Abstract
The long‐term progression of coronary artery disease as defined by the natural disease course years after a myocardial infarction (MI) is an important but poorly studied area of clinical research. The long‐Term rIsk, clinical manaGement, and healthcare Resource utilization of stable coronary artery dISease in post–myocardial infarction patients (TIGRIS) study was designed to address this knowledge gap by evaluating patient management and clinical outcomes following MI in different regions worldwide. TIGRIS (ClinicalTrials.gov Identifier: NCT01866904) is a multicenter, observational, prospective, longitudinal study enrolling patients with history of MI 1 to 3 years previously and high risk of developing atherothrombotic events in a general‐practice setting. The primary objective of TIGRIS is to evaluate clinical events (time to first occurrence of any event from the composite cardiovascular endpoint of MI, unstable angina with urgent revascularization, stroke, or death from any cause), and healthcare resource utilization associated with hospitalization for these events (hospitalization duration and procedures) during follow‐up. Overall, 9225 patients were enrolled between June 2013 and November 2014 and are being followed in 369 different centers worldwide. This will allow for the description of regional differences in patient characteristics, risk profiles, medical treatment patterns, clinical outcomes, and healthcare resource utilization. Patients will be followed for up to 3 years. Here we report the rationale, design, patient distribution, and selected baseline characteristics of the TIGRIS study. TIGRIS will describe real‐world management, quality of life (self‐reported health), and healthcare resource utilization for patients with stable coronary artery disease ≥1 year post‐MI.
Keywords: Coronary artery disease, Healthcare resource utilization, Observational, Trial design, myocardial infarction < Ischemic heart disease
1. INTRODUCTION
Atherosclerotic plaque ulceration, erosion, or rupture, followed by platelet activation and thrombus formation, is thought to constitute the main cause of cardiovascular (CV) events. Medical management of patients after an acute coronary syndrome (ACS) is based on multiple randomized clinical trials defining the acute (typically first‐year) treatment after the index event. Although potent P2Y12 inhibitors are the most important cornerstone, the exact duration of the treatment is still under discussion.1, 2, 3, 4 This evidence‐based management has reduced mortality significantly in recent years, but patients surviving the first year after an ACS remain at high risk for future CV events.5, 6, 7, 8 This risk is aggravated by distinct comorbidities, including hypertension, diabetes mellitus (DM), and chronic renal dysfunction, as well as a history of recurrent myocardial infarction (MI) or documented history of angiographic evidence of multivessel coronary artery disease (CAD).9, 10, 11 These comorbidities will also impact on medical management. Studies prolonging dual antiplatelet therapy for >12 months show improved outcomes in this regard.12, 13 However, other combinations of antiplatelet drugs combined with low‐dose oral anticoagulants appear to be beneficial for long‐term event reduction.14
With better survival of the first year after an ACS, the prevalence of this high‐risk population is growing worldwide, and rehospitalization remains frequent and unchanged over decades.15, 16 Therefore, it is important to establish an understanding of the demographics, treatments, and outcomes of these patients over time to tailor therapy strategies for the individual patient. The availability of the long‐Term rIsk, clinical manaGement, and healthcare Resource utilization of stable coronary artery dISease in post–myocardial infarction patients (TIGRIS) registry will complement the current armament of generally country‐specific registries of variable design that render extrapolation of observations to other countries and regions difficult.17, 18 Furthermore, the limited information that is available suggests that management patterns and outcomes may vary for different nations. Direct comparisons of regional differences in post‐MI management and outcomes would be facilitated through the standardized aggregation of this information across different countries.
The goal of TIGRIS is to provide a standardized prospective and longitudinal description of patient characteristics, events (per person‐years at 12 and 24 months), healthcare resource utilization, and current treatment patterns as seen in a relatively unselected patient population post‐ACS. In this prospective observational study, patients from 25 different countries with a previous MI 1 to 3 years before enrollment in the registry and at high risk of developing future atherothrombotic events will be followed for up to 3 years (minimum of 2 years).
2. METHODS
2.1. Overall study design
TIGRIS (http://www.clinicaltrials.gov NCT01866904) included a follow‐up period of up to 3 years. All patients underwent routine clinical assessment and received the standard medical care, as determined by the treating physician. Patients did not receive any experimental intervention or treatment because of their participation in the study. The overall study design is shown in Figure 1.
