Abstract
Background:
Although many of the proposed mediating processes of self-management interventions are operationally defined as cognitive processes (e.g., acquiring and using information, self-efficacy, motivation, decision-making), little is known about their underlying brain mechanisms. Brain biomarkers of how people process health information may be an important characteristic on which to individualize health information to optimize self-management of chronic conditions.
Objectives:
We describe a program of research addressing the identification of brain biomarkers that differentially predict responses to two types of health information (analytic-focused and emotion-focused) designed to support optimal self-management of chronic conditions.
Methods:
We pooled data from two pilot studies (N = 52) that included functional magnetic resonance imaging (fMRI) during a specially designed, ecologically valid protocol to examine brain activation (task differentiation) associated with two large-scale neural networks—the Analytic Network and the Empathy Network—and the ventral medial prefrontal cortex while individuals responded to different types of health information (analytic and emotional).
Results:
Findings indicate that analytic and emotional information are processed differently in the brain, and the magnitude of this differentiation in response to type of information varies from person to person. Activation in the a priori regions identified in response to both analytic and emotion information was confirmed. The feasibility of obtaining brain imaging data from persons with chronic conditions also is demonstrated.
Discussion:
An understanding of brain signatures related to information processing has potential to assist in the design of more individualized, effective self-management interventions.
Keywords: biomarkers, brain, health education, self-management
The Precision Medicine Initiative is an emerging method for disease treatment and prevention in which an individual’s genes, environment, and lifestyle factors are taken into account in everyday treatment of patients (Collins & Varmus, 2015). Expanding on this definition, the goal of precision health is to integrate data about lifestyle and preferences, health status and behaviors, genomics, other physiological measures of health, and environment to design and deliver targeted, personalized interventions to assist individuals achieve and maintain optimal health and well-being (Khoury, Iademarco, & Riley, 2016). Concurrent with the Precision Medicine Initiative over the past decade, the National Institutes of Health (NIH) launched the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative (Insel, Landis, & Collins, 2013) to identify the neural circuitry associated with the prevention and treatment of disease. Because of this initiative, advances in translational neuroimaging will allow us a deeper understanding of the brain and its potential to aid in the design of customized interventions based on brain biomarkers. Although the contribution of genomics is frequently the focus of precision health initiatives, brain-based approaches are also needed to best achieve precision healthcare for self-management of chronic conditions.
In our center of excellence in self-management research, our team is engaged in research examining the brain-behavior connections underpinning effective self-management of chronic conditions. We have a long-term goal to develop brain-based phenotypes of self-management to assist clinicians in tailoring interventions. There can be many dimensions comprising self-management phenotypes, including characteristics of the individual (e.g., developmental stage, knowledge and beliefs; health condition and its treatment), the social environment (e.g., family support, culture, social capital), and the community (e.g., neighborhood, access to healthcare). An important characteristic influencing self-management is how an individual processes and responds to self-management information (e.g., patient or public health education) (Ryan & Sawin, 2009). Given that cognitive processes are greatly involved in understanding and responding to health information, exploration of brain markers underlying those cognitive responses is desirable. Neural activity based on these brain signatures may represent a neurobiological explanation for why responses to health information and subsequent self-management differ from one individual to another. The purposes of this paper are to: (a) describe a program of research addressing the identification of brain signatures assessable through noninvasive neuroimaging that differentially predict responses to health information designed to support self-management of health and illness; and (b) present early findings indicating the potential usefulness and feasibility of these brain biomarkers for individualization of self-management interventions, thus supporting the future design and delivery of precision health.
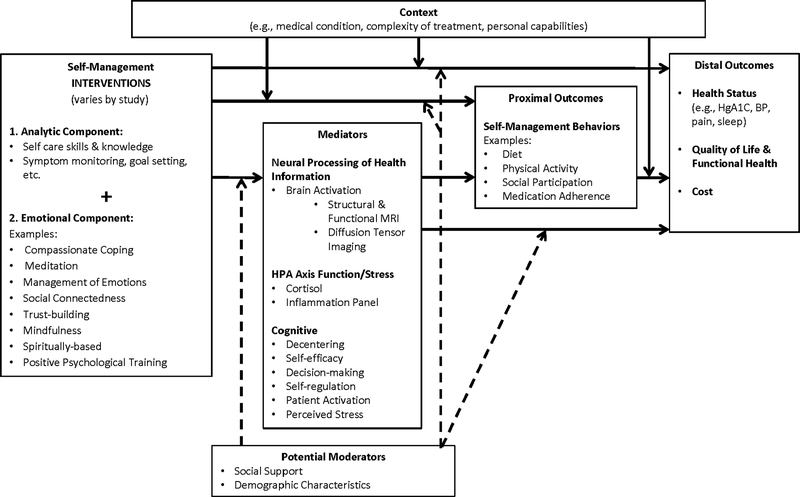
The existing models of self-management (Grey, Knafl, & McCorkle, 2006; Lorig & Holman, 2003; Ryan & Sawin, 2009) are predominantly cognitive-behavioral in nature and do not address the biological processes underlying self-management, such as the influence of brain and hormonal activity on self-management behaviors. Although many of the proposed mediating processes of self-management are cognitive and behavioral (e.g., acquiring and using information, self-efficacy, motivation, decision-making, goal setting, self-monitoring), little is known about the underlying brain-based mechanisms associated with these cognitive-behavioral processes. Figure 1 displays the study model that is currently being used in eight center studies. The central hypothesis of our center studies is that individuals who optimally process health information that comprised both analytic and emotional components will be more likely to act on that information for self-management of their health. Therefore, as shown in the far left of Figure 1, all of our studies are testing the effects of interventions that include both types of information (analytic and emotional/empathic) compared to interventions with less balance in these types of information. As depicted in this figure, all pilot studies include a set of potential mediators that are expected to affect a set of self-management behaviors (e.g., diet, physical activity, medication adherence), which, in turn, will improve health outcomes in chronic conditions. Our biomarker of interest in this paper—brain activation in response to health information—is shown in this model as a mediator (mechanism) of the self-management interventions on the performance of self-management behaviors. The use of a common study model and common data elements provides an opportunity to perform pooled analyses across all center studies, and assists us to generate and test theory-driven hypotheses for the research projects of the center.
