Abstract
Purpose:
Within-subject controlled models in individuals who preferentially load one side of the body enable efficient exploration of the skeletal benefits of physical activity. There is no established model of physical activity-induced side-to-side differences (i.e., asymmetry) at the proximal femur.
Methods:
Proximal femur asymmetry was assessed via dual-energy x-ray absorptiometry in male jumping athletes (JMP, n=16), male baseball pitchers (BB, n=21), female fast-pitch softball pitchers (SB, n=22), and controls (CON, n=42). The jumping leg was the dominant leg in JMP, whereas in BB, SB and CON the dominant leg was contralateral to the dominant/throwing arm.
Results:
BB and SB had 5.5% (95%CI, 3.9 to 7.0%) and 6.5% (95%CI, 4.8 to 8.2%) dominant-to-nondominant leg differences for total hip areal bone mineral density (aBMD), with the asymmetry being greater than both CON and JMP (p<0.05). BB and SB also possessed dominant-to-nondominant leg differences in femoral neck and trochanteric aBMD (p<0.001). SB had 9.7% (95% CI, 6.4 to 13.0%) dominant-to-nondominant leg differences in femoral neck bone mineral content, which was larger than any other group (p≤0.006). At the narrow neck, SB had large (>8%) dominant-to-nondominant leg differences in cross-sectional area, cross-sectional moment of inertia and section modulus, which were larger than any other group (p≤0.02).
Conclusion:
Male baseball and female softball pitchers are distinct within-subject controlled models for exploring adaptation of the proximal femur to physical activity. They exhibit adaptation in their dominant/landing leg (i.e., leg contralateral to the throwing arm), but the pattern differs with softball pitchers exhibiting greater femoral neck adaptation.
Keywords: DXA, exercise, femoral neck, hip, osteoporosis
MINI ABSTRACT:
Individuals who preferentially load one side of the body are used to explore skeletal adaptation to physical activity. No within-subject controlled model of proximal femur adaptation currently exists. The current study demonstrates baseball and softball pitchers are distinct within-subject controlled models of physical activity-induced proximal femur adaptation.
INTRODUCTION
Osteoporotic fractures of the proximal femur account for the majority of fracture-related health care costs and mortality. In Western nations, 20–25% of women who suffer an osteoporotic proximal femur fracture die within 1 year [1] and 10–20% of previously independent individuals are institutionalized [2]. Optimizing peak bone mass when young is advocated to delay osteoporosis and reduce proximal femur fracture risk during aging [3]. Fracture risk during aging doubles for each standard deviation of bone lost from mean peak bone mass [4] and a 10% increase in peak bone mass is predicted to delay the onset of osteoporosis by 13 years [5]. As 25–30% of adult bone mineral is accrued within the 2–3 years around puberty and 95% of adult bone mass has accrued by the end of adolescence [6, 7], the growing years are viewed as a window of opportunity for lifelong bone health.
The growing years appear particularly important to maximize the skeletal benefits of physical activity. This was eloquently shown by Kannus and colleagues [8], and supported by others [9, 10], who explored the upper extremities of racquet sport players to show the skeletal advantage of physical activity during specific phases of growth. We subsequently observed these physical activity-induced skeletal benefits persisted lifelong, particularly the benefits on bone size [11]. In terms of the proximal femur, randomized controlled trials (RCTs) and longitudinal studies have confirmed the benefit of physical activity during growth on proximal femur bone health (see [3] for review). For instance, the Iowa Bone Development Study demonstrated 10–16% greater hip BMC and 8% greater hip aBMD in participants who accumulated the greatest amount of physical activity from childhood through adolescence [12].
RCTs and longitudinal studies are effective means of demonstrating the skeletal benefits of physical activity; however, they are costly in terms of both time and money. Consequently, alternative study designs are often employed, with within-subject controlled studies assessing side-to-side differences (i.e., asymmetry) in individuals who preferentially exercise one side of the body being particularly attractive. Within-subject study designs enable the skeletal effects of physical activity to be explored in the absence of selection bias and with lessened impact of inherited and systemic factors. These factors influence conclusions drawn from traditional cross-sectional study designs comparing between individuals.
Within-subject controlled study designs have almost exclusively been used to address questions regarding the skeletal effects of physical activity at upper extremity sites, with racquet sport players [9, 13] and throwing athletes [11, 14] being popular models. In contrast, there is no established model to explore physical activity-induced side-to-side skeletal differences in the lower extremities and, in particular, at the proximal femur. Lower extremity skeletal asymmetry has been reported in some athletic populations, including handball players (contralateral > ipsilateral leg to throwing arm), soccer players (stance > kicking leg) [15], fencers (lunging > trail leg) [16] and long/high jump athletes (jump > lead leg) [17, 18]. However, to our knowledge, only two previous studies have reported asymmetry at the proximal femur, with rhythmic gymnasts having 4.7% greater aBMD at the femoral neck in their take-off versus landing leg [19] and ten-pin bowlers having 12.2% greater femoral neck aBMD in their slide (i.e., the leg opposite the bowling arm) versus trail leg [20]. These latter models show initial promise, but there is a need for additional within-subject models that enable physical activity benefits at the proximal femur to be explored.
The aim of the current study was to explore jumping (high/long) and throwing (baseball/softball pitchers) athletes as potential within-subject controlled models to study the skeletal effects of physical activity at the proximal femur. Jumping athletes expose their jump leg to vertical ground reaction forces during take-off that are more than double that experienced during maximal sprinting [21–23], whereas baseball and fast-pitch softball pitchers expose their landing leg (i.e., the leg opposite the pitching arm) to elevated ground reaction forces compared to their drive leg [24–26]. Control subjects were also assessed to explore whether any asymmetry observed in the athlete groups were accounted for by crossed asymmetry whereby the lower extremity opposite the dominant arm possesses enhanced proximal femur bone properties [27].