Figure 1.
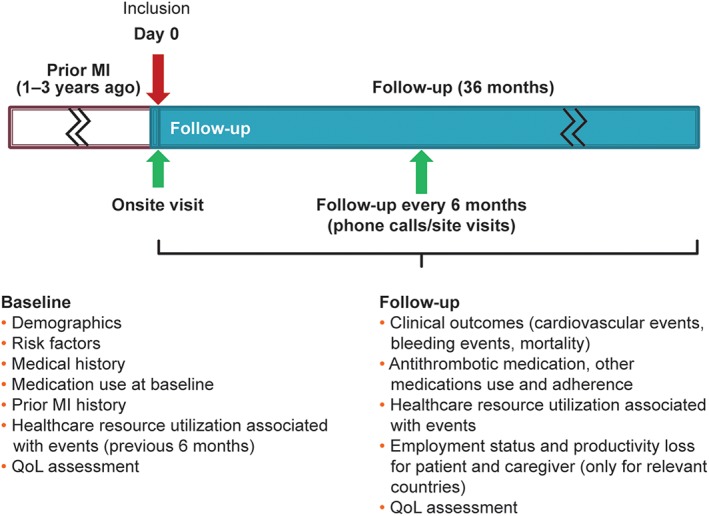
Study design. Abbreviations: MI, myocardial infarction; QoL, quality of life (self‐reported health status)
2.2. Study population
TIGRIS included patients age ≥50 years with a documented history of presumed spontaneous MI with their most recent MI having occurred 1 to 3 years prior to enrollment, and with ≥1 of the following risk factors: age ≥ 65 years, DM treated with medication, documented history of a second MI >1 year prior to enrollment, multivessel CAD, and/or chronic non–end‐stage renal dysfunction (creatinine clearance calculated by Cockcroft‐Gault equation at 15 mL/min to <60 mL/min). The requirement for patients to have experienced a recent MI and have at ≥1 additional risk factor essentially mirrors the patient population from the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis In Myocardial Infarction 54 (PEGASUS‐TIMI 54) trial.12 All patients provided written informed consent for participation. The inclusion and exclusion criteria are described in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age ≥ 65 y | Presence of any condition or circumstance that, in the opinion of the investigator, could significantly limit the complete follow‐up of the patient (eg, tourist, non‐native speaker or does not understand the local language where interpreter services are not reliably available, psychiatric disturbances, alcohol or drug abuse) |
| DM requiring medication | Presence of serious/severe comorbidities that, in the opinion of the investigator, may limit life expectancy to <1 y |
| Documented history of a second prior presumed spontaneous MI (>1 y ago) | Current participation in a blinded RCT |
| Documented history of angiographic evidence of multivessel CAD | Patients receiving treatment with ticagrelor beyond 12 months, or off‐label use of ticagrelor |
| Chronic, non–end‐stage renal dysfunction (CrCl ≥15 mL/min and <60 mL/min, calculated by Cockcroft‐Gault equation) |
Abbreviations: CAD, coronary artery disease; CrCl, creatinine clearance; DM, diabetes mellitus; MI, myocardial infarction; RCT, randomized clinical trial.
2.3. Aim and objectives
The TIGRIS study will evaluate prospectively over a follow‐up period of up to 3 years (minimum of 2 years) the risk for recurrent CV events in this high‐risk stable post‐MI population. The primary objective is to evaluate clinical event rates (time to first occurrence of any event from the composite CV endpoint of MI, unstable angina (UA) with urgent coronary revascularization, stroke, or death from any cause), and healthcare resource utilization associated with hospitalization for CV and bleeding events (duration of hospitalizations, and procedures) over the follow‐up period. Definition of MI is based on the universal definition of Thygesen et al. in 2012.19 Definition of UA is based on the presence of ischemic chest pain and hospitalization within 24 hours of most recent symptoms, without elevation in cardiac biomarkers but with evidence of ischemia. Urgent coronary revascularization for recurrent symptoms of chest pain at rest was defined as percutaneous coronary intervention revascularization within 7 days of symptom onset or surgical revascularization within 14 days of symptoms. Stroke was defined as neurological deficit due to an ischemic or hemorrhagic central nervous system event with residual systems >24 hours after onset or leading to death.
