Abstract
Diabetes mellitus is a highly prevalent metabolic disease affecting 29.1 million people or 9.3% of the population of the United States. The most prevalent form of diabetes is type 2 diabetes (T2D) which comprises 90–95% of all reported cases of diabetes. While the exact cause of T2D remains an enigma, known risk factors include age, weight, sedentary lifestyle, poor dietary habits, and genetic predisposition. However, these risk factors can not sufficiently explain the increasing prevalence of T2D. Recently, environmental exposures have been explored as potential risk factors. Indeed, epidemiological and limited empirical studies have revealed elevated serum concentrations of certain persistent organic pollutants (POPs), including the bioaccumulative metabolite of p,p′-dichlorodiphenyltrichloroethane (DDT), p,p′-dichlorodiphenyldichloroethylene (DDE), are positively correlated with increased T2D prevalence. The goal of the present study is to determine if chronic exposure to DDE promotes T2D in a widely used in vivo model, the high saturated fat-fed mouse. Male C57BL/6H mice were exposed to DDE (2.0 mg/kg) or vehicle (corn oil; 1 ml/kg) via gavage for 5 consecutive days, then every 7 days for the duration of the study. One week following the 5 day consecutive DDE dosing, animals were placed on either a low fat (10% kcal from lard) or high fat (45% kcal from lard) diet (HFD) for 13 weeks. Chronic exposure to DDE promoted fasting hyperglycemia after 4 and 8 weeks on the HFD diet and normalized fasting blood glucose levels at week 13. This DDE-mediated decrease in fasting hyperglycemia was preceded by improved glucose tolerance at week 12. In addition to normalizing fasting hyperglycemia at the end of high fat feeding, DDE exposure decreased HFD-induced fasting hyperinsulinemia, homeostasis model assessment of insulin resistance (HOMA-IR) values, and hepatic steatosis. Therefore, based on the current data, chronic DDE exposure appears to have a biphasic effect on HFD-induced hyperglycemia in the male C57BL/6H mouse characterized by elevated fasting blood glucose at weeks 4 and 8 of HFD intake followed by normoglycemia upon sacrifice. In addition, chronic DDE exposure reduced HFD-induced hepatic steatosis upon sacrifice. These results indicate chronic exposure to DDE can directly affect systemic glucose and hepatic lipid metabolism and that these effects can be diet dependent.
Keywords: persistent organic pollutants, organochlorine compounds, DDE, glucose, diabetes, C57BL/6 mice, hepatic steatosis
2. Introduction
One of the most pressing healthcare issues in the United States (U.S.) and globally is the growing obesity epidemic. According to the National Health and Nutrition Examination Survey (NHANES) conducted over the period of 2009–2010, 35.7% of adults over the age of 20 years were classified as obese (body mass index ≥ 30 kg/m2) and almost more alarmingly 16.9% of children and adolescents in the U.S. were classified as being obese (Flegal et al. 2012; Ogden et al. 2012). While obesity does not automatically make someone develop type 2 diabetes (T2D) and the concept of a metabolically healthy obese phenotype is becoming realized (Gregg et al. 2007; Wildman et al. 2008), being obese is a well-accepted risk factor for the development of other disease processes such as dyslipidemias and hypertension. While the prevalence of diabetes is not as high as the prevalence of obesity, in 2012 approximately 9.1% or 29.1 million people in the U.S. were reported to have either diagnosed or undiagnosed diabetes with approximately 90–95% of these cases of diabetes being T2D. T2D is a state of metabolic dysfunction characterized by fasting hyperglycemia and glucose intolerance due to the development of insulin resistance in the liver, skeletal muscle, and adipose tissue as well as eventual pancreatic decompensation.
While the etiology of T2D is an area of intensive investigation, there are known risk factors for the development of insulin resistance and subsequent T2D such as age, poor dietary habits, sedentary lifestyle, and genetic predisposition. Although these major risk factors for the development of T2D have been identified and well characterized, they appear to be insufficient in accounting for the increasing prevalence of T2D. Within the last 10–15 years, an environmental exposures component to the development of T2D has been proposed and has been actively investigated through epidemiological and limited laboratory based studies. Recent epidemiological studies indicate exposure to certain organochlorine (OC) pesticides, a major class of POPs, is positively associated with the development of insulin resistance, diabetes, and metabolic syndrome. Upon analysis of data from the NHANES from 1999–2002, Lee et al. (2007) reported that in obese individuals the development of insulin resistance is highly correlated with the level of OC pesticides or pesticide derivatives, namely oxychlordane and trans-nonachlor, present in the serum (Lee et al. 2007). However, when exploring the association between diabetes and serum concentration of POPs, a significant association between the prevalence of diabetes and serum concentrations of oxychlordane and DDE was observed (Lee et al. 2006). Interestingly, in this study, there was no association between obesity and diabetes in subjects with non-detectable levels of POPs (when all six categories of POPs were taken collectively). This observation suggests elevated serum concentration of POPs and not obesity promotes diabetes in these subjects. Additional studies by this group have indicated low dose exposure to POPs is also predictive of other metabolic abnormalities such as dyslipidemia and this exposure-response relationship appears to be non-monotonic in nature (Lee et al. 2011). In studies examining the prevalence of diabetes in Swedish men and women, there was a significant correlation between serum DDE and prevalence of diabetes (Rignell-Hydbom et al. 2007; Rylander et al. 2005). In addition to these studies, Turyk et al. (2009) determined in a cohort of Great Lakes sport fish consumers who were followed over a period of ten years from a healthy, non-diabetic state to clinical diabetes that DDE exposure was positively associated with the incidence of diabetes (Turyk et al. 2009a; Turyk et al. 2009b). Recent review of the epidemiological studies examining the association between POPs exposure and diabetes by a panel assembled by the National Toxicology Program determined the strongest positive associations between POPs exposures and diabetes existed for OC pesticides, especially DDE and trans-nonachlor, and polychlorinated biphenyls (PCBs) (Taylor et al. 2013).
While these epidemiological studies implicate exposure to OC pesticides, especially DDE, trans-nonachlor, and oxychlordane in the pathogenesis of T2D, they do not demonstrate causality. Recent animal studies have revealed exposure to an environmentally relevant mixture of OC compounds, including OC pesticides (DDE, trans-nonachlor, and oxychlordane among others with DDE being the most prevalent) and PCBs, in contaminated salmon oil promotes the development of T2D in high fat or western diet fed rodents (Ibrahim et al. 2011; Ruzzin et al. 2010). In these studies, animals exposed to contaminated salmon oil containing POPs in conjunction with a high fat diet (HFD) displayed fasting hyperglycemia, hyperinsulinemia, glucose intolerance, hypertriglyceridemia, and hepatic steatosis compared to animals fed HFD or western diet alone. This effect of contaminated salmon oil was eliminated upon refinement to decrease the concentration of POPs. These animal studies demonstrate exposure to a mixture of POPs, including OC pesticides, promotes the development of T2D and metabolic dysfunction. However, they do not delineate the contribution of individual OC pesticides or PCBs to the observed metabolic dysfunction.
Our recently published studies indicate subacute exposure to DDE alone, one of the most highly implicated POPs in the pathogenesis of T2D, causes mild to moderate transient fasting hyperglycemia that does not appear to be mediated by insulin resistance as indicated by glucose intolerance and defective insulin signaling in male C57BL/6H mice. However, it is not known if chronic exposure to DDE in concert with a known inducer of insulin resistance, high dietary saturated fat intake, will exacerbate diet-induced metabolic dysfunction. Therefore, based on our previous studies and those reported in the literature, the current study seeks to determine if chronic exposure to DDE promotes metabolic dysfunction characterized by insulin resistance, hyperglycemia, and dyslipidemia in a rodent model of T2D, the high fat-fed C57BL/6H mouse.
3. Materials and Methods
3.1 Chemicals
A stock of DDE was made prior to in vivo administration. DDE stock consisted of DDE (98% purity; Chem Service) dissolved in corn oil (Sigma Aldrich) at a concentration of 2 mg/ml. DDE stock was maintained at 4°C when not in use but was equilibrated to room temperature prior to administration. Solvents used for DDE extraction and D-glucose were obtained from Sigma Aldrich.
3.2 Animal Care
Male C57BL/6H mice were purchased from Harlan Laboratories at six weeks of age. Animals were individually housed in polycarbonate cages in an AAALAC-approved animal facility with a 12-hour light/dark cycle and access to food and water ad libitum unless they were being subjected to fasting blood glucose testing or glucose tolerance testing. Prior to being subjected to experimental manipulation, animals were allowed at least a five day acclimation period. The Mississippi State University Animal Care and Use Committee approved all animal protocols prior to implementation.
