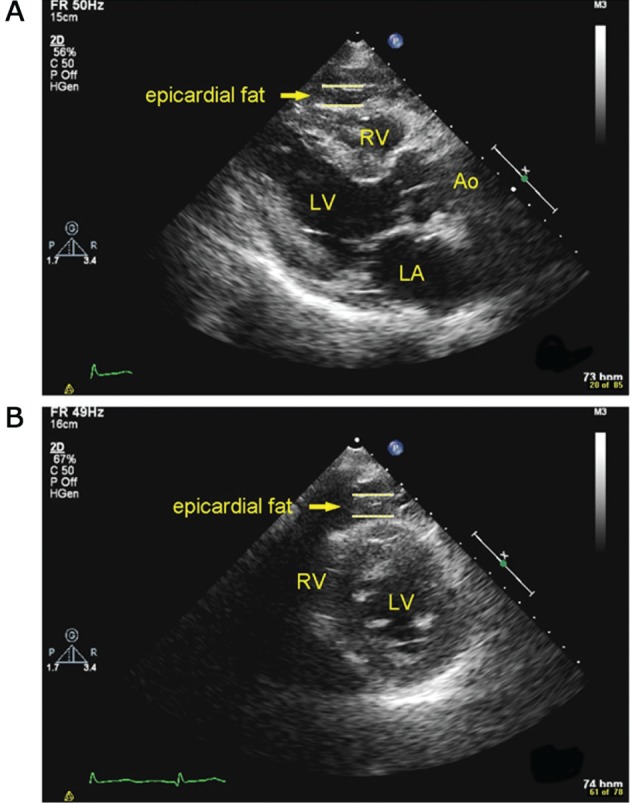
Epicardial fat thickness in echocardiography. EFT was defined as the relatively echo‐free space between the outer wall of the myocardium and the visceral layer of the pericardium. Abbreviations: Ao, aorta; LA, left atrium; LV, left ventricle; RV, right ventricle.
2.5. Measurement of circulating EPCs
Quantification of the circulating EPCs by flow cytometry was performed according to previous study.27 Peripheral blood of patients was incubated with human kinase insert domain receptor (KDR) antibodies (R&D Systems, Minneapolis, MN) for 30 minutes in the dark, and then by allophycocyanin‐conjugated secondary antibody, with the phycoerythrin‐conjugated human CD133 antibodies (Miltenyi Biotec, Bergisch Gladbach, Germany) and fluorescein isothiocyanate (FITC)‐conjugated human CD34 antibodies (Becton Dickinson Pharmingen, San Jose, CA).27 After incubation for 30 minutes, the cells were washed with phosphate‐buffered saline before analysis. Each analysis included 100 000 events. We measured the numbers of circulating EPCs gated with monocytes and defined as CD34+KDR+, CD34+KDR+CD133+. CD34 is known to be expressed on endothelial cells and it also is a marker used to isolate human hematopoietic stem and progenitor cells for transplantation of stem cells. KDR, a receptor for vascular endothelial growth factor, is expressed on cardiac and endothelial cells. CD133 is a cell‐surface glycoprotein that localizes on numerous hematopoietic and various cancer stem cells. Reproducibility of EPC measurements has been confirmed in our previous published studies,28, 29, 30 in which we measured the circulating EPCs from 2 separate blood samples in 10 subjects; we also found a strong correlation between the 2 measurements (r = 0.90, P < 0.001).
2.6. Statistical analysis
Data were expressed in terms of mean ± SD for numeric variables and as numbers and percentages for categorical variables. Subjects were categorized into tertiles according to echocardiographic EFT, as follows: EFT ≤0.274 cm, EFT 0.274 to 0.422 cm, and EFT >0.422 cm. Comparisons of the characteristics among the 3 echocardiographic EFT tertile groups were determined using 1‐way ANOVA or the χ2 test. Association between EFT and EPC levels, SXscore, age, BMI, biochemical parameters, and medications were identified using Pearson correlation analysis. Multivariate linear regression analysis was performed to determine the effect of EPC levels on echocardiographic EFT. Data were analyzed using SPSS version 20.0 (IBM Corp., Armonk, NY). A P value <0.05 was considered to be statistically significant.