Secondary objectives are to describe the rate of ischemic events (MI, UA with urgent revascularization, ischemic stroke, CV‐related death, and death for unknown reasons) and to evaluate the incidence of bleeding events requiring medical attention. The latter were defined as any overt sign of hemorrhage requiring intervention, leading to hospitalization, or prompting an unscheduled evaluation. Other objectives are to describe associations between patient characteristics with quality of life (self‐reported health status) and with the use of evidence‐based therapies, the association between oral antiplatelet therapy and clinical events (ischemic, death for any cause, and bleeding; including in high‐risk subgroups such as elderly patients, patients with DM, renally impaired patients, or patients with recurrent MI), and to describe risk‐factor control in this high‐risk population.
2.4. Site selection and patient enrollment
In total, 334 sites (333 active with ≥1 patient recruited, and 1 site with no patients) have participated in the TIGRIS study in 25 countries worldwide. A National Principal Investigator (NPI) was identified for each participating country. In an attempt to obtain a representative sample at a country level, recruitment of sites and subjects was initially based on predefined selection of physicians (office‐based primary‐care physicians and cardiologists as well as cardiologists based in hospitals with outpatient clinics) by NPIs; this was intended to provide a distribution of physicians across regions and locations (ie, urban, suburban, and rural areas). The aim was for each participating site to enroll ≥10 patients. (A complete list of participating sites and principal investigators is provided in Supporting Information, Appendix I, in the online version of this article.) All patients will be followed for up to 3 years in accordance with the study design shown in Figure 1.
2.5. Study duration and phases
Overall, 9225 patients were enrolled between June 2013 and November 2014, and the planned duration of the study is from June 2013 (first patient in) until September 2017 (last subject's last visit). Data collection is performed during the initial visit and every 6 months such that every patient will have between 2 and 3 years of follow‐up. These follow‐ups are conducted either by a telephone call or personal visit, depending on the approach considered most practical and effective (Figure 1). This provides information on these high‐risk patients for a period from a minimum of 1 year to a maximum of 6 years since the index MI event.
2.6. Study variables
Variables obtained at baseline/enrollment and at the 6‐monthly follow‐up visits included patient demographics (sex, age, race, place and type of residence, education level, and professional status; see Supporting Information, Appendix II, in the online version of this article). Socio‐demographics such as living‐arrangement status, income, and health‐insurance status were also collected. In addition, the prevalence of CV and bleeding risk factors, history of CAD and other CV disease, as well as details of the medical history related to the index MI with a focus on antithrombotic medications have been assessed.
Also captured during the initial visit were variables from routine physical examination (heart rate, blood pressure [BP], weight, height, waist circumference) and from existing routine laboratory tests (renal function tests, lipid profile, hemoglobin, and glycated hemoglobin), if they were available and if they were performed within 3 months prior to the visit and up to 1 month after the visit (assuming no clinical events within this time window).
Emphasis was paid to healthcare resource utilization related to CV or bleeding conditions during the 6 months preceding enrollment.
Measures of health status as assessed by the EuroQol 5‐Dimensions (EQ‐5D) and EuroQol Visual Analog Scale (EQ‐VAS) were collected at baseline. This measures self‐reported health status in 5 dimensions (EQ‐5D: mobility, self‐care, usual activities, pain or discomfort, anxiety or depression) with 3 levels of severity (none, moderate, severe). For each patient, a single health state value, or utility, was calculated (EQ‐Index and EQ‐VAS scores) and set on a scale ranging from 0 (which corresponds to death) to 1 (EQ‐Index) or from 0 to 100 (EQ‐VAS; which corresponds to a best‐imaginable state of health).
Information was also collected at baseline regarding clinics/outpatient center characteristics.
During the follow‐up period, clinical outcomes (death, CV events, and bleeding events), antithrombotic medications and other evidence‐based medications, and patient‐reported health status assessed by the EQ‐5D are collected. Productivity loss, such as sick leave for the patient and the caregiver that is event‐related, is being captured from a subset of approximately 10% of the study population from several countries where this information is considered particularly relevant (Nordic Region and The Netherlands). A complete list of variables captured in the study at baseline and during follow‐up is provided in the Supporting Information, Appendix II, in the online version of this article.