3.3 Experimental Design
To determine the effect of chronic exposure to DDE on the development of obesity and type 2 diabetes, male C57BL/6H mice (7 weeks of age; n = 30/group) were administered either vehicle (corn oil; 1 mL/kg) or DDE (2.0 mg/kg) via oral gavage daily for five consecutive days, then allowed to rest for 7 days. Our previous studies have demonstrated that this subacute treatment paradigm produces a sustained, mild to moderate fasting hyperglycemia (Howell et al. 2014). Thus, the current study was designed to determine if this mild to moderate effect of DDE on fasting hyperglycemia will be exacerbated by consumption of a HFD in conjunction with chronic DDE administration throughout diet intake and to determine if chronic exposure to DDE promotes dyslipidemia and hepatic steatosis, two pathophysiological alterations in lipid metabolism associated with T2D. At the end of the resting period, animals were randomly divided into one of four experimental groups (n = 15/group): 1. vehicle treatment with low fat diet (LFD), 2. DDE (2.0 mg/kg) with LFD, 3. vehicle with HFD, 4. DDE (2.0 mg/kg) with HFD. The LFD (Research Diets, D12450J) consisted of 10% of total kcal from lard whereas the HFD (Research Diets, D12451) consisted of 45% of total kcal from lard. Animals were allowed access to the experimental diet ad libitum for 13 weeks. During the experimental dietary period, animals were administered a single dose of either vehicle or DDE (2.0 mg/kg) via gavage each week for the 13 weeks of LFD or HFD consumption. Based on our previous studies, serum concentrations of DDE decrease dramatically over the course of 14 days following the cessation of subacute DDE administration (Howell et al. 2014). Thus, weekly administration of DDE was utilized to maintain elevated serum concentrations of DDE. Upon assignment to treatment groups, all animals were weighed to obtain an initial baseline value. Following this initial weighing, animals were weighed every 7 days to track weight gain or loss. Once started on the LFD or HFD, weekly food intake was assessed by weighing the amount of food added at the beginning of every week and the amount remaining at the end of the week (7 day period) and subtracting the remaining feed weights from the starting feed weights. Feed was completely replaced on a weekly basis.
3.4 Intraperitoneal glucose tolerance test (IPGTT)
To determine if DDE exposure altered systemic glucose tolerance, animals were subjected to an IPGTT, as previously described, after 12 weeks on the LFD or HFD with or without chronic DDE exposure (Howell et al. 2014). Briefly, animals were fasted for 6 hours prior to the start of the test. After the 6 hour fast, a basal blood glucose measurement was taken with a handheld glucometer (AlphaTrak; Bayer Animal Health) via a tail nick. Following basal blood glucose measurement, animals were injected intraperitoneally with 1 g/kg of glucose. Following glucose injection, blood glucose was measured at 15, 30, 45, 60, 90, and 120 minutes to track circulating glucose concentrations.
3.5 Measurement of systemic insulin resistance, serum adipokines, and serum lipids
The development of insulin resistance in all animals was determined using the homeostasis model assessment of insulin resistance (HOMA-IR) upon sacrifice following a 6 hour fast. Fasting blood glucose was measured immediately prior to sacrifice via handheld glucometer. HOMA-IR was calculated using the following formula: fasting serum insulin (μU/ml) x fasting blood glucose (mg/dL) / 405 (Berglund et al. 2008). Serum insulin (Mercodia Mouse Insulin ELISA) and adiponectin (SPI Bio Mouse Adiponectin) were measured by ELISA. Leptin, resistin, monocyte chemotractant protein-1 (MCP-1), tumor necrosis factor alpha (TNFα), and interleukin 6 (IL-6) were determined by multiplex immunoassay (Milliplex Mouse Adipokine 6-plex immunoassay) as previously performed (Howell and Mangum 2011; Howell et al. 2014). To determine if chronic DDE exposure promotes dyslipidemia, serum triglyceride (triglyceride colorimetric assay kit; Cayman Chemical), free fatty acid (Bioassay), and total cholesterol (Bioassay) concentrations were determined via commercially available assays per the manufacturer’s protocols.
3.6 Measurement of hepatic triglyceride and total cholesterol content
Hepatic levels of triglyceride and total cholesterol were measured as an index of hepatic steatosis. Both hepatic triglyceride (triglyceride colorimetric assay kit, Cayman Chemical) and total cholesterol (Bioassay) concentrations were determined with commercially available assays per the manufacturer’s protocols. Hepatic concentrations of both triglyceride and total cholesterol were normalized to total protein content per lysate and expressed as triglyceride (mg/dL) or cholesterol(umole) per milligram of protein.
3.7 Tissue and serum DDE measurement
Analysis of DDE in mouse serum, liver, and adipose tissue was performed by gas chromatography/mass spectrometry (GC/MS) following organic solvent extraction as previously reported by our lab (Howell et al. 2014). Slight modifications of the CDC method number 6105.01 (Accelerated Solvent Extraction; Isotope Dilution Mass Spectrometry) used in the NHANES studies were used for extraction and analysis. A targeted analysis of extracts using 13C-labeled standards was performed using the single ion monitoring (SIM) mode of the GC/MS (Agilent Technologies). This targeted analysis was used to quantify the levels of DDE. Analyte peaks acquired by SIM were quantified using Agilent ChemStation software. Our current limits of quantitation are 100 pg/L p,p′-DDE. Mean percent recoveries for DDE are higher than 85%. Areas under the curve were converted to ng/mL for serum and ng/g for liver and adipose utilizing a standard curve generated from these tissues spiked with five concentrations of DDE. In our previous study, DDE levels in serum, liver, and adipose tissue of vehicle treated animals was below the limit of detection, therefore DDE levels in only the DDE treated animals were measured.
3.8 Expression of genes involved in systemic glucose and hepatic lipid metabolism
To determine if chronic DDE exposure altered the expression of genes governing systemic insulin-induced glucose uptake, expression of glucose transporter-4 (Glut4) in gastrocnemius muscle and adipose tissue was assessed by real time PCR. To determine if chronic DDE exposure altered the expression of genes governing hepatic lipid and glucose metabolism, expression of sterol response element binding protein-1c (Srebp-1c), fatty acid synthase (Fasn), acetyl CoA carboxylase-1 (Acc-1), stearyl CoA desaturase-1 (Scd-1), fatty acid translocase (Cd36), carnitine palmitoyl transferase-1A (Cpt-1A), acyl CoA oxidase-1 (Acox-1), phosphoenolpyruvate carboxykinase (Pepck), and glucose-6-phosphatase (G6Pase) was assessed in the liver by real time PCR using the ΔΔCt method as previously described with minor modifications (Elam et al. 2009; Howell et al. 2009; Howell and Mangum 2011). Briefly, total RNA (PureLink Total RNA Isolation kit; Ambion) was isolated, cDNA synthesized (Verso cDNA Synthesis kit; Thermo Scientific) from 1 μg of total RNA, and subjected to real time PCR with Sybr Green detection (Sybr Select Master Mix; Life Technologies). All primer pairs were designed with Primer-BLAST software and melting curve analysis performed to detect non-specific amplification. Data are expressed as the fold change from average of vehicle treated, LFD fed animals.
3.9 Immunoblot analysis of Glut4 protein levels
Alterations in Glut4 protein levels in tissues governing insulin induced systemic glucose uptake, the skeletal muscle and the adipose tissue, were determined by SDS-PAGE followed by Western blot analysis as previously described with minor modifications (Howell et al. 2014). Briefly, gastrocnemius muscle and adipose tissue samples (~50 mg each; n=6–8) from vehicle (1 ml/kg) or DDE (2.0 mg/kg) treated animals on LFD or HFD were lysed with RIPA buffer. Protein concentrations were determined (Bradford protein assay; Bio-Rad) and lysates (50 μg protein per well) were subjected to SDS-PAGE using a 4–15% polyacrylamide gel. Proteins were transferred to a PVDF membrane, blocked, and then incubated with mouse anti-Glut4 primary antibodies (Millipore). Mouse anti-tubulin immunoreactivity was used as a loading control for each lane. Following incubation with appropriate HRP labelled secondary antibodies, proteins were visualized with Immobilon chemiluminescent reagent (Millipore) and digital images captured with the ChemiDoc XRS+ imaging system (Bio-Rad). The integrated density of each band was determined using ImageJ (National Institutes of Health) image analysis software. Data are expressed as Glut4 integrated density normalized to tubulin integrated density.