METHODS
Study design and participants
A within-subject controlled cross-sectional study design was used to compare bone properties at the bilateral proximal femurs in male jumping athletes (JMP group), male baseball pitchers (BB group), female fast-pitch softball pitchers (SB group), and male and female controls (CON group). JMP were included if they were currently competing or practicing in long and/or high jump at the collegiate-level. BB and SB were included if they were currently competing or practicing as a pitcher in professional Minor League Baseball or collegiate-level fast-pitch softball, respectively. JMP, BB, SB were excluded if they had a past history of competing in an activity alternate to their primary sport that may have exposed their lower extremities to asymmetrical loading (e.g. soccer, fencing, ten-pin bowling, baseball, softball, etc.). CON were included if they were aged 18–30 years and did not have a past history of competing in an activity that may have exposed their lower extremities to asymmetrical loading. Exclusion criteria for all groups were: 1) known metabolic bone disease; 2) history of a femoral fracture or stress fracture; 3) implanted metal within the femur, and; 4) exposure to lower extremity immobilization for more than 2 weeks within the past 2 years. The study was approved by the Institutional Review Board of Indiana University and written informed consent was obtained from all participants.
Dual-energy x-ray absorptiometry
Dual-energy X-ray absorptiometry (DXA) using a Discovery-W machine (Hologic, Inc., Waltham, MA) equipped with Apex v4.0 software was performed per the manufacturer’s instructions to obtain bilateral hip areal bone mineral density (aBMD; g/cm2), bone mineral content (BMC, g) and projected area (cm2). Hip structural analysis was used to determine cross-sectional area (CSA), cross-sectional moment of inertia (CSMI), section modulus (Z) and cortical thickness (CtTh) at the narrow neck region of the proximal femur. A whole-body scan was performed to assess whole-body fat-free lean mass (kg), fat mass (%), and aBMD (g/cm2).
Statistical analyses
Two-tailed analyses with a level of significance set at 0.05 were performed with IBM SPSS Statistics (v24; SPSS Inc., Chicago, IL). Demographic and anthropometric characteristics in male participants (JMP, BB and male CON) were compared between groups using one-way ANOVAs followed by a Fisher’s least square difference post-hoc test. Demographic and anthropometric characteristics in female participants (SB and female CON) were compared between groups using independent t-tests.
Leg dominance within each group was defined as follows: JMP group—the leg the participant jumps from during long and/or high jump; BB and SB groups—the leg opposite the pitching arm, and; CON group—the leg opposite the dominant arm. Dominant versus nondominant leg differences in proximal femur properties were assessed by calculating mean percent differences ([dominant leg – nondominant leg] / nondominant leg × 100%) and their 95% confidence intervals (CIs). 95% CIs not crossing 0% were considered statistically significant, as determined by single sample t-tests on the mean percent differences with a population mean of 0%. Dominant-to-nondominant leg percent difference values were compared between groups using a one-way ANOVA followed by a Fisher’s least square difference post-hoc test.
RESULTS
Demographic and anthropometric characteristics of the 101 recruited participants are detailed in Table 1. Among males, BB were older, taller, heavier, and had a greater BMI and whole-body lean mass than both JMP and CON (all p < 0.05). They also started competing and had been competing for longer than JMP (all p < 0.05). JMP were younger than CON (p < 0.05), and both BB and JMP had greater whole-body aBMD than CON (all p < 0.001). Among females, SB were younger, taller and heavier than their respective CON group (all p < 0.05). These demographic differences between groups are normalized and are not believed to influence within-subject asymmetry.
Table 1.
Participant demographic and anthropometric characteristics†
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Controls | Jumpers | Baseball | Controls | Softball | |||
| n | 27 | 16 | 21 | 15 | 22 | |||
| Demographics | ||||||||
| Age (yr) | 23.7 ± 3.6JB | 21.1 ± 2.1CB | 27.5 ± 2.6CJ | 22.5 ± 3.3S | 20.6 ± 1.3C | |||
| Dominant leg (R/L) | 2/25 | 4/12 | 7/14 | 0/15 | 2/20 | |||
| Estimated age of adolescent growth spurt (yr) | 13.3 ± 2.1 | 13.8 ± 1.8 | 14.5 ± 1.5 | 12.6 ± 1.7 | 12.9 ± 1.1 | |||
| Jumping sport (long:high jump) | ― | 12:10‡ | ― | ― | ― | |||
| Age started competing (yr) | ― | 13.6 ± 1.5B | 7.7 ± 2.7J | ― | 9.0 ± 1.9 | |||
| Years competing before adolescent growth spurt (yr) | ― | 0.3 ± 2.1B | 8.6 ± 2.0J | ― | 5.5 ± 2.0 | |||
| Total years competing (yr) | ― | 6.3 ± 2.7B | 21.4 ± 4.2J | ― | 13.2 ± 2.9 | |||
| Whole-body anthropometry | ||||||||
| Height (m) | 1.82 ± 0.08B | 1.83 ± 0.07B | 1.88 ± 0.05CJ | 1.64 ± 0.07S | 1.73 ± 0.06C | |||
| Mass (kg) | 75.7 ± 15.9B | 78.3 ± 6.9B | 96.9 ± 8.7CJ | 65.6 ± 13.2S | 76.1 ± 12.6C | |||
| Body mass index (kg/m2) | 22.8 ± 3.8B | 23.5 ± 1.9B | 27.5 ± 2.5CJ | 24.3 ± 4.4 | 25.2 ± 3.6 | |||
| Areal bone mineral density (g/cm2)# | 1.23 ± 0.09JB | 1.38 ± 0.09C | 1.35 ± 0.14C | 1.25 ± 0.11 | 1.18 ± 0.06 | |||
| Lean mass (kg)§ | 55.5 ± 10.0B | 59.2 ± 5.1B | 66.1 ± 5.8CJ | 50.6 ± 6.3 | 48.8 ± 1.0 | |||
| Fat mass (%) | 19.3 ± 6.8J | 14.0 ± 2.0CB | 21.9 ± 2.8J | 29.9 ± 6.8 | 31.2 ± 6.5 | |||
Data are mean ± SD, except for frequencies. Superscript capital letters indicate the data significantly differs from CON (C), JMP (J), BB (B) and SB (S)
Six jumpers competed in both jumping sports
Values corrected for whole-body lean mass
Values corrected for height
Proximal femur properties in each leg in male and female subjects are provided in Tables 2 and 3, respectively. Few parameters revealed bilateral asymmetry in absolute difference values in male and female CON. As such, and as male vs. female differences in proximal femur bone health are normalized when data are expressed in terms of dominant-to-nondominant leg differences, male and female CON groups were condensed into a single CON group when determining and comparing mean percent differences.