2.7. Collection of data
Data are collected using electronic case‐report forms (eCRFs). Initial data collection was performed by the investigator. During the follow‐up period, patients are either contacted via telephone every 6 months or attend for a study visit from the site. Follow‐up phone calls are performed by either trained staff from the enrolling hospital or from a designated call center, in accordance with a standard protocol, to assist in recording of treatments, events, and medical visits.
At each follow‐up time point, predefined clinical outcome events in the study as well as the variables related to associated healthcare resource utilization during hospitalization are collected directly from the treating physician/hospital through a standardized event confirmation eCRF and hospital module in the eCRF, respectively, in accordance with the following protocol. If, during interview, the patient or relative reports a predefined CV or bleeding‐related hospitalization, the treating physician or hospital is contacted for confirmation of this event. Further information allowing determination of final diagnosis, primary cause of hospitalization, duration of hospital stay, procedures and interventions, and related healthcare resource utilization during hospital stay is collected from the treating physician or hospital directly through a standardized hospital module in the eCRF. If a death occurred, efforts are made to identify the cause of death (CV‐related or non‐CV) through the death certificate where available, or through relatives, physicians, or hospitals where it is not.
2.8. Statistical methods and sample‐size determination
This is a descriptive observational study without predefined hypothesis testing. A statistical analysis plan finalized prior to database lock (July 2017) specified the planned evaluation. Occurrence of primary and secondary CV and bleeding endpoints will be presented as incidence rate (number of patients with ≥1 event divided by total event‐free person time) and rate of recurrent events (total number of events divided by total person time). The incidence rate of the primary composite and ischemic composite and their components, and bleeding events, will be stratified for the inclusion risk factors that define high‐risk CAD (age ≥ 65 years, treated DM, documented history of a second MI >1 year prior to enrollment, multivessel CAD, and/or chronic renal dysfunction), index MI characteristics, sex, and region. In addition, the association of these covariates with the primary composite is planned to be explored with a multivariable Cox proportional‐hazards regression.
Use of medications over the study period, and specifically the use of antithrombotic medication, will be described in terms of use at specific time points during follow‐up. EQ‐5D data collected during the study will be reported descriptively. Healthcare resource utilization associated with CV and bleeding events reported during the follow‐up period will be assessed.
No formal power calculation has been undertaken for this descriptive study. Sample size was primarily driven by each country's needs based on payer requirements from their markets. On this basis, an initial sample size of 10 170 patients was estimated; due to delays in ethics approval in some countries, 9225 patients were ultimately recruited into the study. Assuming a 2‐ to 3‐year cumulative incidence or risk ranging from 5% to 10% for both ischemic and hemorrhagic events, this sample size would provide a precision, defined by the 95% confidence interval, of ±0.4% and ±0.6%, respectively. This is supported by the initial data collected at 1 year, where the cumulative incidence in 9225 patients was estimated at 3.8%. This would result in a 2‐year cumulative incidence of 7.5% (±0.5), assuming a constant hazard over time, consistent with the original calculation above.
2.9. Ethics approval
The final protocol was approved by the relevant ethics committee from each country and the site ethics committee. The study is being performed in accordance with ethical principles consistent with the Declaration of Helsinki revision, International Conference on Harmonisation of Good Clinical Practice guidelines, and the applicable legislation on noninterventional studies.
2.10. Funding and responsibilities
TIGRIS is sponsored by AstraZeneca and is noninterventional in nature, with no drugs being supplied or funded. The TIGRIS Executive Committee comprises 12 members (7 cardiologists, 1 clinical pharmacologist, 1 health economist, and 1 statistician, and 2 representatives from AstraZeneca). The executive committee with support from AstraZeneca is responsible for the design and conduct of this study, and all study analyses.