3.10 Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM) for each treatment group or biochemical measurement. To determine statistically significant differences in fasting blood glucose at weeks 0, 4, 8, or 13 of LFD or HFD intake or in weekly body weights and food intake, two-way analysis of variance (ANOVA) with a Tukey’s post hoc test for pairwise comparisons was utilized. Likewise, a two-way ANOVA was used to determine statistically significant differences between groups following sacrifice when physical and biochemical parameters were compared. It should be noted, to determine significant differences in gene expression, statistical analyses were performed on ΔCt values and not fold change values. When comparing DDE levels in serum, liver, or adipose of LFD and HFD fed animals, a Student’s t-test was utilized. A P-value of ≤ 0.05 was used to indicate a statistically significant difference between groups.
4. Results
4.1 Chronic exposure to DDE has a biphasic effect on fasting hyperglycemia
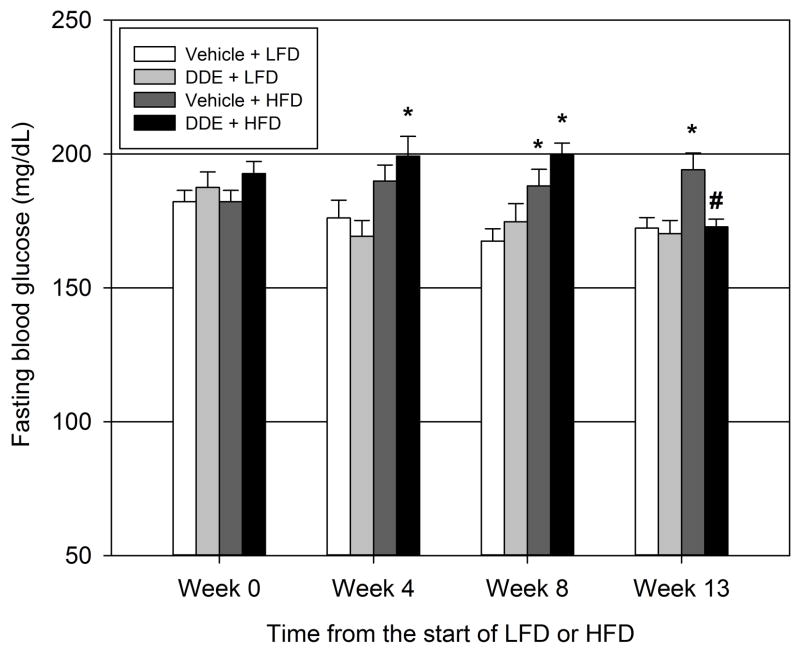
To determine if chronic DDE exposure promotes fasting hyperglycemia in a rodent model of T2D, animals were exposed to DDE for five consecutive days followed by exposures every 7 days for the duration of LFD or HFD intake with fasting blood glucose measurements taken at week 0 (beginning of LFD or HFD), week 4, week 8, or at sacrifice on week 13 (Figure 1). At 4 and 8 weeks from the start of the experimental diet, chronic exposure to DDE in conjunction with HFD produced a mild to moderate, yet significant fasting hyperglycemia when compared to vehicle treated LFD animals at 4 weeks (13.1% increase) and 8 weeks (19.1% increase). Vehicle treated HFD animals were hyperglycemic compared to vehicle treated LFD animals at 8 weeks (12.3% increase) and 13 weeks (12.7% increase). Interestingly, at week 13 from the start of the diet, chronic exposure to DDE in conjunction with HFD significantly decreased (12.4% decrease) fasting blood glucose concentrations when compared to vehicle treated animals on the same diet. Thus, exposure to DDE in conjunction with HFD promoted fasting hyperglycemia at weeks 4 and 8 from the start of the experimental diet but at week 13 animals that were chronically exposed to DDE in conjunction with HFD were normoglycemic compared to vehicle treated LFD fed animals.
Figure 1.
Biphasic effect of DDE exposure on HFD-induced hyperglycemia. Animals were exposed to vehicle (1 ml/kg) or DDE (2.0 mg/kg) for five consecutive days then every 7 days throughout consumption of a LFD or HFD. Fasting blood glucose measurements were taken at the start of the diet (Week 0; 9 weeks of age), at the ends of Weeks 4 (13 weeks of age) and 8 (17 weeks of age) on the diet, and at sacrifice (Week 13; 22 weeks of age). Data represent the mean ± SEM of each group (n=14–15/group) and were analyzed by two-way ANOVA with a Tukey’s post hoc test. * P≤0.05 vs. time matched vehicle + LFD group, # P≤0.05 vs. time matched vehicle + HFD group
4.2 Chronic exposure to DDE decreases HFD-induced glucose intolerance
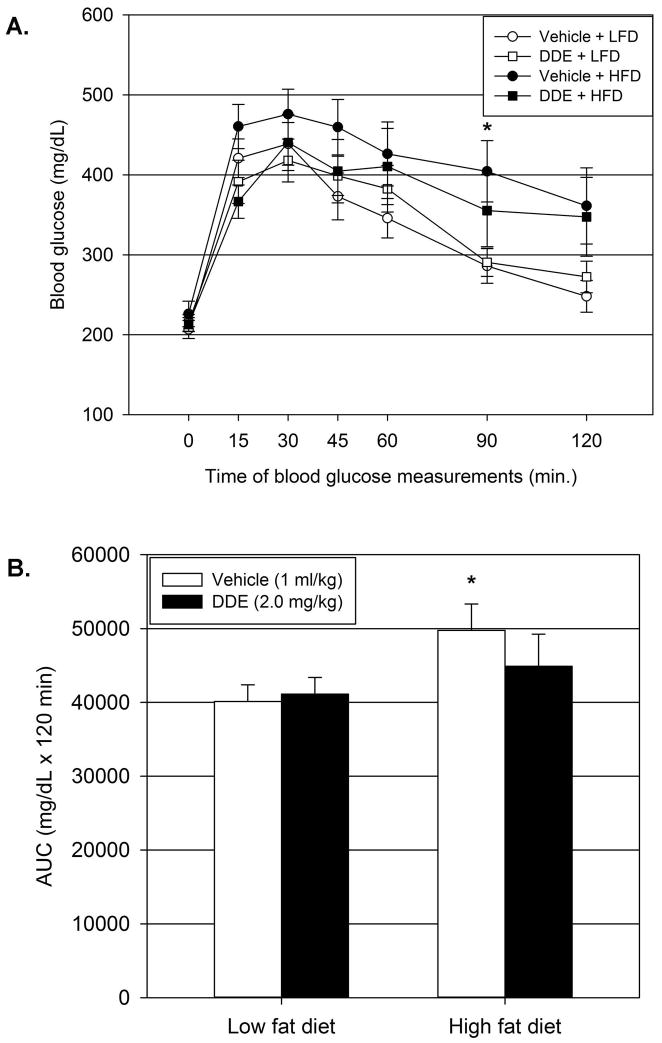
Following 12 weeks of DDE exposure in conjunction with LFD or HFD intake, animals were subjected to an IPGTT to assess glucose tolerance. Maximal elevations in blood glucose concentrations in all groups were reached 30 minutes following glucose injection (Figure 2.A.). As expected, vehicle treated animals fed a HFD had impaired glucose tolerance as indicated by an elevated glucose tolerance curve compared to vehicle treated LFD animals with significant elevations at 90 minutes following glucose injection (Figure 2.A.) and by a significantly elevated area under the curve (AUC; Figure 2.B.) in vehicle treated HFD animals compared to vehicle treated LFD animals (24% increase in AUC). Chronic DDE exposure resulted in a blunting of HFD induced glucose intolerance as indicated by a lower glucose tolerance curve compared to vehicle treated HFD animals and a reduced AUC that was not significantly elevated compared to vehicle treated LFD animals.
Figure 2.