3. RESULTS
The mean age of 213 individuals was 68.8 ± 13.2 years, and 141 of the individuals were male (66%). All patients were divided into 3 tertiles according to EFT levels: group 1, low tertile of EFT, n = 71; group 2, middle tertile of EFT, n = 71; and group 3, high tertile of EFT, n = 71. The baseline characteristics of the study participants are provided in Table 1, stratified by the tertiles of echocardiographic EFT. There were no significant differences in baseline characteristics among the 3 groups, except that patients in the high‐EFT tertile had increased total cholesterol, TG, and LDL‐C levels than did the middle‐ and low‐EFT subjects. There were more patients taking medications, including calcium channel blockers, among middle‐ and high‐EFT subjects than in low‐EFT subjects.
Table 1.
Baseline characteristics of enrolled patients in the echocardiographic EFT tertiles
| Low EFT, n = 71 | Middle EFT, n = 71 | High EFT, n = 71 | P Value | |
|---|---|---|---|---|
| Age, y | 68.1 ± 13.7 | 69.2 ± 12.0 | 69.2 ± 14.0 | NS |
| BMI, kg/m2 | 25.2 ± 3.8 | 25.8 ± 4.5 | 26.2 ± 4.4 | NS |
| Male sex | 49 (69) | 51 (72) | 41 (58) | NS |
| Current smoker | 31 (44) | 26 (37) | 24 (34) | NS |
| HTN | 48 (68) | 53 (75) | 53 (75) | NS |
| DM | 23 (32) | 29 (41) | 27 (38) | NS |
| Previous MI | 5 (7) | 4 (6) | 7 (10) | NS |
| Previous CVA | 3 (4) | 5 (7) | 4 (6) | NS |
| HF | 12 (17) | 17 (24) | 14 (20) | NS |
| AF | 15 (21) | 10 (14) | 12 (17) | NS |
| CKD | 9 (13) | 13 (18) | 13 (18) | NS |
| PAOD | 9 (13) | 9 (13) | 14 (20) | NS |
| Echocardiographic EFT, cm | 0.2 ± 0.04 | 0.34 ± 0.04 | 0.54 ± 0.1 | <0.001 |
| LVEF, % | 54 ± 13 | 54 ± 11 | 52 ± 11 | NS |
| Mitral valve E/A ratio | 1.05 ± 0.58 | 0.88 ± 0.34 | 0.87 ± 0.53 | NS |
| Mitral annulus medial E′, cm/s | 6.03 ± 2.4 | 6.18 ± 2.1 | 5.8 ± 2.3 | NS |
| Mitral annulus medial E/E′ | 16.3 ± 7.3 | 15.1 ± 7.6 | 15.6 ± 9.2 | NS |
| Hgb | 12.6 ± 1.8 | 12.7 ± 1.8 | 12.7 ± 2.1 | NS |
| TC, mg/dL | 156.6 ± 35.7 | 157.1 ± 37.5 | 176.3 ± 36.8 | 0.002 |
| HDL‐C, mg/dL | 49.0 ± 29.0 | 43.9 ± 21.0 | 46.3 ± 31.0 | NS |
| LDL‐C, mg/dL | 83.5 ± 31.0 | 93.4 ± 30.4 | 105.2 ± 35.8 | 0.001 |
| Cr, mg/dL | 2.0 ± 2.9 | 1.5 ± 1.4 | 1.6 ± 1.8 | NS |
| eGFR, mL/min | 63.1 ± 29.1 | 65.1 ± 27.8 | 62.3 ± 26.6 | NS |
| Uric acid, mg/dL | 6.8 ± 2.4 | 6.1 ± 2.0 | 6.5 ± 2.2 | NS |
| Medications | ||||
| ASA/Plavix | 42 (59) | 45 (63) | 49 (69) | NS |
| ACEI/ARB | 18 (25) | 23 (32) | 32 (45) | 0.055 |
| β‐Blocker | 24 (34) | 19 (27) | 24 (34) | NS |
| CCB | 10 (14) | 25 (35) | 17 (24) | 0.014 |
| Diuretics | 9 (13) | 20 (28) | 17 (24) | 0.068 |
| Statins | 15 (21) | 23 (32) | 23 (32) | NS |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; ASA, acetylsalicylic acid (aspirin); BMI, body mass index; CAD, coronary artery disease; CCB, calcium channel blocker; CKD, chronic kidney disease; Cr, creatinine; CVA, cerebrovascular accident; DM, diabetes mellitus; EFT, epicardial fat thickness; eGFR, estimated glomerular filtration rate; EPC, endothelial progenitor cell; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; Hgb, hemoglobin; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NS, not significant; PAOD, peripheral arterial occlusive disease; SD, standard deviation; TC, total cholesterol.