2.11. Participating regions
The following is a list of the participating regions. Asia‐Pacific, with 5 countries: Australia, China, India, Japan, and South Korea (n = 2850 patients [31%]). Europe, with 13 countries: Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Portugal, Romania, Spain, Turkey, and the UK (n = 4240 patients [46%]). North America: 2 countries, Canada and the United States (n = 1024 patients [11%]). South America, with 5 countries: Argentina, Brazil, Colombia, Mexico, and Venezuela (n = 1111 patients [12%]; Table 2 and Figure 2).
Table 2.
Patient characteristics
| All | Asia‐Pacific and Australia | Europe | North America | South America | P Valuea | |
|---|---|---|---|---|---|---|
| Patients | 9225 (100) | 2850 (30.9) | 4240 (46.0) | 1024 (11.1) | 1111 (12.0) | |
| Inclusion risk factors | ||||||
| Age ≥ 65 y | 5766 (62.5) | 1652 (58.0) | 2814 (66.4) | 666 (65.0) | 634 (57.1) | <0.001 |
| DM requiring medication | 2805 (30.4) | 999 (35.1) | 1118 (26.4) | 307 (30.0) | 381 (34.3) | <0.001 |
| Second prior MI | 943 (10.2) | 210 (7.4) | 491 (11.6) | 131 (12.8) | 111 (10.0) | <0.001 |
| Multivessel CAD | 6052 (65.6) | 1921 (67.4) | 2714 (64.0) | 715 (69.8) | 702 (63.2) | <0.001 |
| Chronic renal dysfunction (CrCl 15–60 mL/min) | 707 (7.7) | 212 (7.4) | 327 (7.7) | 78 (7.6) | 90 (8.1) | 0.898 |
Abbreviations: CAD, coronary artery disease; CrCl, creatinine clearance; DM, diabetes mellitus; MI, myocardial infarction.
Data are presented as n (%).
P values estimated using χ2 test.
Figure 2.
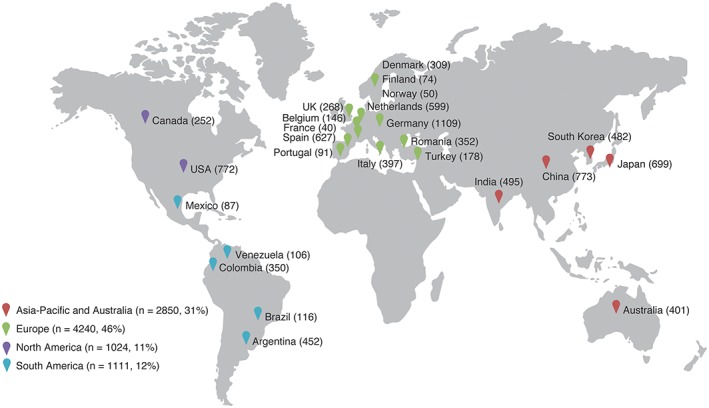
Patient enrollment in long‐Term rIsk, clinical manaGement, and healthcare Resource utilization of stable coronary artery dISease in post–myocardial infarction patients (TIGRIS) by country and region. Abbreviations: UK, United Kingdom; USA, United States of America
2.12. Patient characteristics
Characteristics of patients in TIGRIS are shown in Table 2. Patients in Asia‐Pacific and South America were younger; hence, the risk factor of age > 65 years was less frequently met as an inclusion criterion. There was a higher percentage of patients with treated DM in these 2 regions compared with patients from Europe or North America. On the other hand, the percentage of patients with a second prior MI (before the index MI) was higher in Europe and North America, whereas multivessel CAD was seen more frequently in patients from North America and Asia‐Pacific. The rates of chronic renal dysfunction were similarly distributed worldwide.
3. DISCUSSION
This report describes the rationale and design of the TIGRIS study. TIGRIS will investigate patients with stable CAD who are at high risk of adverse events due to multiple risk factors. Specifically, TIGRIS will address regional differences in patient characteristics, clinical outcomes, medical treatment patterns, and healthcare resource utilization of patients with high risk of future CV events 1 to 3 years post‐MI.
The risk of future CV events in patients with a history of MI is increased by comorbidities such as DM and chronic kidney disease as well as the age and the complexity of CAD. Although many randomized clinical trials have addressed this risk with emphasis on the first 12 months after an MI, little is known about the risk of subsequent ischemic events beyond this time in a “real‐world” population. The improved survival post‐MI and the aging population are creating an emerging patient group for whom there is a problematic knowledge gap. Specifically, there is a growing number of patients with a remote history of MI for whom there is little evidence to guide appropriate treatment.