Table 2.
Proximal femur properties in the male control, jumper and baseball groups
| Site | Measure | Control (male) | Jumper | Baseball | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nondominanta | Dominanta | Abs. diff. (95% CI)b | Nondominanta | Dominanta | Abs. diff. (95% CI)b | Nondominanta | Dominanta | Abs. diff. (95% CI)b | ||
| Total hip | aBMD (g/cm2) | 1.11 ± 0.12 | 1.10 ± 0.11 | 0.01 (−0.01, 0.02) | 1.29 ± 0.12 | 1.31 ± 0.12 | 0.02 (−0.01, 0.04) | 1.28 ± 0.12 | 1.35 ± 0.10 | 0.07 (0.05, 0.09)‡ |
| BMC (g) | 46.5 ± 7.9 | 47.4 ± 8.3 | 0.9 (−0.3, 2.1) | 55.6 ± 7.2 | 57.5 ± 7.3 | 1.9 (−0.3, 4.0) | 62.4 ± 7.6 | 66.2 ± 6.8 | 3.9 (2.1, 5.6)‡ | |
| Area (cm2) | 42.1 ± 5.4 | 42.7 ± 5.0 | 0.6 (−0.2, 1.4) | 44.3 ± 4.9 | 45.0 ± 4.3 | 0.7 (−0.7, 2.0) | 48.7 ± 4.9 | 49.2 ± 4.7 | 0.4 (−0.5, 1.4) | |
| Femoral neck | aBMD (g/cm2) | 1.01 ± 0.11 | 1.01 ± 0.11 | 0.00 (−0.02, 0.02) | 1.20 ± 0.18 | 1.22 ± 0.18 | 0.02 (−0.01, 0.05) | 1.20 ± 0.12 | 1.24 ± 0.12 | 0.04 (0.03, 0.06)‡ |
| BMC (g) | 5.62 ± 0.81 | 5.66 ± 0.80 | 0.04 (−0.09, 0.17) | 6.76 ± 0.97 | 7.01 ± 0.96 | 0.25 (0.05, 0.44)* | 6.94 ± 0.75 | 7.22 ± 0.58 | 0.28 (0.07, 0.49)† | |
| Area (cm2) | 5.58 ± 0.48 | 5.64 ± 0.47 | 0.06 (−0.06, 0.18) | 5.67 ± 0.31 | 5.75 ± 0.33 | 0.09 (−0.02, 0.19) | 5.83 ± 0.45 | 5.85 ± 0.50 | 0.02 (−0.12, 0.15) | |
| Trochanteric | aBMD (g/cm2) | 0.82 ± 0.14 | 0.83 ± 0.14 | 0.01 (−0.01, 0.02) | 0.96 ± 0.10 | 0.97 ± 0.10 | 0.01 (−0.01, 0.03) | 0.95 ± 0.09 | 1.01 ± 0.10 | 0.06 (0.04, 0.07)‡ |
| BMC (g) | 11.1 ± 3.8 | 11.3 ± 3.8 | 0.2 (−0.1, 0.5) | 13.4 ± 2.2 | 13.9 ± 2.1 | 0.5 (0.2, 0.8)† | 13.7 ± 1.9 | 14.9 ± 2.1 | 1.3 (0.7, 1.8)‡ | |
| Area (cm2) | 13.4 ± 2.4 | 13.6 ± 2.3 | 0.3 (−0.1, 0.6) | 14.0 ± 2.1 | 14.3 ± 1.7 | 0.3 (−0.1, 0.7) | 14.6 ± 1.5 | 14.8 ± 1.4 | 0.2 (−0.3, 0.7) | |
| Narrow neck | CSA (cm2) | 4.11 ± 0.55 | 4.16 ± 0.58 | 0.05 (−0.03, 0.14) | 5.23 ± 0.50 | 5.34 ± 0.46 | 0.11 (0.01, 0.22) | 5.23 ± 0.50 | 5.34 ± 0.46 | 0.11 (0.01, 0.22)* |
| CSMI (cm4) | 4.26 ± 1.11 | 4.48 ± 1.22 | 0.23 (0.01, 0.44)* | 6.18 ± 1.06 | 6.08 ± 0.95 | −0.10 (−0.37, 0.17) | 6.18 ± 1.06 | 6.08 ± 0.95 | −0.10 (−0.37, 0.17) | |
| Z (cm3) | 2.19 ± 0.40 | 2.25 ± 0.46 | 0.06 (−0.03, 0.15) | 3.02 ± 0.45 | 2.95 ± 0.35 | −0.07 (−0.19, 0.06) | 3.02 ± 0.45 | 2.95 ± 0.35 | −0.07 (−0.19, 0.06) | |
| CtTh (cm) | 0.23 ± 0.03 | 0.23 ± 0.03 | 0.00 (−0.01, 0.01) | 0.28 ± 0.04 | 0.28 ± 0.04 | 0.01 (−0.01, 0.01) | 0.28 ± 0.04 | 0.28 ± 0.04 | 0.01 (−0.01, 0.01) | |
Data are mean ± SD
Mean absolute side-to-side differences were assessed using single sample t-tests with a population mean of 0%. Significance is indicated by:
P<0.05,
P<0.01,
P<0.001
Table 3.