Data are presented as n (%) or mean ± SD.
Among the 3 groups, CAD disease severity assessed by SXscore was negatively correlated with the EFT, but the difference did not reach statistical significance (P = 0.066). There was also an inverse association between the circulating EPC levels determined by CD34+KDR+, CD34+KDR+CD133+, and EFT (Table 2, Figure 2). Levels of CD34+ tended to be higher in patients with low EFT than in those in the middle‐ and high‐EFT groups, but the difference did not show statistical significance (P = 0.069).
Table 2.
Angiographic severity of CAD and EPC level among echocardiographic EFT tertiles
| Low EFT, n = 71 | Middle EFT, n = 71 | High EFT, n = 71 | P Value | |
|---|---|---|---|---|
| SXscore | 9.98 ± 13.1 | 9.9 ± 11.3 | 14.5 ± 14.6 | 0.066 |
| Insignificant CAD | 43.3 | 38.8 | 26.9 | — |
| CAD with SVD | 17.9 | 17.9 | 11.9 | — |
| CAD with DVD | 16.4 | 20.9 | 25.4 | — |
| CAD with TVD ± LM | 22.4 | 22.4 | 35.8 | — |
| CD34+, % | 0.031 ± 0.028 | 0.025 ± 0.023 | 0.022 ± 0.018 | 0.069 |
| CD34+KDR+, % | 0.0089 ± 0.007 | 0.0057 ± 0.004 | 0.005 ± 0.007 | 0.001 |
| CD34+KDR+CD133+, % | 0.0086 ± 0.007 | 0.0052 ± 0.003 | 0.0047 ± 0.006 | <0.001 |
Abbreviations: CAD, coronary artery disease; DVD, double‐vessel disease; EFT, epicardial fat thickness; EPC, endothelial progenitor cell; KDR, kinase insert domain receptor; LM, left main; SD, standard deviation; SVD, single‐vessel disease; SXscore, SYNTAX score; TVD, triple‐vessel disease.
Data are presented as % or mean ± SD.
Figure 2.
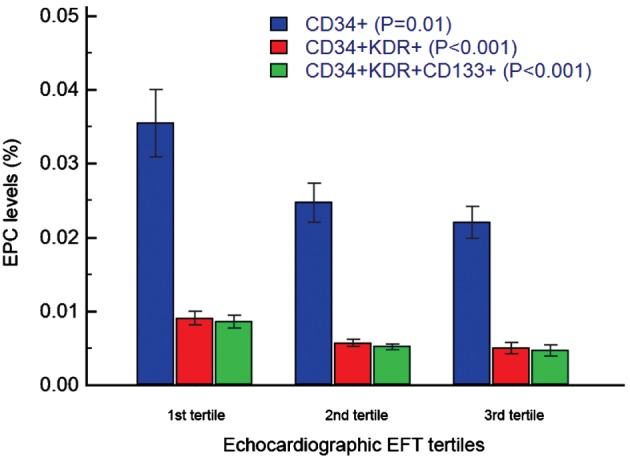
EPC levels (%) among echocardiographic EFT tertiles. There was an inverse association between the circulating EPC levels determined by CD34+KDR+, CD34+KDR+CD133+, and EFT. Abbreviations: EFT, epicardial fat thickness; EPC, endothelial progenitor cell; KDR, kinase insert domain receptor.
To identify the independent predictors of EFT, univariate and multivariate logistic regression analyses were performed. Univariate analysis of BMI, circulating EPC levels, SXscore, serum LDL‐C, serum TG, and angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker medications are significantly associated with echocardiographic EFT (Table 3 and Supporting Information, Figure 2, in the online version of this article). To address concerns over the potential for confounding variables to affect the prognostic performance of the circulating EPCs, we constructed a multivariate linear regression analysis and adjusted several covariates, such as BMI, circulating EPCs, serum LDL‐C, serum TG, and angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker. Circulating EPC level (CD34+KDR+CD133+) is an independent predictor of echocardiographic EFT (standardized β = −0.233, P = 0.001) after adjustment of multiple covariates (Table 3). Both circulating EPC level (CD34+KDR+CD133+) and echocardiographic EFT are independent predictors of SXscore after adjustment of multiple covariates (see Supporting Information, Table, in the online version of this article).
