Capsule Summary
We demonstrate for the first time that EROS (CYBC1/C17ORF62) regulates abundance of the gp91phox-p22phox heterodimer of the phagocyte NADPH oxidase in human cells and that EROS mutations are a novel cause of chronic granulomatous disease.
Keywords: EROS, C17ORF62, CYBC1, Chronic granulomatous disease, Nox2, gp91phox
Short Summary (for the Editor only)
The phagocyte respiratory burst is mediated by the phagocyte NADPH oxidase, a multi-protein subunit complex that facilitates production of reactive oxygen species and which is essential for host defence. Monogenic deficiency of individual subunits leads to chronic granulomatous disease (CGD), which is characterized by an inability to make reactive oxygen species, leading to severe opportunistic infections and auto-inflammation. However, not all cases of CGD are due to mutations in previously identified subunits. We recently showed that Eros, a novel and highly conserved ER-resident transmembrane protein, is essential for the phagocyte respiratory burst in mice because it is required for expression of gp91phox-p22phox heterodimer, which are the membrane bound components of the phagocyte NADPH oxidase. Eros has a human orthologue, CYBC1/EROS. We now show that the function of CYBC1/EROS is conserved in human cells and describe a case of CGD secondary to a homozygous CYBC1/EROS mutation that abolishes EROS protein expression. This work demonstrates the fundamental importance of CYBC1/EROS in human immunity and describes a novel cause of CGD.
To the editor:
The multi-subunit phagocyte NADPH oxidase generates reactive oxygen species and is crucial for host defence (1). Deficiencies in individual subunits (gp91phox, p22phox, p47phox, p67phox and p40phox) cause chronic granulomatous disease (CGD) but some patients with CGD do not have mutations in these genes (2). We recently found that Eros (3), a hitherto undescribed protein, is essential for the generation of reactive oxygen species because it is necessary for protein (but not mRNA) expression of the gp91phox-p22phox heterodimer, which is almost absent in Eros-deficient mice. Eros−/− animals succumb quickly following infection with Salmonella Typhimurium or Listeria Monocytogenes. Eros is highly conserved and has a human orthologue CYBC1 (alias C17ORF62), hereafter referred to as CYBC1 gene and EROS protein). We asked whether the gene fulfilled the same function in humans. We performed CRISPR-mediated deletion of CYBC1/EROS in PLB-985 cells, (Fig. S1A) and identified two clones with 8bp and 1bp deletions respectively (Fig. S1B and C). Neither clone expressed EROS protein (Fig. 1A) or detectable gp91phox (Fig. 1B). p22phox expression was also much lower in both EROS-deficient clones than in control cells (Fig. 1C). We verified the lack of surface gp91phox expression by flow cytometry (Fig. 1D). Both CYBC1/EROS-deficient clones had a severely impaired respiratory burst (Fig. 1E). In addition, CYBC1/EROS-deficient clones differentiated towards a neutrophil phenotype also demonstrated an impaired respiratory burst (data not shown). As expected, re-introduction of CYBC1/EROS using a lentiviral vector restored gp91phox expression to CYBC1/EROS-deficient clones (Figure 1F) and oxidase activity as measured by Nitro blue tetrazolium chloride (NBT) test (Figure 1G) and DIOGENES assay (data not shown).
Figure 1: EROS function is conserved in humans.