Proximal femur properties in the female control and softball groups
| Site | Measure | Control (female) | Softball | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nondominanta | Dominanta | Abs. diff. (95% CI)b | Nondominanta | Dominanta | Abs. diff. (95% CI)b | ||||
| Total hip | aBMD (g/cm2) | 1.02 ± 0.13 | 1.13 ± 0.12 | 0.01 (−0.01, 0.03) | 1.12 ± 0.12 | 1.19 ± 0.13 | 0.07(0.05, 0.09)‡ | ||
| BMC (g) | 32.6 ± 5.7 | 32.6 ± 4.9 | −0.1 (−1.1, 1.1) | 40.2 ± 6.8 | 43.3 ± 7.6 | 3.1 (2.1, 4.1)‡ | |||
| Area (cm2) | 31.8 ± 3.3 | 31.4 ± 2.7 | −0.3 (−1.2, 0.6) | 35.9 ± 4.0 | 36.3 ± 4.5 | 0.5 (−0.1, 0.9) | |||
| Femoral neck | aBMD (g/cm2) | 0.94 ± 0.15 | 0.95 ± 0.15 | 0.01 (−0.01, 0.03) | 1.01 ± 0.10 | 1.08 ± 0.11 | 0.06 (0.04, 0.09)‡ | ||
| BMC (g) | 4.33 ± 0.79 | 4.42 ± 0.72 | 0.09 (−0.07, 0.25) | 5.08 ± 0.61 | 5.58 ± 0.69 | 0.50 (0.35, 0.65)‡ | |||
| Area (cm2) | 4.62 ± 0.51 | 4.64 ± 0.31 | 0.02 (−0.15, 0.19) | 5.02 ± 0.35 | 5.19 ± 0.29 | 0.17 (0.06, 0.28)† | |||
| Trochanteric | aBMD (g/cm2) | 0.77 ± 0.13 | 0.78 ± 0.13 | 0.02 (0.00, 0.03)* | 0.84 ± 0.10 | 0.90 ± 0.12 | 0.05 (0.04, 0.07)‡ | ||
| BMC (g) | 7.6 ± 1.5 | 7.6 ± 1.6 | 0.0 (−0.3, 0.3) | 9.3 ± 1.4 | 10.1 ± 1.8 | 0.8 (0.3, 1.3)† | |||
| Area (cm2) | 9.8 ± 0.9 | 9.7 ± 1.0 | −0.2 (−0.6, 0.2) | 11.0 ± 1.1 | 11.2 ± 1.3 | 0.2 (−0.3, 0.7) | |||
| Narrow neck | CSA (cm2) | 3.26 ± 0.59 | 3.35 ± 0.57 | 0.09 (0.02, 0.15)* | 3.72 ± 0.50 | 4.07 ± 0.54 | 0.34 (0.23, 0.46)‡ | ||
| CSMI (cm4) | 2.37 ± 0.76 | 2.43 ± 0.64 | 0.05 (−0.11, 0.22) | 3.21 ± 0.90 | 3.58 ± 0.97 | 0.38 (0.19, 0.56)‡ | |||
| Z (cm3) | 1.46 ± 0.36 | 1.49 ± 0.37 | 0.02 (−0.03, 0.07) | 1.87 ± 0.35 | 2.03 ± 0.39 | 0.17 (0.09, 0.25)‡ | |||
| CtTh (cm) | 0.22 ± 0.04 | 0.22 ± 0.04 | 0.00 (−0.01, 0.01) | 0.23 ± 0.03 | 0.25 ± 0.03 | 0.02 (0.01, 0.03)† | |||
Data are mean ± SD
Mean absolute side-to-side differences were assessed using single sample t-tests with a population mean of 0%. Significance is indicated by:
P<0.05,
P<0.01,
P<0.001
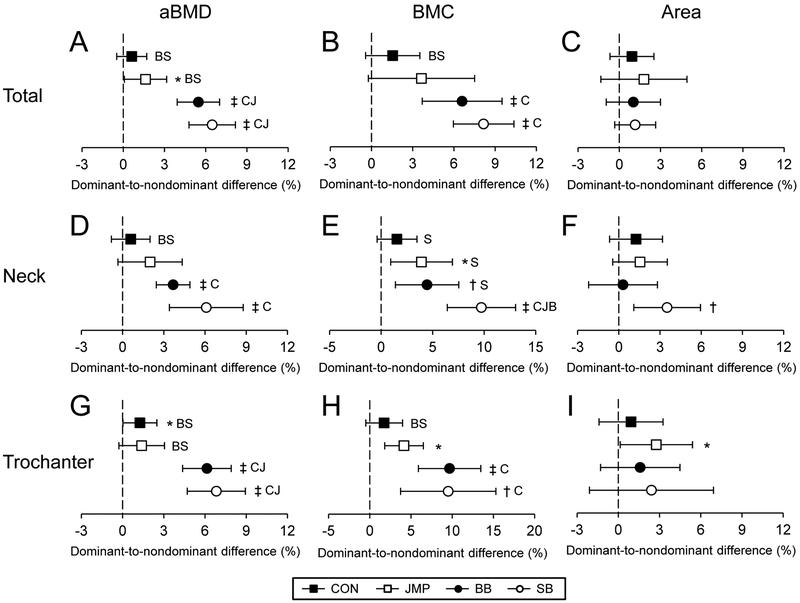
CON did not exhibit dominant-to-nondominant leg differences in aBMD, BMC or area in any region (all p = 0.12 to 0.43), except aBMD at the greater trochanter (p = 0.04) (Fig. 1). JMP, BB and SB had 1.6% (95% CI, 0.1 to 3.2%), 5.5% (95% CI, 3.9 to 7.0%) and 6.5% (95% CI, 4.8 to 8.2%) dominant-to-nondominant leg differences for total hip aBMD, with the bilateral asymmetry in BB and SB being greater than in both CON and JMP (all p < 0.05; Fig. 1A). The greater total hip aBMD in BB and SB resulted from dominant-to-nondominant leg differences in BMC (all p < 0.001; Fig. 1B), as opposed to differences in projected bone area (all p > 0.12; Fig. 1C).
Figure 1.
Percent dominant-to-nondominant leg differences for areal bone mineral density (aBMD), bone mineral content (BMC), and bone area in the total proximal femur (A-C), femoral neck (D-F), and trochanter (G-I) regions. Data represent mean percent difference between the dominant and nondominant legs, with error bars indicating 95% confidence intervals. Confidence intervals greater than 0% indicate greater bone properties within the dominant leg compared to nondominant leg (*p < 0.05; †p < 0.01; ‡p < 0.001). Capital letters indicate the group data differs significantly from CON (C), JMP (J), BB (B) and SB (S) (p < 0.05).