Table 3.
Univariate and multivariate associations with echocardiographic EFT
| Univariate Analysis | Multivariate Analysis1 | |||
|---|---|---|---|---|
| Coefficient | P Value | Coefficient | P Value | |
| Age | 0.049 | NS | ||
| BMI | 0.183 | 0.008 | 0.195 | 0.004 |
| CD34+KDR+CD133+, % | −0.279 | <0.001 | −0.233 | 0.001 |
| SXscore | 0.154 | 0.03 | 0.147 | 0.03 |
| TG | 0.174 | 0.012 | 0.104 | 0.131 |
| LDL‐C | 0.222 | 0.002 | 0.183 | 0.007 |
| HDL‐C | −0.035 | NS | ||
| Cr | −0.074 | NS | ||
| eGFR | −0.023 | NS | ||
| Uric acid | −0.03 | NS | ||
| ACEI/ARB | 0.182 | 0.008 | 0.151 | 0.025 |
| Statins | 0.390 | NS | ||
| CCB | 0.079 | NS | ||
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CCB, calcium channel blocker; Cr, creatinine; EFT, epicardial fat thickness; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; KDR, kinase insert domain receptor; LDL‐C, low‐density lipoprotein cholesterol; NS, not significant; SXscore, SYNTAX score; TG, triglycerides.
The multivariate regression model included all available variables with P < 0.1 in the univariate analysis.
4. DISCUSSION
To the best of our knowledge, this is the first study to show that echocardiographic EFT is associated with circulating EPC levels in patients with stable angina, independent of multiple covariates. These findings suggest that epicardial adipose tissue may contribute to the pathogenesis of coronary atherosclerosis and increase risk of cardiovascular events by modulation of EPC‐related endothelial repair capacity.
Accumulating evidence has suggested perivascular adipose tissue may act as a proatherogenic trigger by promoting vascular inflammation and impairing endothelial function. Because of its anatomical contact with the myocardium and the fact that the myocardium and epicardial adipose tissue share the same blood circulation, researchers have been paying much attention to the potential role of epicardial adipose tissue on coronary atherosclerosis. Moreover, epicardial adipose tissue is metabolically active and responsible for the secretion of several cytokines and adipokines such as tumor necrosis factor‐α, interleukin‐6, and leptin.31, 32 Accumulation of excessive epicardial adipose tissue within the pericardial sac may play a pivotal role in coronary artery atherosclerosis development through potential paracrine or endocrine effects. With the progression of imaging modalities, echocardiography, MDCT, and cardiac MRI are capable of measuring epicardial adipose tissue accurately.1, 2, 3 EFT measurement determined by echocardiography is a simpler and less expensive method than MDCT or cardiac MRI and can be reproducibly used to assess the EFT.
Several observational studies have reported that epicardial adipose tissue correlated to risk factors of CAD and severity of coronary artery atherosclerosis.13 Eroglu et al showed that EFT was significantly increased in patients with CAD compared with those with normal coronary arteries.13 Moreover, with respect to subclinical atherosclerosis, Iacobellis et al presented that subepicardial adipose tissue is associated with carotid intima‐media thickness.16 Recent evidence also showed an independent relationship between EFT and arterial stiffness18 and endothelial function.19 These findings provide evidence that regional fat deposits on myocardium have a variety of functions, other than simply a storage depot for fat, and are associated with coronary atherosclerosis. Therefore, the quantification of epicardial adipose tissue could be a helpful practical tool for clinicians managing patients at high risk with cardiovascular disease. However, the pathophysiologic mechanisms underlying the impact from epicardial fat tissue to coronary atherosclerosis and enhancement of cardiovascular risk remain unclear.