Recently, Jernberg and colleagues published outcome data from a large Swedish national registry including 108 315 patients after MI with long‐term follow‐up.20 Overall, 10% of the patients died during the hospital stay and within 1 week after hospital discharge, and an additional 12.3% died over the following year. The cumulative rate of the CV composite outcome, including recurrent MI and stroke and CV death, was 18.3% in the first year after a MI. The CV risk remained high, with 9.0% in the next 12 months and 20.0% in the following 36 months. Similarly, Rapsomaniki and colleagues investigated patients age ≥ 65 years from 1 year following MI in the 4‐nation APOLLO study (Study of an Investigational Drug, Patisiran, for the Treatment of Transthyretin‐Mediated Amyloidosis). The adjusted risk of mortality over the subsequent 3 years ranged from 12.8% to 19.5%; the corresponding risks of hospitalized bleeding events ranged from 2.7% to 4.9%.21 These data, derived from datasets from France, Sweden, the United States, and the United Kingdom, were generated for a variety of purposes using different collection and validation methodologies; hence, it is difficult to ascertain whether the variation in event rates between countries reflected real differences or variations in methodology.
In 2 recently reported acute dual antiplatelet therapy randomized clinical trials, mortality risk (Kaplan–Meier) in the 12 months following randomization was generally low; for example, for the PLATO study (Comparison of ticagrelor and clopidogrel in patients with acute coronary syndrome), rates were 4.5% for ticagrelor and 5.9% for clopidogrel1; and in TRITON‐TIMI 38 (Prasugrel versus clopidogrel in patients with acute coronary syndromes–Thrombolysis In Myocardial Infarction), rates were 3% for prasugrel and 3.2% for clopidogrel.22 PEGASUS‐TIMI 54 (Prevention with ticagrelor of secondary thrombotic events in high‐risk patients with prior acute coronary syndrome–Thrombolysis In Myocardial Infarction) investigated the long‐term addition of ticagrelor to aspirin, initiating treatment 1 to 3 years after a MI.23 The 3‐year Kaplan–Meier mortality risk was 5.2% in the aspirin arm, substantially lower than that observed in the APOLLO program. Therefore, it is clear, compared with more selected and generally younger patients within randomized clinical trials, that the unselected population has a substantially higher risk.24 This emphasizes the need for real‐world data collected using a standardized, rigorous, consistent methodology to access outcomes during the years following a MI. In addition to the provision of representative, general practice evidence, a major strength of TIGRIS is the focus on different countries. Notably, 31% of study patients derive from Asia‐Pacific and 12% from South America, areas where the availability of such information is relatively limited.
TIGRIS has already shown that post‐MI patients from Asia‐Pacific and South America are younger and have a higher incidence of DM compared with patients from Europe or North America. Given these baseline differences in populations, TIGRIS will provide important information on comparative outcomes and healthcare resource utilization in the years after a MI. Interpretation of these outcomes will be greatly aided through the recording of medical management patterns in different international settings and their association with clinical outcomes.
3.1. Study limitations
It should perhaps be noted that the selection of site investigators was not random; general physicians or cardiologists were selected because they have access to sufficient resources to facilitate patient enrollment. Although consecutive patient selection was encouraged, TIGRIS employed specific inclusion and exclusion criteria that may have resulted in a cohort not wholly representative of all post‐MI survivors. Additionally, central adjudication was not used in the TIGRIS study, with patient‐reported outcomes confirmed by the event‐treating physician.
4. CONCLUSION
TIGRIS is a study that documents demographics, comorbidities, treatment patterns, and outcome data in unselected stable CAD patients with previous MI in general‐practice settings. Data are being collected internationally in a rigorous, standardized, and consistent fashion, providing a relevant picture of both the burden of recurrent events and variability in management and outcomes in this poorly studied patient population.