BB and SB possessed 3.7% (95% CI, 2.5 to 4.9%) and 6.1% (95% CI, 3.4 to 8.8%) dominant-to-nondominant leg differences in aBMD at the femoral neck (all p < 0.001; Fig. 1D). In SB, the bilateral asymmetry in aBMD resulted from a 9.7% (95% CI, 6.4 to 13.0%) dominant-to-nondominant leg difference in BMC (p < 0.001; Fig. 1E), whose effect on aBMD was tempered by a dominant-to-nondominant leg difference in projected bone area (p = 0.005; Fig. 1F). The bilateral asymmetry in femoral neck BMC in SB was greater than that observed in all other groups (all p ≤ 0.006, Fig. 1E).
BB and SB exhibited 6.1% (95% CI, 4.4 to 7.9%) and 6.8% (95% CI, 4.7 to 8.9%) dominant-to-nondominant leg differences in trochanteric aBMD (all p < 0.001; Fig. 1G). These dominant-to-nondominant leg differences were greater than those observed in both JMP and CON (all p ≤ 0.001) and resulted from large dominant-to-nondominant leg differences in trochanteric BMC (all p ≤ 0.005, Fig. 1H).
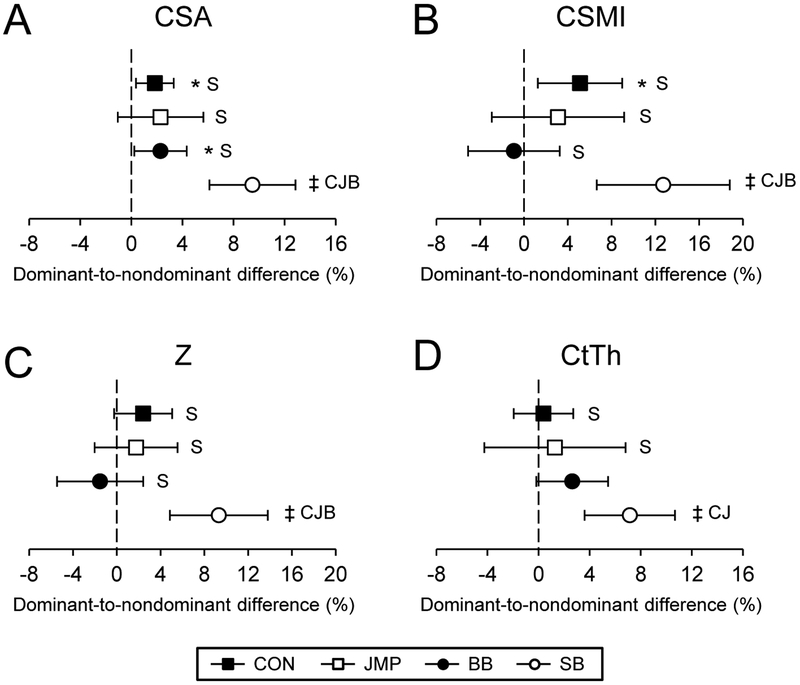
CON exhibited dominant-to-nondominant leg differences in CSA and CSMI favoring the dominant leg at the narrow neck region (all p ≤ 0.02, Fig. 2A,B). JMP and BB did not exhibit any dominant-to-nondominant leg differences at the narrow neck (all p > 0.07), except for CSA in BB (p = 0.03, Fig. 2A). There were no differences between CON, JMP or BB for any measure at the narrow neck (all p > 0.08, Fig. 2). In contrast, SB had large dominant-to-nondominant leg differences, with the largest difference being for CSMI which was 12.7% (95% CI, 6.6 to 18.8%) greater in the dominant leg (p < 0.001, Fig. 2B). Dominant-to-nondominant leg differences for CSA, CSMI and Z were greater in SB than all other groups (all p < 0.02, Fig. 2A–C), with SB also having greater dominant-to-nondominant leg differences for CtTh than both CON and JMP (all p < 0.03, Fig. 2D).
Figure 2.
Percent dominant-to-nondominant leg differences at the narrow neck region of the proximal femur for A) cross-sectional area (CSA), B) cross-sectional moment of inertia (CSMI), C) section modulus (Z) and D) cortical thickness (CtTh). Data represent mean percent difference between the dominant and nondominant legs, with error bars indicating 95% confidence intervals. Confidence intervals greater than 0% indicate greater bone properties within the dominant leg compared to nondominant leg (*p < 0.05; †p < 0.01; ‡p < 0.001). Capital letters indicate the group data differs significantly from CON (C), JMP (J), BB (B) and SB (S) (p < 0.05).
DISCUSSION
The current data indicate that male baseball and female fast-pitch softball pitchers are distinct within-subject controlled models for exploring the impact of physical activity on the proximal femur. Both groups of throwing athletes exhibited 5.5–6.5% and 6.1–6.8% greater total proximal femur and trochanteric aBMD in their dominant/landing leg (i.e., leg contralateral to their throwing arm) compared to their nondominant/drive leg (i.e., leg ipsilateral to their throwing arm), respectively. These differences were larger than dominant-to-nondominant leg differences observed in both jumping athletes and controls. Baseball and softball pitchers also had significant dominant-to-nondominant leg differences in aBMD at the femoral neck; however, softball pitchers interestingly had greater differences in femoral neck BMC at this site than all other groups, including baseball pitchers. In addition, softball pitchers were the only group that exhibited significant dominant-to-nondominant leg differences in projected bone area at the femoral neck. Exploring the femoral neck region further, softball pitchers had large (>8%) and greater dominant-to-nondominant leg differences in narrow neck CSA, CSMI and Z than all other groups. These cumulative data indicate that both baseball and softball pitchers load and adapt the proximal femur in their dominant/landing leg, but the pattern of adaptation differs with softball pitchers exhibiting greater adaptation within the femoral neck region.