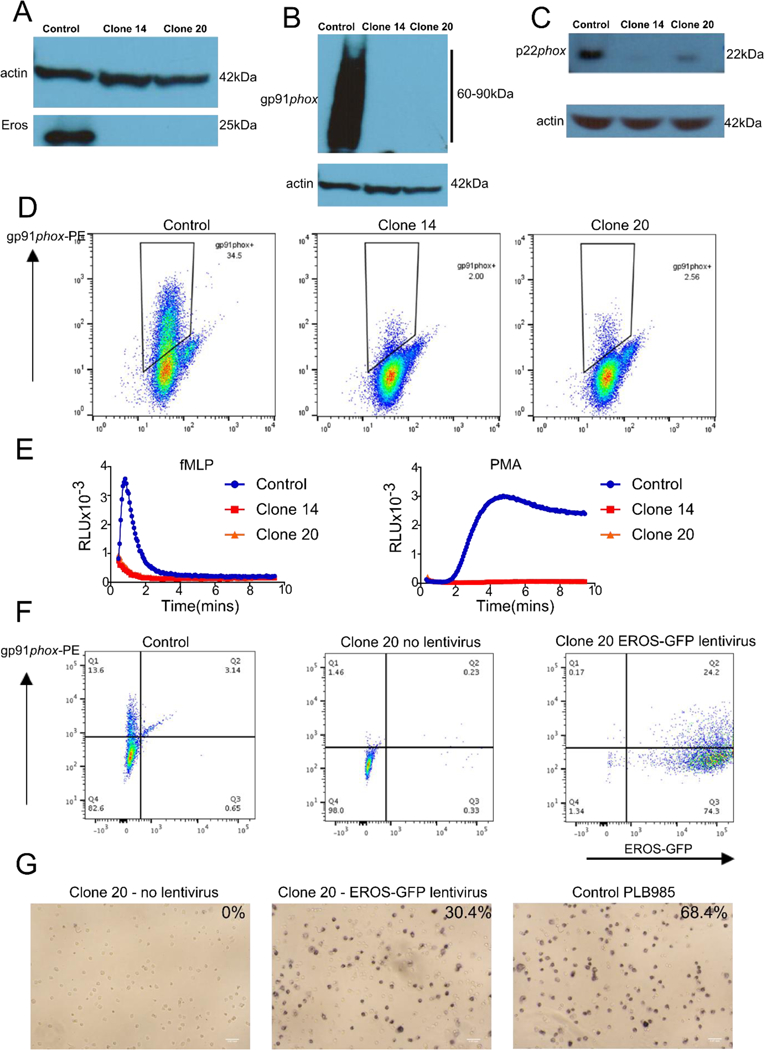
(A-C) Western blot of (A) EROS (B) gp91phox (C) p22phox, (D) surface gp91phox expression and (E) phagocyte respiratory burst in CRISPR targeted PLB-985 clones (F) surface expression of gp91phox in CRISPR targeted cells +/− overexpression of EROS-GFP. (G) NBT reduction following reconstitution with EROS-GFP lentiviral vector. Representative of 3 independent experiments.
We then identified a patient with a homozygous CYBC1/EROS mutation in a resource paper that details a thousand Saudi Arabian families with genetic disease(4). He presented with fever, splenomegaly, lymphadenopathy and short stature, but no immuno-phenotyping was detailed at that time. His full clinical history is as follows. He is a Saudi Arabian boy, born in 2007, the son of parents in a consanguineous marriage. He has three healthy older sisters. At 2 months of age, he developed a localized abscess following BCG vaccination. He was then relatively well until 8 years of age but was noted to be of short stature and experienced recurrent pulmonary infections and tonsillitis/pharyngitis despite tonsillectomy.
In August 2015, he became unwell with a febrile illness and an abnormal dihydrorhodamine (DHR) test was noted (Fig. 2A,B). He has a severely impaired DHR in response to both PMA and zymosan. He was also profoundly lymphopenic. He subsequently developed an acute episode of hemolytic anemia in November 2015 and again in January 2017. During 2015, his fevers, infection, lymphopenia and elevated inflammatory markers met criteria for a diagnosis of haemophagocytic lymphohistiocytosis (HLH). He had no mutations previously implicated in HLH pathogenesis. He was treated with cyclosporine and steroids and was transferred to Boston Children’s Hospital (BCH) in December 2016 for consideration of bone marrow transplantation. The DHR test was repeated and rhodamine fluoresence was again virtually absent. Further assessment demonstrated granulomatous inflammation in his lungs, and discrete granulomata in his bone marrow, with no evidence of infection. Following his open lung biopsy at BCH, he developed hemolytic anemia and required Intensive Care. He recovered with steroids, however on weaning of this therapy he developed recurrent pleural effusions. Due to his autoimmunity, features of lymphopenia with granulomata, and pleural effusions/hemolytic anemia he was started on sirolimus and he remains in this therapy. His steroids have been weaned to 4mg daily. While reasonably well clinically, he developed diminished anti-pneumococcal antibody responses, worsening lymphopenia, and declining immunoglobulin levels. He therefore underwent a myeloablative bone marrow transplant and has recovered well.