Supporting information
AppendixS 1 Supplementary Materials
ACKNOWLEDGMENTS
The authors thank the patients, their families, and all investigators involved in this study. Assistance with project management, site management, data management, and regulatory affairs was provided by Worldwide Clinical Trials Evidence Group, Nottingham, England, United Kingdom. Medical writing support was provided by Carl V. Felton, PhD, of Prime, Knutsford, England, United Kingdom, in accordance with Good Publication Practice guidelines and funded by AstraZeneca. AstraZeneca was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Author contributions
DW: Data analysis and interpretation, drafting and revision of the manuscript. SGG: Study design, data analysis and interpretation, revision of the manuscript. SB: Study design, data analysis and interpretation, drafting and revision of the manuscript. JCN, GR, AM, JYC, CBG, RG, SJP, AMV, SY, TS, DB: Study design, data analysis and interpretation, manuscript revision.
Conflicts of interest
Dirk Westermann reports speaker/consulting honoraria and/or research grant support from AstraZeneca, Bayer, Berlin‐Chemie, Biotronik, and Novartis. Shaun G. Goodman reports speaker/consulting honoraria and/or research grant support from Amgen Inc., Amilyn, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Ferring Pharmaceuticals, GlaxoSmithKline, Matrizyme, Merck, Novartis, Pfizer, Regeneron, Revalesio, Sanofi, Servier, and Tenax Pharmaceuticals. José C. Nicolau reports speaker/consulting honoraria and/or research grant support from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, GlaxoSmithKline, Merck, Novartis, Pfizer, and Sanofi. Gema Requena and Andrew Maguire are employed by Oxon Epidemiology Ltd. (which has received funding from AstraZeneca). Christopher B. Granger reports consulting honoraria and/or research grant support from Armetheon, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Eli Lilly, Gilead, GlaxoSmithKline, Hoffmann‐La Roche, Janssen Pharmaceuticals, Medtronic, Pfizer, Salix Pharmaceuticals, Sanofi, Takeda, and The Medicines Company. Stuart Pocock reports statistical consulting honoraria from AstraZeneca. Stefan Blankenberg reports speaker/consulting honoraria and/or research grant support from Abbott, Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Pfizer, Roche, Siemens, Siemens Diagnostics, and Thermo Fisher Scientific. Tabassome Simon reports speaker/consulting honoraria and/or research grant support from Astellas, Amgen Inc., AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, and Sanofi. Satoshi Yasuda reports speaker/consulting honoraria and/or research grant support from Takeda, Daiichi‐Sankyo, AstraZeneca, Boehringer Ingelheim, and Bristol‐Myers Squibb. Ana Maria Vega is employed by AstraZeneca. David Brieger reports speaker/consulting honoraria and/or research grant support from Amgen Inc., AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Merck, and Sanofi. The authors declare no other potential conflicts of interest.
Westermann D, Goodman SG, Nicolau JC, et al. Rationale and design of the long‐Term rIsk, clinical manaGement, and healthcare Resource utilization of stable coronary artery dISease in post–myocardial infarction patients (TIGRIS) study. Clin Cardiol. 2017;40:1197–1204. 10.1002/clc.22837
Funding information Long‐Term rIsk, clinical manaGement, and healthcare Resource utilization of stable coronary artery dISease in post–myocardial infarction patients (TIGRIS) study is supported by AstraZeneca AB, Södertälje, Sweden. The sponsor was involved in the study conception and design, data collection, interpretation of the data and review of the manuscript. The authors and other executive committee members were involved in the study design, analysis and interpretation of the data, in the writing and review of the manuscript, and in the decision to submit the manuscript for publication.