Few physical activities asymmetrically load the lower limbs and there is no accepted within-subject controlled model system to explore proximal femur adaptation to mechanical loads associated with physical activity. To our knowledge, only two previous studies have demonstrated bilateral asymmetry at the proximal femur in athlete groups [19, 20]. The dominant-to-nondominant leg differences in femoral neck aBMD observed in baseball and softball pitchers in the current study (3.7–6.1%) are comparable to the 4.5% difference observed between the take-off and landing legs in rhythmic gymnasts [19], but they are less than the 12.2% difference observed between the slide and trail legs in competitive ten-pin bowlers [20]. Further work is required to confirm the large bilateral asymmetry observed in ten-pin bowlers and their relative utility as a within-subject controlled model compared to baseball and softball pitchers.
The magnitude of the dominant-to-nondominant leg differences observed in baseball and softball pitchers in the current study is translatable to studies exploring the between-subject skeletal benefits of physical activity. While throwing athletes exhibit extreme upper extremity adaptation at levels beyond those achievable within the general population (e.g., bone strength is up to double in their throwing vs. nonthrowing arm [11]), the dominant-to-nondominant leg differences observed in proximal femur aBMD and BMC are equivalent to those observed with physical activity in between-subject intervention and prospective observational studies [28, 29]. Thus, throwing athletes may be a useful, efficient model for studying clinically achievable skeletal benefits of physical activity prior to performing a more definitive and costly longitudinal study.
The reason for greater dominant-to-nondominant leg differences in bone mass and structural properties at the femoral neck in softball pitchers compared to baseball pitchers was unexpected. Both groups started competing well prior to their adolescent growth spurt, and the professional baseball pitchers had been competing for longer and at a higher level than the collegiate-level fast-pitch softball pitchers (21.4 vs. 13.2 years). The latter would be considered to favor greater adaptation in baseball pitchers, with inclusion of age or years training as a covariate in analyses accentuating differences in proximal femur asymmetry the between softball and baseball pitchers (data not shown). It is possible there are sex-related differences in mechanosensitivity and adaptation, with some reports indicating males have a longer ‘window of opportunity’ for physical activity induced adaptation across the pubertal growth period [9, 30]. However, this would again favor greater adaptation in male baseball pitchers which was not observed.
The greater adaptation in softball pitchers most likely resulted from differences in mechanical loading between fast-pitch softball and baseball pitching. No studies have directly compared lower extremity biomechanics during baseball and softball pitching, and few have assessed bilateral loading of both the landing and drive legs in either baseball or softball pitching. However, from the available data, softball pitching may be associated with greater ground reaction forces. Baseball pitchers generate maximum vertical ground reaction forces in their landing leg (1.5–2.0X body weight (BW) [25, 31, 32]) that are 1.5-to-2 times greater than in their drive leg (1.0–1.3X BW [25, 33, 34]). In contrast, softball pitchers may generate larger landing leg maximum vertical ground reaction forces (3.0–3.5X BW) [35, 36], although other studies report similar forces (1.4–1.8X BW [26, 37, 38]) to those observed in baseball pitchers.
In addition to potentially generating greater ground reaction forces, softball pitchers exhibit different lower extremity kinematics than baseball pitchers. They stride on a flat surface to land their landing/dominant leg with the knee in relative extension (29–33° knee flexion [26, 37, 39]) and foot closed (i.e., facing 3rd base for a right-handed pitcher [26]). In contrast, baseball pitchers stride downhill from an elevated mound to land with their knee in greater knee flexion (41–64° knee flexion [24, 40–42]) and foot more open (i.e., approximately facing the batter [40]). The reduced knee flexion position in softball pitchers has the potential to reduce shock absorption as forces are propagated proximally to the proximal femur, while a more closed foot position would influence hip rotation position to potentially alter the distribution of forces at the proximal femur.
Finally, differences in pitching frequency, intensity, and volume when young may have contributed to the different proximal femur adaptation between baseball and softball pitchers. We deliberately did not collect information on pitching volumes when young as it would not be possible to validate historically recalled data, particularly for throwing across the adolescent growth period. However, pitching volume is regulated in youth baseball by pitch count limits and required days of rest between pitching outings. In contrast, no such regulations have historically been present in youth softball, where it has been common for pitchers to pitch up to 100 times within a single game, and to pitch in multiple games per day and on consecutive days [43–45]. Thus, it is possible that greater throwing volumes when young in softball pitchers contributed to their greater bilateral asymmetry at the proximal femur relative to baseball pitchers.
In comparison to baseball and softball pitchers, there were limited dominant-to-nondominant leg differences observed in jumping athletes. Jumping athletes exhibited dominant-to-nondominant leg differences in total proximal femur aBMD, and femoral neck and trochanteric BMC; however, the differences were not greater than dominant-to-nondominant leg differences observed in controls. The limited dominant-to-nondominant leg differences in jumping athletes may relate to the timing, duration and frequency of jumping exposure in the athletes tested, as we know jumping is an osteogenic stimulus [28, 29]. Jumping athletes begun competing in jump events at the approximately the same time as their self-reported adolescent growth spurt and had been competing for less than a half to a third as long in their chosen sport compared to the throwing athletes (Table 1). The later introduction and shorter duration of unilateral preferential loading compared to baseball and softball pitchers may have tempered the side-to-side differences in jumpers. For instance, differences in proximal femur asymmetry the between jumpers and baseball pitchers (but, not softball pitchers) disappeared when years training was used as a covariate (data not shown). We also hypothesize that the jumping athletes performed less frequent and less repetitions of unilateral loading compared to throwing athletes who perform hundreds of weekly repetitions. Finally, jumping athletes perform a high volume of bilateral loading activities such as sprinting which may enhance proximal femur bone health bilaterally. The net result is enhancement of the denominator in calculations of side-to-side differences and a subsequent reduction in dominant-to-nondominant leg percent differences.