Chronic DDE exposure increases glucose tolerance after 12 weeks on HFD. At the end of twelve weeks of LFD or HFD consumption with chronic DDE exposure, animals (21 weeks of age) were subjected to an IPGTT to assess glucose tolerance. Blood glucose measurements (2.A) at the indicated time following IP glucose (1g/kg) administration. AUC measurements (2.B) for each blood glucose curve. Data represent the mean ± SEM per group (n=13/group) and were analyzed by two-way ANOVA with a Tukey’s post hoc test. * P≤0.05 vs. vehicle + LFD group
4.3 Chronic DDE exposure decreases HFD-induced hyperinsulinemia and insulin resistance
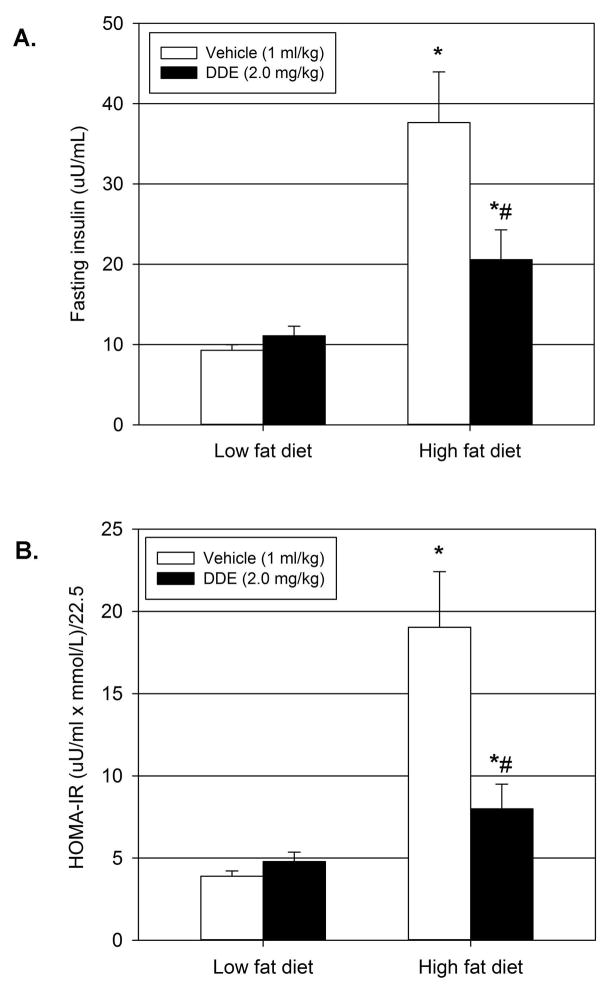
Prolonged exposure (12–13 weeks) to DDE in conjunction with HFD resulted in a reduction of HFD-induced fasting hyperglycemia and glucose intolerance. To determine if these reductions were a result of decreased insulin resistance, fasting serum insulin levels (Figure 3.A.) and HOMA-IR (Figure 3.B.) were calculated following sacrifice after 13 weeks of vehicle or DDE exposure in concert with LFD or HFD. As expected, animals treated with vehicle fed a HFD for 13 weeks were hyperinsulinemic (305.7% increase) and had significantly elevated HOMA-IR values (386.9% increase) compared to animals treated with vehicle and fed a LFD. These data indicate HFD-induced fasting hyperglycemia is due to insulin resistance as illustrated by hyperinsulinemia and elevated HOMA-IR, an index of systemic insulin resistance. Following 13 weeks of chronic DDE exposure in addition to LFD or HFD, fasting serum insulin levels and HOMA-IR values following chronic DDE exposure in conjunction with HFD were significantly elevated compared to vehicle treatment in LFD fed animals but were significantly decreased compared to vehicle treated HFD fed animals.
Figure 3.
Decreased hyperinsulinemia and homeostasis model assessment of insulin resistance (HOMA-IR) following chronic DDE exposure with HFD. Upon sacrifice (22 weeks of age), fasting serum insulin (3.A) was measured and HOMA-IR values (3.B) were calculated. Data represent the mean ± SEM (n=15/group) of each group and were analyzed by two-way ANOVA with a Tukey’s post hoc test. * P≤0.05 vs. vehicle + LFD group, # P≤0.05 vs. vehicle + HFD group
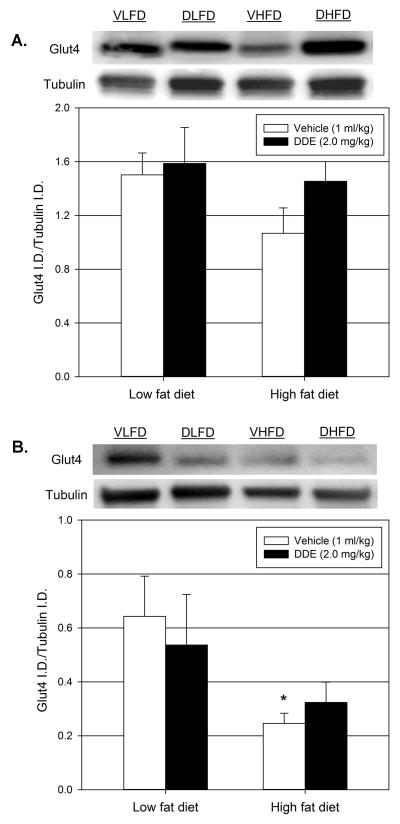
4.4 Chronic DDE exposure differentially effects HFD-induced alterations in Glut4 mRNA expression and protein production in skeletal muscle and adipose tissue
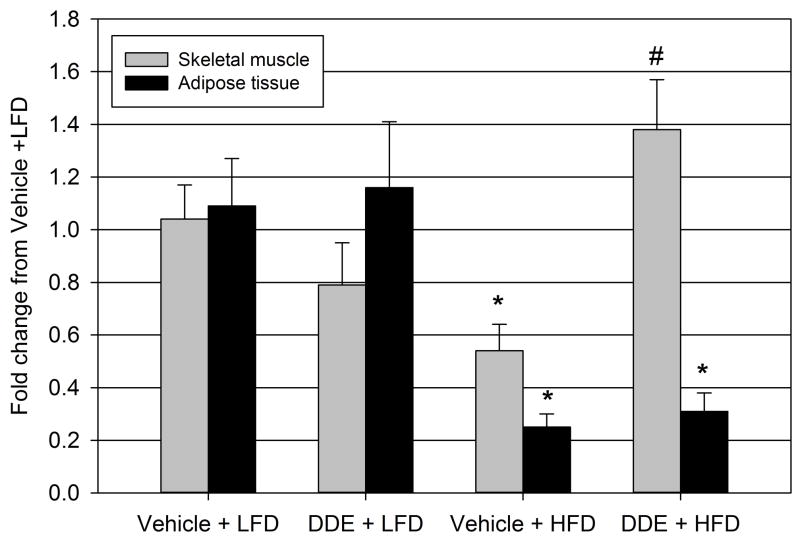
The effect of DDE exposure on the expression of Glut4 transporter in the primary tissues governing insulin stimulated glucose uptake, the skeletal muscle and adipose tissue, was explored by real time PCR (Figure 4). Prolonged administration of vehicle in conjunction with HFD (vehicle + HFD) significantly decreased the expression of Glut4 in both skeletal muscle and adipose tissue when compared to the vehicle treated LFD (vehicle + LFD) animals for each tissue. In the adipose tissue, chronic exposure to DDE along with the HFD (DDE + HFD) did not significantly affect Glut4 expression when compared to the vehicle + HFD group. However, the chronic exposure to DDE along with HFD did significantly increase skeletal muscle Glut4 expression in the DDE + HFD group compared to vehicle + HFD group.
Figure 4.
Effect of diet and chronic DDE exposure on skeletal muscle and adipose tissue Glut4 expression. Following sacrifice (22 weeks of age), expression of Glut4 was determined by real-time PCR in the gastrocnemius muscle (skeletal) and epididymal fat pads (adipose tissue). Data represent the mean ± SEM of the fold change from the average of the vehicle + LFD group for each group (n=5–6/group) in the appropriate tissue. Statistically significant differences between groups were determined from the ΔCt values for each group by two-way ANOVA with a Tukey’s post hoc test. * P≤0.05 vs. vehicle + LFD group, # P≤0.05 vs. vehicle + HFD group
To determine if the observed alterations in Glut4 mRNA expression corresponded with changes in Glut4 protein production, Glut4 protein production in the skeletal muscle (Figure 5.A.) and the adipose tissue (Figure 5.B.) was assessed via Western blot. The Glut4/tubulin ratios for skeletal muscle and adipose tissue decreased in the vehicle + HFD groups compared to the vehicle + LFD groups, however, these decreases were only statistically significant in the adipose tissue. In the skeletal muscle, the Glut4 levels are similar in the DDE + HFD group compared to the vehicle + LFD group.
Figure 5.