Endothelial dysfunction and injury are thought to be early stages in atherogenesis. In a traditional view, endothelium integrity is believed to be maintained by neighboring endothelial cells migrating and proliferating to restore the damaged endothelial cells. However, a series of clinical and basic studies indicates that the injured endothelial monolayer can be regenerated by circulating EPCs. The amount of circulating EPCs also has been reported to inversely correlate with the presence of risk factors of CAD. The severity of coronary atherosclerosis assessed by the SXscore inversely correlates with circulating EPCs.33, 34 Reduced EPC levels also have been linked to occurrence of ischemic cardiovascular events in patients with CAD.24, 25 In the current study, we first showed that increased EFT in patients is associated with lower circulating EPC levels, which implied reduced endothelial repair capacity in patients with increased epicardial fat tissue. This is in agreement with a previous study showing that patients with increased EFT had endothelial dysfunction and advanced atherosclerosis,19 which suggests that local epicardial fat deposits on myocardium exert local and systemic effects on coronary atherosclerosis. The association between epicardial fat tissue and circulating EPC level may contribute to coronary atherogenesis and higher incidence of cardiovascular events.
The mechanism associated with epicardial fat tissue and EPC remains to be determined. Epicardial adipose tissue is an abundant source of cytokines and adipokines. The adipokines derived from epicardial adipose tissue may act locally and contribute to the deterioration of coronary vessel inflammation and promote the progression of atherosclerosis via outside‐to‐inside signaling.4 Aydin et al demonstrated that increased EFT is associated with high‐sensitivity C‐reactive protein levels in patients with metabolic syndrome, suggesting a link between low‐grade systemic inflammation and increased epicardial fat tissue volume.19 Numerous studies have demonstrated that excessive inflammation may impair EPC level and functions.35, 36 Taken together, persistent and excessive inflammatory stimulation from epicardial adipose tissue may attenuate endothelial repair capacity provided by EPCs and contribute to deteriorated endothelial function and further progression of coronary atherosclerosis.
4.1. Study limitations
Some limitations of this study should be mentioned. First, the sample size is rather small, and patients were assembled from a single center. This result may be difficult to apply to a more general population. Therefore, further larger confirmative studies are needed to verify the current result. Second, our study had a cross‐sectional design, so we could not establish a cause‐and‐effect relationship between EFT and EPCs. Third, we did not measure the EPC functions in the current study. However, a decreased EPC number has been demonstrated to be correlated with pathogenesis of atherosclerosis37 and also independently associated with major adverse cardiovascular events.38, 39 Thus we suggested that decreased EPC counts in our study also represented the advanced atherosclerosis that was associated with EFT. Fourth, although having been mentioned in previous literature,40 we did not measure representative inflammatory markers in our study. Therefore, we could not clarify the association between echocardiographic EFT, inflammatory biomarkers, and the risk of cardiovascular disease. Fifth, association between epicardial fat and severity of CAD did not reach statistical significance (P = 0.066), and this may be attributed to the interaction between several risk factors of CAD in terms of interaction between visceral fat, lipids, and EPC levels and also the small number of cases (n = 213) in this study. Further prospective studies should be arranged to clarify the cause‐and‐effect relationship and test whether quantification of EPC levels could provide additional information over the current risk factors to predict future cardiovascular events in CAD patients with different echocardiographic EFT.
5. CONCLUSION
In patients with stable angina, echocardiographic EFT is associated with circulating EPC levels, irrespective of confounding factors. There is an association between epicardial fat and severity of CAD, although analysis does not reach statistical significance, and this may be attributed to the interaction between several risk factors of CAD. These findings may pave the way for further basic and prospective clinical research studies to confirm the mechanism of association between epicardial adipose tissue and coronary atherogenesis.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Figure S1. Correlation between echocardioraphic EFT and CT epicardial fat volume. There was significant association between echocardiographic EFT and CT epicardial fat volume (r = 0.433, p = 0.012)
Figure S2. Correlation between echocardiographic EFT and EPC levels, BMI and lipid profiles. Univariate analysis indicated that BMI, circulating EPCs levels, serum LDL‐C, serum triglycerides are significantly associated with echocardiographic EFT. BMI: body mass index; LDL‐C: low‐density lipoprotein cholesterol; TG: triglycerides.
Table S1. Univariate and multivariate associations with SXscore.