Our study had a number of strengths, including the use of a within-subject controlled model to control selection bias and minimize the impact of inherited and systemic factors, and the inclusion of a control group not exposed to unilaterally elevated loads to account for any normal crossed asymmetry. However, the study also possesses a number of limitations. Analyses were restricted to low-resolution, areal-based DXA outcomes which have known limitations when assessing the skeletal benefits of physical activity [46]. We did not assess hip muscle properties. Muscle and bone are intricately linked and the increased loading of the dominant leg in baseball and softball pitchers may be associated with increased muscle mass and strength. However, we did not observe any dominant-to-nondominant differences in DXA-derived lower extremity fat-free lean mass in either baseball (1.2%; 95% CI, −0.3 to 2.7%, p = 0.12) or softball (0.1%; 95% CI, −1.6 to 1.8%, p = 0.88) pitchers and, interestingly, the hip musculature in the nondominant leg of softball pitchers has actually been shown to be 6–7% stronger than in the dominant leg [43]. We also did not quantify loading during pitching or retrospective and current training volumes (including frequency, intensity, and volume of throwing) which may provide insight into the differences in adaptation between baseball and softball pitchers.
In summary, the current data demonstrate that male baseball and female fast-pitch softball pitchers are useful and efficient within-subject controlled models for exploring adaptation of the proximal femur to the mechanical loading associated with physical activity. Both athlete groups preferentially load their landing/dominant leg (i.e., leg contralateral to their throwing arm) during each pitch. Repeated many times over the course of an athletic career, the net result is enhanced proximal femur bone properties when compared to the contralateral drive/non-dominant leg (i.e., leg ipsilateral to their throwing arm). Interestingly, collegiate-level female softball pitchers exhibited greater adaptation in the femoral neck region compared to professional-level male baseball pitchers. Additional studies are required to explore the mechanism/s for this discrepancy and to fully establish the models’ utility in addressing deeper questions regarding mechanoadaptation at the clinically relevant proximal femur.
ACKNOWLEDGEMENTS
This contribution was made possible by support from the National Institutes of Health (R01 AR057740 and P30 AR072581). The authors have no conflicts of interest.
REFERENCES
- 1.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB (2009) Incidence and mortality of hip fractures in the United States. JAMA 302:1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyer SM, Crotty M, Fairhall N, Magaziner J, Beaupre LA, Cameron ID, Sherrington C, for the Fragility Fracture Network Rehabilitation Research Special Interest G (2016) A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr 16:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O’Karma M, Wallace TC, Zemel BS (2016) The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 27:1281–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ 3rd, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A (2005) Predictive value of BMD for hip and other fractures. J Bone Miner Res 20:1185–1194. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez CJ, Beaupre GS, Carter DR (2003) A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int 14:843–847. [DOI] [PubMed] [Google Scholar]
- 6.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA (1999) A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J Bone Miner Res 14:1672–1679. [DOI] [PubMed] [Google Scholar]
- 7.Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA (2011) Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res 26:1729–1739. [DOI] [PubMed] [Google Scholar]
- 8.Kannus P, Haapasalo H, Sankelo M, Sievänen H, Pasanen M, Heinonen A, Oja P, Vuori I (1995) Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med 123:27–31. [DOI] [PubMed] [Google Scholar]
- 9.Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S (2002) The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res 17:2274–2280. [DOI] [PubMed] [Google Scholar]
- 10.Ireland A, Maden-Wilkinson T, Ganse B, Degens H, Rittweger J (2014) Effects of age and starting age upon side asymmetry in the arms of veteran tennis players: a cross-sectional study. Osteoporos Int 25:1389–1400. [DOI] [PubMed] [Google Scholar]
- 11.Warden SJ, Mantila Roosa SM, Kersh ME, Hurd AL, Fleisig GS, Pandy MG, Fuchs RK (2014) Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci U S A 111:5337–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janz KF, Letuchy EM, Burns TL, Eichenberger Gilmore JM, Torner JC, Levy SM (2014) Objectively measured physical activity trajectories predict adolescent bone strength: Iowa Bone Development Study. Br J Sports Med 48:1032–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannus P, Haapasalo H, Sievanen H, Oja P, Vuori I (1994) The site-specific effects of long-term unilateral activity on bone mineral density and content. Bone 15:279–284. [DOI] [PubMed] [Google Scholar]
- 14.Warden SJ, Bogenschutz ED, Smith HD, Gutierrez AR (2009) Throwing induces substantial torsional adaptation within the midshaft humerus of male baseball players. Bone 45:931–941. [DOI] [PubMed] [Google Scholar]
- 15.Anliker E, Sonderegger A, Toigo M (2013) Side-to-side differences in the lower leg muscle-bone unit in male soccer players. Med Sci Sports Exerc 45:1545–1552. [DOI] [PubMed] [Google Scholar]
- 16.Chang G, Regatte RR, Schweitzer ME (2009) Olympic fencers: adaptations in cortical and trabecular bone determined by quantitative computed tomography. Osteoporos Int 20:779–785. [DOI] [PubMed] [Google Scholar]
- 17.Ireland A, Korhonen M, Heinonen A, Suominen H, Baur C, Stevens S, Degens H, Rittweger J (2011) Side-to-side differences in bone strength in master jumpers and sprinters. J Musculoskelet Neuronal Interact 11:298–305. [PubMed] [Google Scholar]