Effect of diet and chronic DDE exposure on skeletal muscle and adipose tissue Glut4 protein levels. Following sacrifice (22 weeks of age), Glut4 protein levels were determined by Western blot in the gastrocnemius muscle (5.A.) and epididymal fat pads (5.B.). Data represent the mean ± SEM of the Glut4/tubulin ratio for each group (n=6–8/group). Representative western blots are shown above each graph. VLFD = vehicle + LFD, DLFD = DDE + LFD, VHFD = vehicle + HFD, DHFD = DDE + HFD. Statistically significant differences between groups were determined by two-way ANOVA with a Tukey’s post hoc test. * P≤0.05 vs. vehicle + LFD group
4.5 Effect of chronic DDE administration and on serum lipids and adipokines
The effect of chronic DDE administration in concert with HFD on serum biochemical indices normally altered during T2D was examined (Table 1). With regards to serum lipids, vehicle + HFD and DDE + HFD groups had significantly lower levels of circulating triglyceride compared to the vehicle + LFD group. A similar effect was observed following measurement of serum free fatty acids. Free fatty acid levels in vehicle + HFD and DDE + HFD groups were decreased compared to vehicle + LFD with the DDE + HFD group being statistically significant. There were no statistically significant differences in serum total cholesterol levels between any of the treatment groups.
Table 1.
Serum lipid and adipokine concentrations following chronic DDE exposure with HFD. Following sacrifice (22 weeks of age), serum total cholesterol, triglyceride, and free fatty acids were measured to determine effects of DDE and HFD on serum lipid profiles. Adipokines including leptin, resistin, and adiponectin were measured to determine if DDE exposure altered adipokine release. Data represent the mean ± SEM per group (n=13–15/group) and were analyzed by two-way ANOVA with a Tukey’s post hoc test.
| Analyte | Vehicle + LFD | DDE + LFD | Vehicle + HFD | DDE + HFD |
|---|---|---|---|---|
| Cholesterol (mg/dL) | 36.0 ± 5.6 | 35.7 ± 5.7 | 35.8 ± 6.3 | 41.4 ± 6.6 |
| Triglyceride (mg/dL) | 88.5 ± 3.6 | 89.8 ± 2.5 | 72.1 ± 4.5 * | 70.5 ± 3.2 * |
| Free fatty acid (uM) | 906.9 ± 33.6 | 868.3 ± 25.6 | 800.8 ± 25.0 | 743.7 ± 43.3 * |
| Leptin (pg/mL) | 6579.6 ± 752 | 5647.7 ± 1012 | 8544.7 ± 1771 | 8624.2 ± 1473 |
| Resistin (pg/mL) | 8554.4 ± 761 | 7305.5 ± 858 | 10704.2 ± 1460 | 9470.5 ± 906 |
| Adiponectin (ng/mL) | 2.0 ± 0.03 | 1.9 ± 0.06 | 1.82 ± 0.04 * | 1.74 ± 0.04 * |
P≤0.05 vs. vehicle + LFD group
The effect of chronic DDE exposure on circulating adipokines was determined by measuring leptin, resistin, TNFα, IL-6, and MCP-1 via multiplex immunoassay and measuring adiponectin by traditional ELISA. Adiponectin levels were significantly decreased in both the vehicle + HFD and the DDE + HFD groups compared to the vehicle + LFD group with no significant differences due to DDE exposure in the HFD groups. Leptin and resistin were elevated in the vehicle + HFD and DDE + HFD groups compared to the vehicle + LFD group although these differences did not reach statistical significance. Serum TNFα, IL-6, and MCP-1 levels were below the limit of detection of the multiplex immunoassay in all treatment groups (data not shown).
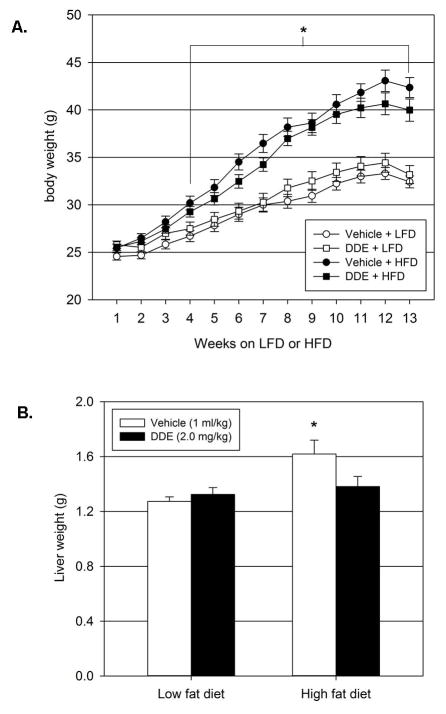
4.6 Chronic exposure to DDE does not affect body weight but decreases elevated liver weight following HFD
Animals consuming the HFD had significantly elevated body weights compared to animals on the LFD (Figure 6.A). This increase in body weight became significant after 4 weeks on the HFD and persisted for the duration of the experiment for both vehicle and DDE treated animals when compared to animals on the LFD that were treated with vehicle. This increase in body weight does not appear to be related to increased food intake. Consumption of the HFD caused a significant increase in food intake and DDE exposure significantly decreased HFD-induced hyperphagia for the first 7 weeks of HFD consumption (Supplementary Figure 1). After 7 weeks, HFD intake in the vehicle group was comparable to LFD intake in the corresponding vehicle group. There were no significant differences in body weight between vehicle and DDE treated animals in the HFD or LFD groups. Upon sacrifice, animals in the vehicle + HFD group had significantly higher liver weights compared to animals in the vehicle + LFD group (Figure 6.B.). Exposure to DDE in concert with HFD intake resulted in decreased liver weights compared to vehicle treated animals consuming the HFD. The liver weights of the DDE + HFD animals were not significantly elevated when compared to the vehicle + LFD animals. Thus, chronic DDE exposure blunted HFD-induced hepatic weight gain but did not affect whole body HFD-induced weight gain.
Figure 6.
Effect of chronic DDE exposure on body weight and liver weight. Weekly body weights (6.A.) were recorded to determine if DDE exposure alters body weight of animals on LFD or HFD. Upon sacrifice following 13 weeks of LFD or HFD intake (22 weeks of age), livers were removed and weighed to determine if DDE exposure alters liver weight of animals on LFD or HFD. Data represent the mean ± SEM of each group (n=15/group) and were analyzed by two-way ANOVA with a Tukey’s post hoc test. * P≤0.05 vs. vehicle + LFD group at the corresponding time point.
4.7 Chronic exposure to DDE alleviates HFD-induced hepatic steatosis
To determine if HFD-induced elevations in liver weight were due to hepatic steatosis, hepatic accumulation of both triglyceride and cholesterol were measured. The increase in raw liver weight in the vehicle + HFD group compared to the vehicle + LFD group and subsequent decrease in liver weight following chronic DDE administration was accompanied by corresponding alterations in normalized (mg/dL triglyceride per mg protein) hepatic triglyceride levels (Figure 7.A) and normalized (μmole cholesterol per mg protein) total cholesterol levels (Figure 7.B.). Animals in the vehicle + HFD group had significantly elevated normalized triglyceride levels compared to animals in all other groups including the vehicle + LFD (80.2% increase) and the DDE + HFD (54.0% increase) groups. Animals in the vehicle + HFD group had significantly elevated normalized cholesterol levels compared to animals in all other groups including the vehicle + LFD (63.9% increase) and the DDE + HFD (50.3% increase) groups. Thus, the DDE mediated decrease in liver weight appears to be due, at least in part, to decreases in hepatic triglyceride and cholesterol levels in the HFD group.
Figure 7.
Decreased hepatic triglyceride and cholesterol content following chronic DDE exposure with HFD. Following sacrifice (22 weeks of age), hepatic triglyceride (7.A.) and total cholesterol (7.B.) concentrations were determined, normalized to hepatic protein content, and expressed as normalized triglyceride (TG/mg protein) or normalized cholesterol (μmole cholesterol/mg protein). Data represent the mean ± SEM of each group (n=10/group) and were analyzed by two-way ANOVA with a Tukey’s post hoc test. * P≤0.05 vs. vehicle + LFD group, # P≤0.05 vs. vehicle + HFD group
4.8 Effects of diet and chronic DDE exposure on expression of genes involved in hepatic lipid and glucose metabolism
The effect of chronic DDE exposure on diet-induced alterations in the expression of genes involved in hepatic lipid metabolism including de novo lipogenesis (Srebp-1c, Fasn, Acc-1, and Scd-1), fatty acid oxidation (Cpt-1α and Acox-1), and fatty acid uptake (Cd36) as well as hepatic glucose production (Pepck and G6pase) were determined as possible mechanisms for DDE-mediated reductions in diet-induced hepatic steatosis and hyperglycemia (Table 2). Animals in the vehicle + HFD and DDE + HFD groups had significantly reduced expression of Acc-1 and Scd-1 compared to animals in the vehicle + LFD group. Expression of Fasn was reduced in the vehicle + HFD group and significantly reduced in the DDE + HFD compared to the vehicle + LFD animals. Interestingly, there was no change in the expression of Srepb-1c, the master transcriptional regulator of de novo lipogenesis. Expression of Acox-1, the rate-limiting enzyme in peroxisomal fatty acid oxidation, was significantly increased in the DDE + HFD compared to the vehicle + LFD and the vehicle ± HFD groups. Expression of Cd36 was significantly increased in the vehicle + HFD group compared to the vehicle + LFD group and this increase was significantly blunted by chronic DDE exposure. As expected, consumption of the HFD in vehicle treated animals increased the fasting expression of both Pepck and G6Pase compared to vehicle treated LFD animals with the increase in Pepck being statistically significant. These diet-induced increases were blunted by chronic exposure to DDE. Pepck expression was significantly decreased in the DDE + HFD group compared to the vehicle + HFD group whereas G6Pase expression in the DDE + HFD was decreased compared to the vehicle + HFD group but did not reach statistical significance.