Chang T‐Y, Hsu C‐Y, Chiu C‐C, Chou R‐H, Huang H‐L, Huang C‐C, Leu H‐B, Huang P‐H, Chen J‐W and Lin S‐J. Association between echocardiographic epicardial fat thickness and circulating endothelial progenitor cell level in patients with stable angina pectoris. Clinical Cardiology. 2017;40:697–703. 10.1002/clc.22717
Funding information UST‐UCSD International Center of Excellence in Advanced Bioengineering; Taiwan Ministry of Science and Technology (https://www.most.gov.tw/ch/public); I‐RiCE, Grant/Award number: MOST 103‐2911‐I‐009‐101; Taipei Veterans General Hospital (http://www.vghtpe.gov.tw/), Grant/Award number: V103A‐009; ministry of Health and Welfare (http://www.mohw.gov.tw/CHT/Ministry/Index.aspx), Grant/Award number: MOHW 104‐TDU‐B‐211‐113‐003; Ministry of Education's “Aim for the Top University” Plan (http://www.edu.tw/).
Author contributions: Research idea and study design, T‐YC, C‐YH, P‐HH; data acquisition, T‐YC, C‐YH, C‐CC, R‐HC; data analysis/interpretation, H‐LH, C‐YH, P‐HH; statistical analysis, C‐CH, H‐BL; manuscript writing, T‐YC, P‐HH; supervision or mentorship, P‐HH, J‐WC, S‐JL. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. P‐HH and S‐JL take responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
REFERENCES
- 1. Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–1418. [DOI] [PubMed] [Google Scholar]
- 2. Sarin S, Wenger C, Marwaha A, et al. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am J Cardiol. 2008;102:767–771. [DOI] [PubMed] [Google Scholar]
- 3. Flüchter S, Haghi D, Dinter D, et al. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity (Silver Spring) . 2007;15:870–878. [DOI] [PubMed] [Google Scholar]
- 4. Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 5. Hirata Y, Kurobe H, Akaike M, et al. Enhanced inflammation in epicardial fat in patients with coronary artery disease. Int Heart J. 2011;52:139–142. [DOI] [PubMed] [Google Scholar]
- 6. Hirata Y, Tabata M, Kurobe H, et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol. 2011;58:248–255. [DOI] [PubMed] [Google Scholar]
- 7. Hartiala O, Magnussen CG, Bucci M, et al. Coronary heart disease risk factors, coronary artery calcification and epicardial fat volume in the Young Finns Study. Eur Heart J Cardiovasc Imaging. 2015;16:1256–1263. [DOI] [PubMed] [Google Scholar]
- 8. Mahabadi AA, Reinsch N, Lehmann N, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–199. [DOI] [PubMed] [Google Scholar]
- 9. Bettencourt N, Toschke AM, Leite D, et al. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol. 2012;158:26–32. [DOI] [PubMed] [Google Scholar]
- 10. Alexopoulos N, McLean DS, Janik M, et al. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210:150–154. [DOI] [PubMed] [Google Scholar]
- 11. Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harada K, Amano T, Uetani T, et al. Cardiac 64‐multislice computed tomography reveals increased epicardial fat volume in patients with acute coronary syndrome. Am J Cardiol. 2011;108:1119–1123. [DOI] [PubMed] [Google Scholar]
- 13. Eroglu S, Sade LE, Yildirir A, et al. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardiovasc Dis. 2009;19:211–217. [DOI] [PubMed] [Google Scholar]
- 14. Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–1395. [DOI] [PubMed] [Google Scholar]
- 16. Iacobellis G, Pellicelli AM, Sharma AM, et al. Relation of subepicardial adipose tissue to carotid intima‐media thickness in patients with human immunodeficiency virus. Am J Cardiol. 2007;99:1470–1472. [DOI] [PubMed] [Google Scholar]
- 17. Natale F, Tedesco MA, Mocerino R, et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr. 2009;10:549–555. [DOI] [PubMed] [Google Scholar]
- 18. Kim BJ, Kim BS, Kang JH. Echocardiographic epicardial fat thickness is associated with arterial stiffness. Int J Cardiol. 2013;167:2234–2238. [DOI] [PubMed] [Google Scholar]
- 19. Aydin H, Toprak A, Deyneli O, et al. Epicardial fat tissue thickness correlates with endothelial dysfunction and other cardiovascular risk factors in patients with metabolic syndrome. Metab Syndr Relat Disord. 2010;8:229–234. [DOI] [PubMed] [Google Scholar]