Figure 2: Homozygous EROS mutation has deleterious effects.
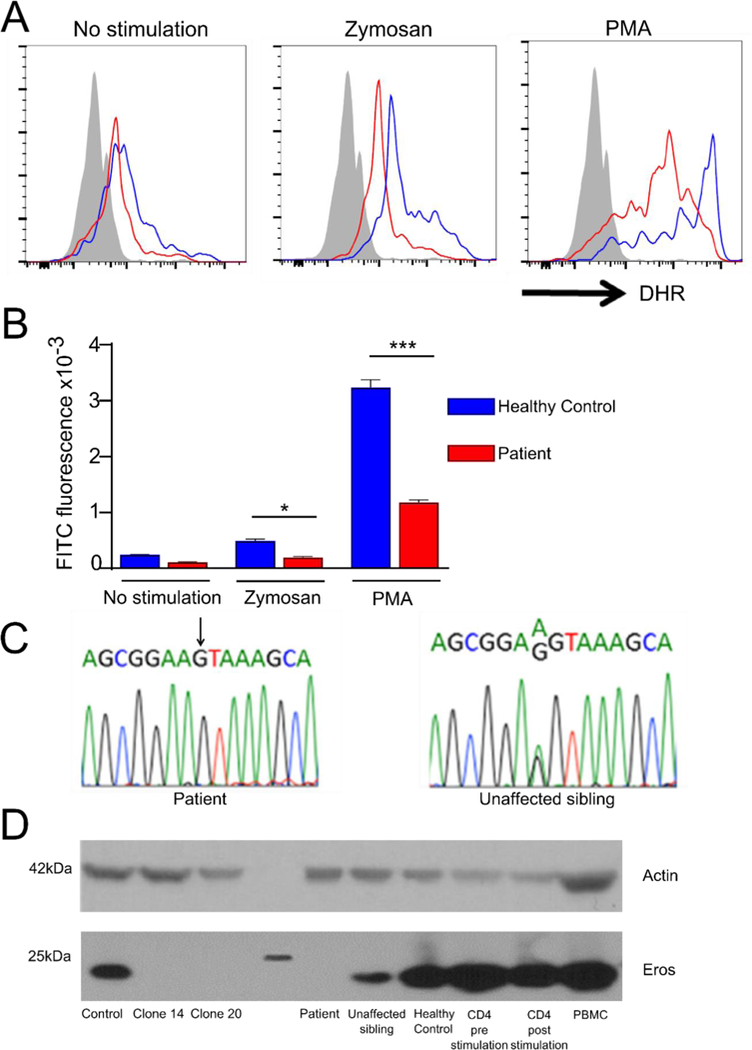
(A, B): respiratory burst in patient and healthy control neutrophils. (C) homozygous c.127 A to G mutation (D) EROS protein expression in CD3/CD2/CD28 expanded PBMC from patient, sister and a healthy control, control CD4+ T cells pre and post CD3/CD2/CD28 or PBMC from a further healthy control. Representative of 2–3 independent experiments.
Whole exome sequencing demonstrated that the patient had a homozygous (c.127 A to G, NM_001033046) mutation in CYBC1/EROS. His sisters were all heterozygous for this mutation, confirmed by Sanger sequencing (Fig. 2C). Based on analysis of other family members and the likely important role of CYBC1/EROS in immunity, this mutation was identified as the most likely cause of the patient’s disease. The mutation was not present in 10,000 whole genomes from the United Kingdom National Institute for Health Research BioResource - Rare Disease cohort (which includes 1000 patients with primary immunodeficiency), nor in gnomAD. The variant was also absent in 3,300 ethnically matched exomes. It is, therefore, not seen in seen across 110,579 individuals with sequence data coverage across this position. There were no deleterious mutations in known NADPH oxidase subunits.
Splice site prediction algorithms including Mutation Tasting (www.mutationtaster.org) and Human Splicing Finder (http://www.umd.be/HSF3/) predict that the variant both disrupts an exonic splice enhancer (ESE) and creates an exonic splice silencer (ESS) which is likely to lead to a retained intron. This intron has 4 in-frame stop codons. It is therefore likely that translation would cease downstream of exon 4. Even if splicing is not disrupted, PolyPhen (http://genetics.bwh.harvard.edu/pph2/) predicts that the D43N mutation that would occur in the translated protein is also damaging.