Table 2.
Effect of diet and chronic DDE exposure on genes involved in hepatic lipid and glucose metabolism. Following sacrifice (22 weeks of age), the effect of HFD and chronic DDE exposure was determined on genes governing hepatic de novo lipogenesis, fatty acid oxidation, fatty acid uptake, and gluconeogenesis by real time PCR following sacrifice. Data represent the mean ± SEM of the fold change from the vehicle + LFD group for each group (n=5–6/group) in the appropriate tissue. Statistically significant differences between groups were determined from the ΔCt values for each group by two-way ANOVA with a Tukey’s post hoc test.
| Function | Gene | Vehicle + LFD | DDE + LFD | Vehicle + HFD | DDE + HFD |
|---|---|---|---|---|---|
| Lipogenesis | Srebp-1c | 1.04 ± 0.13 | 1.23 ± 0.11 | 1.05 ± 0.13 | 1.20 ± 0.05 |
| Fas | 1.09 ± 0.21 | 1.03 ± 0.13 | 0.63 ± 0.12 | 0.48 ± 0.09 * | |
| Acc-1 | 1.08 ± 0.21 | 0.99 ± 0.13 | 0.60 ± 0.05 * | 0.57 ± 0.08 * | |
| Scd-1 | 1.03 ± 0.12 | 0.65 ± 0.13 * | 0.49 ± 0.04* | 0.27 ± 0.05 *# | |
| FA oxidation | Cpt-1a | 1.09 ± 0.19 | 0.83 ± 0.15 | 0.85 ± 0.09 | 1.12 ± 0.23 |
| Acox | 1.04 ± 0.06 | 0.94 ± 0.08 | 1.03 ± 0.09 | 1.63 ± 0.17 *# | |
| FA uptake | Cd36 | 1.14 ± 0.26 | 0.78 ± 0.29 | 2.38 ± 0.45 * | 1.11 ± 0.13 # |
| Glucose production | Pepck | 1.03 ± 0.12 | 1.22 ± 0.12 | 1.78 ± 0.35 * | 0.94 ± 0.09 # |
| G6pase | 1.07 ± 0.19 | 1.27 ± 0.21 | 1.83 ± 0.39 | 1.18 ± 0.22 |
P≤0.05 vs. vehicle + LFD group,
P≤0.05 vs. vehicle + HFD group
4.9 Effect of diet on serum and tissue concentrations of DDE
Serum, liver, and adipose concentrations of DDE were measured following sacrifice to determine if HFD intake altered storage in these depots. Animals which consumed HFD had significantly elevated serum (67.5% increase), liver (52.4% increase), and adipose (48.6% increase) concentrations of DDE when compared to animals on the LFD (Table 3). Thus, based on the current data, it appears consumption of a HFD along with chronic DDE exposure results in a higher body burden of DDE compared to consumption of a LFD during chronic DDE exposure.
Table 3.
Tissue concentrations of DDE following chronic exposure and LFD or HFD intake. Serum, liver, and adipose tissue (epididymal fat pad) DDE concentrations were measured by GC/MS following sacrifice (22 weeks of age) as previously described. Values are expressed as wet weights. Data represent the mean ± SEM for 3–4 pooled samples for each tissue depot with 2–3 animals per pooling. Data were analyzed by Student’s t-test for comparison of DDE levels between LFD and HFD groups.
| Tissue depot | Low fat diet | High fat diet |
|---|---|---|
| Serum (ng/mL) | 795.6 ± 27.1 | 1333.2 ± 76.8 * |
| Liver (ng/g) | 61.6 ± 2.4 | 93.9 ± 6.4 * |
| Adipose (ng/g) | 60,586.9 ± 888.1 | 90,031.8 ± 8849.2 * |
P≤0.05 vs LFD group.
5. Discussion
Our previous studies demonstrate subacute exposure to DDE (2.0 mg/kg) for 5 consecutive days produced a mild/moderate fasting hyperglycemia for up to 21 days following cessation of administration in C57BL/6H mice consuming normal rodent chow (Howell et al. 2014). Therefore, in the present study we examined the effect of chronic exposure to DDE on the development of HFD-induced T2D. Specifically, the effects of chronic DDE exposure on HFD-induced weight gain, hyperglycemia, glucose tolerance, hyperinsulinemia, dyslipidemia, and hepatic steatosis were explored in male C57BL/6H mice. The current data indicate exposure to DDE has a biphasic effect on fasting blood glucose where chronic exposure initially facilitates HFD-induced hyperglycemia followed by DDE-mediated reductions in fasting blood glucose at sacrifice. In addition, chronic DDE exposure decreased HFD-induced fasting hyperinsulinemia, HOMA-IR values, and hepatic steatosis as indicated by increased hepatic triglyceride and cholesterol levels at sacrifice following 13 weeks of HFD intake. Unfortunately, based on the current data, we cannot determine if these effects were biphasic over the course of the HFD intake like the observed effect on fasting hyperglycemia.
In the present study, chronic exposure to DDE in conjunction with HFD promotes fasting hyperglycemia at weeks 4 and 8 from the start of the diet when compared to vehicle treated LFD animals at the corresponding time points. These data are in agreement with our previous studies indicating subacute exposure to DDE for 5 days, as utilized in the current study prior to the start of LFD or HFD along with weekly DDE (2.0 mg/kg) maintenance dosing for chronic exposure, caused a mild to moderate fasting hyperglycemia for up to 21 days following cessation of DDE exposure (Howell et al. 2014). Interestingly, the current hyperglycemia effect of DDE at weeks 4 and 8 was restricted to the HFD group and thus appears to be diet specific. This type of diet specific effect has been observed in studies with other POPs, including PCB-153, where chronic exposure promoted HFD-induced weight gain and hepatic steatosis (Wahlang et al. 2013). In addition, studies by Ibrahim et al. (2011) determined exposure to an environmentally relevant concentration of POPs in contaminated salmon oil in conjunction with a high saturated fat diet for 8 weeks or a high saturated fat/high carbohydrate “Western” diet for 6 weeks promoted fasting hyperglycemia and glucose intolerance in male C57BL/6 mice (Ibrahim et al. 2011). However, it is unclear whether this effect of contaminated salmon oil was diet dependent because it was only administered to the HFD group and not the low fat or control diet groups.
While the current fasting blood glucose data indicate DDE exposure in conjunction with a HFD promotes hyperglycemia at weeks 4 and 8, more prolonged exposures to DDE appear to correct most of the pathophysiological alterations, including fasting hyperglycemia, glucose intolerance, insulin resistance, and hepatic steatosis, associated with HFD-induced T2D at 12–13 weeks of concomitant DDE exposure and HFD intake with the exception of increased body weight (Figure 6). After 12 weeks of vehicle exposure plus HFD intake we observed an elevation in the IPGTT curve compared to vehicle exposure plus LFD intake. Indeed, when AUC for these curves were calculated, the vehicle treated HFD group had a significantly higher AUC compared to the corresponding LFD group indicating HFD-induced impairment of glucose tolerance and the development of insulin resistance (Ayala et al. 2010; Berglund et al. 2008). Chronic exposure to DDE in conjunction with HFD intake (≥12 weeks) appeared to decrease glucose intolerance indicated by a slightly lower IPGTT curve and corresponding AUC compared to vehicle treated HFD animals. However, DDE exposure along with HFD did appear to produce mildly impaired glucose homeostasis indicated by an elevated IPGTT curve and corresponding AUC compared to vehicle treated animals on a LFD. Thus, after 12 weeks on the LFD or HFD, vehicle treated animals appeared to have mild/moderate glucose intolerance as evident by abnormal IPGTT and chronic DDE exposure in conjunction with HFD appeared to improve glucose tolerance. Given this finding, animals were fasted then sacrificed one week later, after 13 weeks on LFD or HFD with or without chronic DDE exposure.