- 18.Weatherholt AM, Warden SJ (2016) Tibial bone strength is enhanced in the jump leg of collegiate-level jumping athletes: a within-subject controlled cross-sectional study. Calcif Tissue Int 98:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Ishizaki S, Kato Y, Kuroda Y, Fukashiro S (1998) The side-to-side differences of bone mass at proximal femur in female rhythmic sports gymnasts. J Bone Miner Res 13:900–906. [DOI] [PubMed] [Google Scholar]
- 20.Young KC, Sherk VD, Bemben DA (2011) Inter-limb musculoskeletal differences in competitive ten-pin bowlers: a preliminary analysis. J Musculoskelet Neuronal Interact 11:21–26. [PubMed] [Google Scholar]
- 21.Coh M, Supej M (2008) Biomechanical model of the take-off action in the high jump: a case study. New Stud Athlet 23:63–73. [Google Scholar]
- 22.Luhtanen P, Komi PV (1979) Mechanical power and segmental contribution to force impulses in long jump take-off. Eur J Appl Physiol Occup Physiol 41:267–274. [DOI] [PubMed] [Google Scholar]
- 23.Mero A, Komi PV (1986) Force-, EMG-, and elasticity-velocity relationships at submaximal, maximal and supramaximal running speeds in sprinters. Eur J Appl Physiol Occup Physiol 55:553–561. [DOI] [PubMed] [Google Scholar]
- 24.Kageyama M, Sugiyama T, Kanehisa H, Maeda A (2015) Difference between adolescent and collegiate baseball pitchers in the kinematics and kinetics of the lower limbs and trunk during pitching motion. J Sports Sci Med 14:246–255. [PMC free article] [PubMed] [Google Scholar]
- 25.MacWilliams BA, Choi T, Perezous MK, Chao EY, McFarland EG (1998) Characteristic ground-reaction forces in baseball pitching. Am J Sports Med 26:66–71. [DOI] [PubMed] [Google Scholar]
- 26.Werner SL, Guido JA, McNeice RP, Richardson JL, Delude NA, Stewart GW (2005) Biomechanics of youth windmill softball pitching. Am J Sports Med 33:552–560. [DOI] [PubMed] [Google Scholar]
- 27.Gümüştekin K, Akar S, Dane S, Yildirim M, Seven B, Varoglu E (2004) Handedness and bilateral femoral bone densities in men and women. Int J Neurosci 114:1533–1547. [DOI] [PubMed] [Google Scholar]
- 28.Hind K, Burrows M (2007) Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone 40:14–27. [DOI] [PubMed] [Google Scholar]
- 29.Tan VP, Macdonald HM, Kim S, Nettlefold L, Gabel L, Ashe MC, McKay HA (2014) Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Miner Res 29:2161–2181. [DOI] [PubMed] [Google Scholar]
- 30.Ducher G, Daly R, Bass S (2009) The effects of repetitive loading on bone mass and geometry in young male tennis players: a quantitative study using magnetic resonance imaging. J Bone Miner Res 24:1686–1692. [DOI] [PubMed] [Google Scholar]
- 31.Guido JA Jr., Werner SL (2012) Lower-extremity ground reaction forces in collegiate baseball pitchers. J Strength Cond Res 26:1782–1785. [DOI] [PubMed] [Google Scholar]
- 32.McNally MP, Borstad JD, Onate JA, Chaudhari AM (2015) Stride leg ground reaction forces predict throwing velocity in adult recreational baseball pitchers. J Strength Cond Res 29:2708–2715. [DOI] [PubMed] [Google Scholar]
- 33.Elliott B, Grove JR, Gibson B (1988) Timing of the lower limb drive and throwing limb movement in baseball pitching. Int J Sports Biomech 4:59–67. [Google Scholar]
- 34.Oyama S, Myers JB (2018) The relationship between the push off ground reaction force and ball speed in high school baseball pitchers. J Strength Cond Res 32:1324–1328. [DOI] [PubMed] [Google Scholar]
- 35.Chang JH, Tseng WM, Tseng JS, Huang SL (2008) Ground reaction force analysis of softball windmill pitch. In: Kwon YH, Shim J, Shim JK, & Shin IS (eds) International Conference on Biomechanics in Sport, p 685. [Google Scholar]
- 36.Huang CF, Wang LI, Chien CJ (2001) Characteristic ground reaction forces in softball pitching. In: Blackwell JR & Sanders RH (eds) International Symposium on Biomechanics in Sport, pp 104–107. [Google Scholar]
- 37.Guido JA Jr., Werner SL, Meister K (2009) Lower-extremity ground reaction forces in youth windmill softball pitchers. J Strength Cond Res 23:1873–1876. [DOI] [PubMed] [Google Scholar]
- 38.Oliver GD, Plummer H (2011) Ground reaction forces, kinematics, and muscle activations during the windmill softball pitch. J Sports Sci 29:1071–1077. [DOI] [PubMed] [Google Scholar]
- 39.Oliver GD, Plummer HA, Washington JK, Saper MG, Dugas JR, Andrews JR (2018) Pitching mechanics in female youth fastpitch softball. Int J Sports Phys Ther 13:493–500. [PMC free article] [PubMed] [Google Scholar]
- 40.Escamilla RF, Fleisig GS, Groeschner D, Akizuki K (2017) Biomechanical comparisons among fastball, slider, curveball, and changeup pitch types and between balls and strikes in professional baseball pitchers. Am J Sports Med 45:3358–3367. [DOI] [PubMed] [Google Scholar]
- 41.Escamilla RF, Fleisig GS, Zheng N, Barrentine SW, Andrews JR (2001) Kinematic comparisons of 1996 Olympic baseball pitchers. J Sports Sci 19:665–676. [DOI] [PubMed] [Google Scholar]
- 42.Fleisig GS, Barrentine SW, Zheng N, Escamilla RF, Andrews JR (1999) Kinematic and kinetic comparison of baseball pitching among various levels of development. J Biomech 32:1371–1375. [DOI] [PubMed] [Google Scholar]
- 43.Corben JS, Cerrone SA, Soviero JE, Kwiecien SY, Nicholas SJ, McHugh MP (2015) Performance demands in softball pitching: a comprehensive muscle fatigue study. Am J Sports Med 43:2035–2041. [DOI] [PubMed] [Google Scholar]
- 44.Skillington SA, Brophy RH, Wright RW, Smith MV (2017) Effect of pitching consecutive days in youth fast-pitch softball tournaments on objective shoulder strength and subjective shoulder symptoms. Am J Sports Med 45:1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith MV, Davis R, Brophy RH, Prather H, Garbutt J, Wright RW (2015) Prospective player-reported injuries in female youth fast-pitch softball players. Sports Health 7:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Järvinen TLN, Kannus P, Sievänen H (1999) Have the DXA-based exercise studies seriously underestimated the effects of mechanical loading on bone? J Bone Miner Res 14:1634–1635. [DOI] [PubMed] [Google Scholar]