We therefore performed western blot analysis on anti-CD3-CD28-CD2 expanded T cells from the patient, his sister and a healthy control, as well as primary T cells (either pre or post polyclonal stimulation) and peripheral blood mononuclear cells from healthy volunteers. The CRISPR targeted clones described above were used as positive and negative controls respectively. The patient had undetectable levels of EROS protein compared with cells from the healthy control or the primary T cells/PBMC, while his heterozygous sister had intermediate levels (Fig. 2D).
This work demonstrates that the function of the novel transmembrane protein, Eros, is conserved in humans. It also represents the first description of an immunodeficiency syndrome secondary to mutations in CYBC1/EROS. The severity of the disease seen in this patient underlines the importance of human EROS is for normal immunity. The patient has a clinical history that is compatible with a diagnosis of autosomal recessive CGD, in that he had both infectious and auto-inflammatory manifestations, together with histo-pathological evidence of granuloma formation in the context of an impaired DHR response. While recurrent infections, BCG-it is (2, 5), granulomatous inflammation and HLH (6) are all recognised sequelae of CGD, this patient some unusual features such as autoimmune haemolytic anaemia. This is uncommon in CGD and may represent an effect of EROS-deficiency that is independent of its effects on the NAPDH oxidase.
The high sequence similarity between mouse and human EROS and the loss of gp91phox expression and the phagocyte respiratory burst that accompanies its absence suggests that EROS plays an almost identical role in human and murine immunity. In summary, we have shown that the function of EROS is fully conserved between human and mouse, and that homozygous mutations in EROS underlie a novel sixth cause of chronic granulomatous disease.
Supplementary Material
Acknowledgements
D.C.T is funded by a Wellcome-Beit Prize Clinical Research Career Development Fellowship. JCL is a Wellcome Trust Intermediate Fellow. E.C and S.C. are funded by the Wellcome Trust (grant code 098051). A.J.T., GS and AS are supported by both the Wellcome Trust (104807/Z/14/Z) and by the NIHR Biomedical Research Centres of both Cambridge and Great Ormond Street Hospital for Children NHS Foundation Trust/University College London. HAM is funded by the National Science, Technology and Innovation Plan’s (NSTIP) strategic technologies program in Saudi Arabia, (KACST: 13-BIO-755–20). FSA is funded by the Saudi Human Genome Program (King Abdulaziz City for Science and Technology). T.A.C. is funded by a grant from the National Institutes of Health (grant R01 AI085090). K.G.C.S. is funded by the Medical Research Council (program grant MR/L019027) and is a Wellcome Investigator and NIHR Senior Investigator.
Abbreviations
- CGD
Chronic Granulomatous Disease
- EROS
Essential for Reactive Oxygen Species
- HLH
haemophagocytic lymphohistiocytosis
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NBT
Nitro blue tetrazolium chloride
- DHR
Dihydrorhodamine
- PBMC
Peripheral Blood Mononuclear Cell
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
References:
- 1.Segal AW. How neutrophils kill microbes. Annual review of immunology. 2005;23:197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, et al. Chronic granulomatous disease: the European experience. PloS one. 2009;4(4):e5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas DC, Clare S, Sowerby JM, Pardo M, Juss JK, Goulding DA, et al. Eros is a novel transmembrane protein that controls the phagocyte respiratory burst and is essential for innate immunity. The Journal of experimental medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monies D, Abouelhoda M, AlSayed M, Alhassnan Z, Alotaibi M, Kayyali H, et al. The landscape of genetic diseases in Saudi Arabia based on the first 1000 diagnostic panels and exomes. Hum Genet. 2017;136(8):921–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conti F, Lugo-Reyes SO, Blancas Galicia L, He J, Aksu G, Borges de Oliveira E Jr., et al. Mycobacterial disease in patients with chronic granulomatous disease: A retrospective analysis of 71 cases. The Journal of allergy and clinical immunology. 2016;138(1):241–8 e3. [DOI] [PubMed] [Google Scholar]
- 6.Parekh C, Hofstra T, Church JA, Coates TD. Hemophagocytic lymphohistiocytosis in children with chronic granulomatous disease. Pediatric blood & cancer. 2011;56(3):460–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