Our current data indicate exposure to DDE decreases the initial hyperphagia observed in animals consuming a HFD (Supplementary Figure 1) without affecting HFD-induced weight gain (Figure 6.A) and that this hyperphagia normalizes after 7 weeks in vehicle treated animals. Thus, although DDE exposure initially decreases HFD intake, it does not appear to significantly affect HFD-induced weight gain. Previous studies have demonstrated that this HFD-induced hyperphagia may not be directly associated with increased weight gain (for review see (West and York 1998)). Therefore, it does not appear that decreased food intake or decreased body weight is responsible for DDE mediated reductions in HFD-induced hyperglycemia, hyperinsulinemia, insulin resistance, and hepatic steatosis upon sacrifice. However, since DDE decreased the initial HFD-induced hyperphagia, we cannot rule out the possibility that this increased HFD consumption altered systemic physiology to promote metabolic dysfunction or insulin resistance which was manifested as hyperglycemia, hyperinsulinemia, and hepatic steatosis following 13 weeks of HFD consumption. Interestingly, the DDE-induced hyperglycemia at 4 and 8 weeks in animals on the HFD does not appear to be related to increased HFD intake or increased weight gain. The possibility arises DDE exposure may promote HFD-induced insulin resistance at 4 and 8 weeks of HFD consumption, but based on the current data we cannot determine if insulin resistance mediates the observed hyperglycemic effect at these time points.
To examine possible mechanisms through which DDE reduced HFD-induced hyperglycemia, we determined the effect of DDE and HFD on expression and subsequent production of Glut4, the major insulin sensitive glucose transporter in skeletal muscle and adipose tissue, as well as the hepatic expression of the gluconeogenic genes Pepck and G6pase (Hanson and Garber 1972; Kahn 1992). High fat feeding significantly decreased Glut4 expression in skeletal muscle and adipose tissue following 13 weeks of HFD intake. This decreased expression was reversed by chronic exposure to DDE in concert with HFD in the skeletal muscle. Alterations in Glut4 protein levels in the skeletal muscle followed the same trend as Glut4 expression however, the HFD-induced reduction in Glut4 protein was not statistically significant. Glut4 protein levels in the adipose were significantly reduced following HFD intake and this reduced protein production was not fully reversed by concomitant DDE exposure. These HFD-mediated reductions in skeletal muscle and adipose Glut4 expression/production are similar to those reported by Rossmeisl et al. (2003) following 8 weeks of HFD in C57BL/6 mice (Rossmeisl et al. 2003). Thus, these data indicate chronic DDE exposure may prevent HFD-induced reductions in Glut4 expression/production in skeletal muscle. Increased Glut4 levels in the skeletal muscle may facilitate increased insulin-stimulated glucose disposal and decrease glucose intolerance as observed in our IPGTT and decrease fasting hyperglycemia as observed following 13 weeks of HFD intake. While chronic DDE exposure in conjunction with HFD increased Glut4 expression/production in skeletal muscle, chronic DDE exposure also reduced HFD-induced increases in the expression of the gluconeogenic genes Pepck and G6pase. Taken together, the current data indicate chronic DDE exposure reduces HFD-induced alterations in hepatic glucose production as well as systemic glucose uptake.
Abnormal lipid metabolism characterized by dyslipidemia and hepatic steatosis is a common pathophysiological alteration associated with T2D and the associated metabolic dysfunction. To determine if chronic DDE exposure promoted abnormal lipid metabolism, we measured fasting serum and hepatic triglyceride and total cholesterol levels as well as serum free fatty acids following LFD or HFD consumption. Interestingly, HFD intake reduced fasting serum triglyceride and free fatty acid concentrations at week 13 which is contradictory to the dyslipidemia observed in human type 2 diabetics. The reduction in serum triglyceride content may be the result of decreased hepatic de novo lipogenesis as indicated by significant reductions in the expression of Fasn, Acc-1, and Scd-1 at week 13 of high fat feeding. Similar reductions in serum triglyceride levels and hepatic expression of lipogenic genes have been reported following high fat feeding in rodents (Albers et al. 1999; Biddinger et al. 2005; Guo et al. 2009; Jump 2011).
Although lipogenic gene expression was decreased following HFD intake (Table 2) these animals had significantly heavier livers than LFD animals (Figure 6.B). This increase in hepatic weight appears to be due, at least in part, to hepatic lipid accumulation as indicated by a significant increase in hepatic triglyceride levels which is a defining feature of hepatic steatosis (for reviews see (Anderson and Borlak 2008; Angulo 2002). Chronic exposure to DDE for the duration of HFD intake significantly reduced hepatic triglyceride (Figure 7.A) and cholesterol (Figure 7.B) accumulation. This DDE-mediated decrease in hepatic lipid accumulation coincided with a decrease in HFD-induced fatty acid translocase, Cd36, expression. Cd36 facilitates fatty acid uptake in the hepatocyte and has been shown to be a major mediator of hepatic steatosis (Bradbury 2006; Zhou et al. 2008). Expression of Acox-1, the rate limiting enzyme in peroxisomal fatty acid oxidation, was significantly up regulated in the DDE + HFD animals compared to the vehicle + HFD animals (Fan et al. 1996; Lazarow and De Duve 1976). Therefore, chronic DDE exposure may reduce HFD-mediated elevations in hepatic triglyceride content by decreasing hepatic fatty acid uptake and increasing fatty acid oxidation.
Upon sacrifice, the serum concentration of DDE in the LFD group was 795.6 ± 27.1 ng/ml (whole serum) or 795.6 ppb and 1333.2 ± 76.8 ng/ml (whole serum) or 1333.2 ppb in the HFD group. These levels are substantially higher than human serum levels measured in the 2003–2004 NHANES study which is a large, random sampling of the U.S. population (CDC 2009). In the LFD group, the serum concentrations of DDE are approximately 65.8 fold higher than the 95th percentile of total subjects in the NHANES study (12.1 ppb) and 34.7 fold higher than the 95th percentile of Mexican Americans (22.9 ppb), the group with the overall highest serum DDE levels. In the HFD group, the serum concentrations of DDE are approximately 110.2 fold higher than the 95th percentile of total subjects and 58.2 fold higher than the 95th percentile of Mexican Americans. Thus, the DDE levels in our animals at sacrifice are substantially higher than those observed in the general population of the U.S. However, sensitivities to chronic DDE exposure between rodents and humans, especially in terms of disruption of metabolic function leading to diabetes and related metabolic defects have not been characterized and may differ.
Interestingly, chronic administration of DDE, resulting in serum concentrations of DDE comparable to those previously observed in animals following subacute DDE exposure which produced mild/moderate hyperglycemia, did not promote mild to moderate hyperglycemia in animals consuming a LFD (Howell et al. 2014). It is unclear whether the change from regular rodent chow to a LFD could have prevented the development of this mild to moderate hyperglycemia. In the HFD group, DDE treated animals did display a mild to moderate fasting hyperglycemia at weeks 4 and 8 compared to vehicle treated LFD animals. Based on the present data, we cannot determine if serum concentrations of DDE fluctuated over the course of the chronic, weekly exposure period. However, our previous studies indicate serum concentrations of DDE will decrease over the course of 7 days between dosing therefore, the serum DDE concentration at the end of the 13 week feeding period may not be at steady state.
In conclusion, the current study was designed to determine if chronic administration of DDE promotes HFD-induced metabolic dysfunction resulting in T2D in vivo. The present data indicate chronic exposure to DDE in conjunction with HFD has a biphasic effect on fasting blood glucose where it is initially increased at weeks 4–8 of HFD but normalized at week 13. This normalization of fasting glucose levels appears to be a product of increased insulin sensitivity, increased glucose disposal possibly mediated by normalization of skeletal muscle Glut4 protein levels, and decreased hepatic glucose production as indicated by decreased expression of gluconeogenic genes. This biphasic effect of chronic DDE administration on fasting blood glucose is reminiscent of the non-monotonic concentration response relationships reported in epidemiological studies where low to midrange serum concentrations of POPs have been positively associated with various aspects of metabolic dysfunction including insulin resistance and dyslipidemia whereas this association becomes weaker or even decreases with increasing concentrations (Lee et al. 2011; Taylor et al. 2013). In addition to reducing or even normalizing HFD-induced (≥ 12 weeks feeding) perturbations in glucose homeostasis, chronic DDE exposure decreased HFD-induced hepatic steatosis upon sacrifice. More in depth studies are needed to determine if the currently observed biphasic effect of chronic DDE exposure on fasting blood glucose during HFD is associated with insulin resistance and other aspects of metabolic dysfunction such as hepatic steatosis during the hyperglycemic phase.