REFERENCES
- 1. Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 2. Mariani M, Mariani G, De Servi S. Efficacy and safety of prasugrel compared with clopidogrel in patients with acute coronary syndromes: results of TRITON‐TIMI 38 trials. Exp Rev Cardiovasc Ther. 2009;7:17–23. [DOI] [PubMed] [Google Scholar]
- 3. Sibbing D, Aradi D, Jacobshagen C, et al; TROPICAL‐ACS Investigators . Guided de‐escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL‐ACS): a randomised, open‐label, multicentre trial. Lancet. 2017;390:1747–57. [DOI] [PubMed] [Google Scholar]
- 4. Udell JA, Bonaca MP, Collet JP, et al. Long‐term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta‐analysis of randomized trials. Eur Heart J. 2016;37:390–399. [DOI] [PubMed] [Google Scholar]
- 5. Simms AD, Weston CF, West RM, et al. Mortality and missed opportunities along the pathway of care for ST‐elevation myocardial infarction: a national cohort study. Eur Heart J Acute Cardiovasc Care. 2015;4:241–253. [DOI] [PubMed] [Google Scholar]
- 6. Puymirat E, Schiele F, Steg PG, et al; FAST‐MI Investigators. Determinants of improved one‐year survival in non–ST‐segment elevation myocardial infarction patients: insights from the French FAST‐MI program over 15 years. Int J Cardiol. 2014;177:281–286. [DOI] [PubMed] [Google Scholar]
- 7. Puymirat E, Simon T, Steg PG, et al; USIK USIC 2000 Investigators, FAST‐MI Investigators. Association of changes in clinical characteristics and management with improvement in survival among patients with ST‐elevation myocardial infarction. JAMA. 2012;308:998–1006. [DOI] [PubMed] [Google Scholar]
- 8. Fox KA, Carruthers KF, Dunbar DR, et al. Underestimated and under‐recognized: the late consequences of acute coronary syndrome (GRACE UK‐Belgian Study). Eur Heart J. 2010;31:2755–2764. [DOI] [PubMed] [Google Scholar]
- 9. Nauta ST, Deckers JW, van der Boon RM, et al. Risk factors for coronary heart disease and survival after myocardial infarction. Eur J Prev Cardiol. 2014;21:576–583. [DOI] [PubMed] [Google Scholar]
- 10. Min JK, Dunning A, Lin FY, et al; CONFIRM Investigators. Age‐ and sex‐related differences in all‐cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23 854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–860. [DOI] [PubMed] [Google Scholar]
- 11. Zengin E, Bickel C, Schnabel RB, et al; AtheroGene–Study Investigators. Risk factors of coronary artery disease in secondary prevention—results from the AtheroGene–Study. PLoS One. 2015;10:e0131434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS‐TIMI 54 Steering Committee and Investigators. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
- 13. Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med. 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eikelboom JW, Connolly SJ, Bosch J, et al; COMPASS Investigators. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 15. Krumholz HM, Nuti SV, Downing NS, et al. Mortality, hospitalizations, and expenditures for the Medicare population aged 65 years or older, 1999–2013. JAMA. 2015;314:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen HY, Tisminetzky M, Yarzebski J, et al. Decade‐long trends in the frequency of 90‐day rehospitalizations after hospital discharge for acute myocardial infarction. Am J Cardiol. 2016;117:743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan MY, Du X, Eccleston D, et al. Acute coronary syndrome in the Asia‐Pacific region. Int J Cardiol. 2016;202:861–869. [DOI] [PubMed] [Google Scholar]
- 18. Gitt AK, Bueno H, Danchin N, et al. The role of cardiac registries in evidence‐based medicine. Eur Heart J. 2010;31:525–529. [DOI] [PubMed] [Google Scholar]
- 19. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Glob Heart. 2012;7:275–295. [DOI] [PubMed] [Google Scholar]
- 20. Jernberg T, Hasvold P, Henriksson M, et al. Cardiovascular risk in post‐myocardial infarction patients: nationwide real world data demonstrate the importance of a long‐term perspective. Eur Heart J. 2015;36:1163–1170. [DOI] [PubMed] [Google Scholar]
- 21. Rapsomaniki E, Thuresson M, Yang E, et al. Using big data from health records from four countries to evaluate chronic disease outcomes: a study in 114 364 survivors of myocardial infarction. Eur Heart J Qual Care Clin Outcomes. 2016;2:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiviott SD, Braunwald E, McCabe CH, et al; TRITON‐TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 23. Bonaca MP, Braunwald E, Sabatine MS. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;373:1274–1275. [DOI] [PubMed] [Google Scholar]
- 24. Chung SC, Gedeborg R, Nicholas O, et al. Acute myocardial infarction: a comparison of short‐term survival in national outcome registries in Sweden and the UK. Lancet. 2014;383:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS 1 Supplementary Materials