- 20. Payne GA, Borbouse L, Kumar S, et al. Epicardial perivascular adipose‐derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C‐β pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fandiño‐Vaquero R, Fernández‐Trasancos A, Álvarez E, et al. Orosomucoid secretion levels by epicardial adipose tissue as possible indicator of endothelial dysfunction in diabetes mellitus or inflammation in coronary artery disease. Atherosclerosis. 2014;235:281–288. [DOI] [PubMed] [Google Scholar]
- 22. Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. [DOI] [PubMed] [Google Scholar]
- 23. Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt‐Lucke C, Rössig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. [DOI] [PubMed] [Google Scholar]
- 25. Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. [DOI] [PubMed] [Google Scholar]
- 26. Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 27. Wu CC, Huang PH, Lai CL, et al. The impact of endothelial progenitor cells on restenosis after percutaneous angioplasty of hemodialysis vascular access. PLoS One. 2014;9:e101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiang CH, Huang PH, Chung FP, et al. Decreased circulating endothelial progenitor cell levels and function in patients with nonalcoholic fatty liver disease. PLoS One. 2012;7:e31799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chiang CH, Huang PH, Leu HB, et al. Decreased circulating endothelial progenitor cell levels in patients with heart failure with preserved ejection fraction. Cardiology. 2013;126:191–201. [DOI] [PubMed] [Google Scholar]
- 30. Chiang CH, Huang PH, Chiu CC, et al. Reduction of circulating endothelial progenitor cell level is associated with contrast‐induced nephropathy in patients undergoing percutaneous coronary and peripheral interventions. PLoS One. 2014;9:e89942. [Google Scholar]
- 31. Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. [DOI] [PubMed] [Google Scholar]
- 32. Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. [DOI] [PubMed] [Google Scholar]
- 33. Chiang CH, Leu HB, Huang PH, et al. Syntax score is associated with circulating endothelial progenitor cells in patients with coronary artery disease. Acta Cardiol Sin. 2012;28:216–224. [Google Scholar]
- 34. Chi J, Hong X, Wang Y, et al. Inverse correlation between circulating endothelial progenitor cells with CD34 + CD133+ and the severity of coronary atherosclerosis assessed by SYNTAX score. Am J Med Sci. 2014;347:457–462. [DOI] [PubMed] [Google Scholar]
- 35. Verma S, Kuliszewski MA, Li SH, et al. C‐reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C‐reactive protein and cardiovascular disease. Circulation. 2004;109:2058–2067. [DOI] [PubMed] [Google Scholar]
- 36. Suh W, Kim KL, Choi JH, et al. C‐reactive protein impairs angiogenic functions and decreases the secretion of arteriogenic chemo‐cytokines in human endothelial progenitor cells. Biochem Biophys Res Commun. 2004;321:65–71. [DOI] [PubMed] [Google Scholar]
- 37. Hayek SS, MacNamara J, Tahhan AS, et al. Circulating progenitor cells identify peripheral arterial disease in patients with coronary artery disease. Circ Res. 2016;119:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fadini GP, Maruyama S, Ozaki T, et al. Circulating progenitor cell count for cardiovascular risk stratification: a pooled analysis. PLoS One. 2010;5:e11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rigato M, Avogaro A, Fadini GP. Levels of circulating progenitor cells, cardiovascular outcomes and death: a meta‐analysis of prospective observational studies. Circ Res. 2016;118:1930–1939. [DOI] [PubMed] [Google Scholar]
- 40. Cherian S, Lopaschuk GD, Carvalho E. Cellular cross‐talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab. 2012;303:E937–E949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Correlation between echocardioraphic EFT and CT epicardial fat volume. There was significant association between echocardiographic EFT and CT epicardial fat volume (r = 0.433, p = 0.012)
Figure S2. Correlation between echocardiographic EFT and EPC levels, BMI and lipid profiles. Univariate analysis indicated that BMI, circulating EPCs levels, serum LDL‐C, serum triglycerides are significantly associated with echocardiographic EFT. BMI: body mass index; LDL‐C: low‐density lipoprotein cholesterol; TG: triglycerides.
Table S1. Univariate and multivariate associations with SXscore.