Supplementary Material
Supplementary Figure 1. Chronic exposure to DDE decreases HFD-induced hyperphagia. Weekly food intake was determined over the course of 13 weeks of LFD or HFD intake in animals chronically exposed to DDE. Data represent the mean ± SEM of each group (n=15/group) and were analyzed by two-way ANOVA with a Tukey’s post hoc test. # P≤0.001 vs. all other groups and * P≤0.05 vs all other groups at the corresponding time point.
Acknowledgments
The authors would like to thank Mr. Shane Bennett, Mr. Alper Coban, and Mrs. Lauren Mangum for their assistance in performing the presently described animal experiments and routine animal monitoring. The present work was funded by the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH) under award number R15ES019742.
Abbreviations
- DDE
p,p′-dichlorodiphenyldichloroethylene
- DDT
p,p′-dichlorodiphenyltrichloroethane
- T2D
type 2 diabetes mellitus
- POPs
persistent organic pollutants
- OC
organochlorine compound
- NHANES
National Health and Nutrition Examination Survey
- AAALAC
Association for the Assessment and Accreditation of Laboratory Animal Care
- ACE
accelerated solvent extractor
- HOMA-IR
homeostasis model assessment value for insulin resistance
- TNFα
tumour necrosis factor alpha
- IL-6
interleukin 6
- MCP-1
monocyte/macrophage chemotractant protein 1
- i.p
intraperitoneal
- IPGTT
intraperitoneal glucose tolerance test
- GC/MS
gas chromatography/mass spectroscopy
Footnotes
7. Conflict of interest statement: There are no conflicts of interest by any authors.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Literature cited
- Albers JJ, Pitman W, Wolfbauer G, Cheung MC, Kennedy H, Tu AY, Marcovina SM, Paigen B. Relationship between phospholipid transfer protein activity and HDL level and size among inbred mouse strains. Journal of lipid research. 1999;40:295–301. [PubMed] [Google Scholar]
- Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacological reviews. 2008;60:311–357. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3:525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Li CY, Poffenberger G, Ayala JE, Fueger PT, Willis SE, Jewell MM, Powers AC, Wasserman DH. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57:1790–1799. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes. 2005;54:1314–1323. doi: 10.2337/diabetes.54.5.1314. [DOI] [PubMed] [Google Scholar]
- Bradbury MW. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G194–198. doi: 10.1152/ajpgi.00413.2005. [DOI] [PubMed] [Google Scholar]
- CDC; C.f.D.C.a.P. Department of Health and Human Services, editor. Fourth national report on human exposure to environmental chemicals. Atlanta, GA: 2009. [Google Scholar]
- Elam MB, Cowan GS, Jr, Rooney RJ, Hiler ML, Yellaturu CR, Deng X, Howell GE, Park EA, Gerling IC, Patel D, Corton JC, Cagen LM, Wilcox HG, Gandhi M, Bahr MH, Allan MC, Wodi LA, Cook GA, Hughes TA, Raghow R. Hepatic gene expression in morbidly obese women: implications for disease susceptibility. Obesity (Silver Spring) 2009;17:1563–1573. doi: 10.1038/oby.2009.49. [DOI] [PubMed] [Google Scholar]
- Fan CY, Pan J, Chu R, Lee D, Kluckman KD, Usuda N, Singh I, Yeldandi AV, Rao MS, Maeda N, Reddy JK. Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme A oxidase gene. The Journal of biological chemistry. 1996;271:24698–24710. doi: 10.1074/jbc.271.40.24698. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Cheng YJ, Narayan KM, Thompson TJ, Williamson DF. The relative contributions of different levels of overweight and obesity to the increased prevalence of diabetes in the United States: 1976–2004. Prev Med. 2007;45:348–352. doi: 10.1016/j.ypmed.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Guo J, Jou W, Gavrilova O, Hall KD. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. PLoS One. 2009;4:e5370. doi: 10.1371/journal.pone.0005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson RW, Garber AJ. Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. Am J Clin Nutr. 1972;25:1010–1021. doi: 10.1093/ajcn/25.10.1010. [DOI] [PubMed] [Google Scholar]
- Howell G, 3rd, Deng X, Yellaturu C, Park EA, Wilcox HG, Raghow R, Elam MB. N-3 polyunsaturated fatty acids suppress insulin-induced SREBP-1c transcription via reduced trans-activating capacity of LXRalpha. Biochim Biophys Acta. 2009;1791:1190–1196. doi: 10.1016/j.bbalip.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell G, 3rd, Mangum L. Exposure to bioaccumulative organochlorine compounds alters adipogenesis, fatty acid uptake, and adipokine production in NIH3T3- L1 cells. Toxicology in vitro : an international journal published in association with BIBRA. 2011;25:394–402. doi: 10.1016/j.tiv.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GE, 3rd, Meek E, Kilic J, Mohns M, Mulligan C, Chambers JE. Exposure to p,p′-dichlorodiphenyldichloroethylene (DDE) induces fasting hyperglycemia without insulin resistance in male C57BL/6H mice. Toxicology. 2014;320:6–14. doi: 10.1016/j.tox.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Fjaere E, Lock EJ, Naville D, Amlund H, Meugnier E, Le Magueresse Battistoni B, Froyland L, Madsen L, Jessen N, Lund S, Vidal H, Ruzzin J. Chronic consumption of farmed salmon containing persistent organic pollutants causes insulin resistance and obesity in mice. PLoS One. 2011;6:e25170. doi: 10.1371/journal.pone.0025170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump DB. Fatty acid regulation of hepatic lipid metabolism. Current opinion in clinical nutrition and metabolic care. 2011;14:115–120. doi: 10.1097/MCO.0b013e328342991c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB. Facilitative glucose transporters: regulatory mechanisms and dysregulation in diabetes. The Journal of clinical investigation. 1992;89:1367–1374. doi: 10.1172/JCI115724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow PB, De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR., Jr Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2007;30:622–628. doi: 10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR., Jr A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. Jama. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rignell-Hydbom A, Rylander L, Hagmar L. Exposure to persistent organochlorine pollutants and type 2 diabetes mellitus. Hum Exp Toxicol. 2007;26:447–452. doi: 10.1177/0960327107076886. [DOI] [PubMed] [Google Scholar]
- Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003;52:1958–1966. doi: 10.2337/diabetes.52.8.1958. [DOI] [PubMed] [Google Scholar]
- Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, Malde MK, Du ZY, Chavey C, Fajas L, Lundebye AK, Brand CL, Vidal H, Kristiansen K, Froyland L. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010;118:465–471. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander L, Rignell-Hydbom A, Hagmar L. A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environ Health. 2005;4:28. doi: 10.1186/1476-069X-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, Jacobs D, Kohrle J, Lee DH, Rylander L, Rignell-Hydbom A, Tornero-Velez R, Turyk ME, Boyles AL, Thayer KA, Lind L. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013;121:774–783. doi: 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk M, Anderson H, Knobeloch L, Imm P, Persky V. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes sport fish consumers. Environ Health Perspect. 2009a;117:1076–1082. doi: 10.1289/ehp.0800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk M, Anderson HA, Knobeloch L, Imm P, Persky VW. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p′-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere. 2009b;75:674–679. doi: 10.1016/j.chemosphere.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Wahlang B, Falkner KC, Gregory B, Ansert D, Young D, Conklin DJ, Bhatnagar A, McClain CJ, Cave M. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. The Journal of nutritional biochemistry. 2013;24:1587–1595. doi: 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DB, York B. Dietary fat, genetic predisposition, and obesity: lessons from animal models. Am J Clin Nutr. 1998;67:505S–512S. doi: 10.1093/ajcn/67.3.505S. [DOI] [PubMed] [Google Scholar]
- Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Archives of internal medicine. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, Lee JH, Khadem S, Ren S, Li S, Silverstein RL, Xie W. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556–567. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Chronic exposure to DDE decreases HFD-induced hyperphagia. Weekly food intake was determined over the course of 13 weeks of LFD or HFD intake in animals chronically exposed to DDE. Data represent the mean ± SEM of each group (n=15/group) and were analyzed by two-way ANOVA with a Tukey’s post hoc test. # P≤0.001 vs. all other groups and * P≤0.05 vs all other groups at the corresponding time point.