FIGURE 1.
Model of Brain-Behavior Connection in Self-Management of Health and illness. Dotted lines indicate moderating effects on the proposed relationships between the intervention and its effects. MRI = Magnetic Resonance Imaging; HPA = Hypothalamic Pituitary Adrenal Axis; HgA1C = Hemoglobin A1C; BP = Blood Pressure
Scientific Premise for Our Selection of Brain Markers and Their Relationship to Information Processing for Self-Management
Precision health involves precision in intervention target discovery, design, and delivery. An important early step in this process is the characterization of a biomarker to further develop precision in the characterization of phenotypes. Thus, to increase our understanding of precision in tailoring self-management information to individuals, our team is focused on the characterization of a brain biomarker representing the magnitude of neural activity that individuals exhibit as they process different types of health information for self-management of chronic conditions. Our central hypothesis is that individuals who optimally process health information—that is they are equally adept at attending to and responding to health information that comprised both analytic and emotion/empathic components—will be more likely to effectively act on that information for self-management. Relatedly, we propose that different types of health information might have markedly different effects on brain areas that predict people’s actions on that information and engagement in self-management behaviors, and that individuals who show the strongest differential neural response to analytic versus empathic health information will exhibit optimal self-management.
The theoretical basis for our work derives from the opposing domains hypothesis (Friedman, Jack, Rochford, & Boyatzis, 2015; Jack, Dawson et al., 2013). The opposing domains hypothesis suggests that information that is emotion-focused will focally engage brain areas associated with motivation, valuing, and self- and social-referencing, and disengage brain areas associated with task performance and nonsocial reasoning (analytic thinking). Conversely, analytic information will focally engage brain areas associated with task performance and nonsocial reasoning, and disengage brain areas associated with motivation, valuing, and self- and social-referencing (emotional/empathic thinking). The strength of the differential engagement of the empathic and analytic brain networks represents our brain signatures.
The opposing domains hypothesis is supported by a number of prior studies examining neural processing of health information and subsequent behavior change (Cooper, Bassett, & Falk, 2017; Falk, Berkman, Mann, Harrison, & Lieberman, 2010; Tompson, Lieberman, & Falk, 2015; Vezich, Falk, & Lieberman, 2016; Whelan, Morgan, Sherar, Orme, & Esliger, 2017). In particular, activity in the medial parietal and medial prefrontal regions in response to health information processing predict the performance of health-promoting behaviors (Falk et al., 2010; Vezich et al., 2016). These brain regions are positively associated with social and emotional cognition and are part of what is commonly known as the default mode network (DMN). Our investigations focus on the dorsal (superior) parts of the DMN, which has been described and termed the Empathy Network (Boyatzis, Rochford, & Jack, 2014; Friedman et al., 2015; Jack, Boyatzis, Khawaja, Passarelli, & Leckie, 2013). This work serves as the rationale for selection of our brain regions of interest to assess the neural response to emotional/empathic self-management health information, the medial parietal/precuneus and dorso-medial prefrontal cortex. In addition to the Empathy Network, another core of the DMN, the ventromedial prefrontal cortex (vmPFC), which is implicated in the valuation of information and rewards (e.g., weighing the benefits vs. barriers for behavior change), was selected as a region of interest due to its association with the prediction of health-promoting behaviors (Falk & Bassett, 2017; Tompson et al., 2015).
In contrast to the Empathy Network, the Analytic Network, which is also known as the Task Positive Network (TPN), involves activation of the lateral parietal and dorsolateral prefrontal cortices of the brain during executive functioning, non-social reasoning, logical and scientific reasoning, and inhibitory control (Duncan & Owen, 2000; Shulman et al., 1997). Hence, to assess neural response to analytic self-management health information, the brain regions of interest investigated are the lateral parietal and dorsolateral prefrontal cortex. Ordinarily, when activation of the Analytic Network is prominent, activity in the Empathy Network diminishes and vice versa. This pattern of differential activation indicates that the networks are anticorrelated with one other. Importantly, according to the Opposing Domains hypothesis, we predict that the strength of the anticorrelation of the Empathy and the Analytic Networks represents our brain biomarker of optimal self-management. In effect, we are hypothesizing that individuals who strongly activate the Empathy Network when the emotional tone of health information is high, and strongly activate the Analytic Network when the emotional tone of information is low, will optimally process all health information and engage more in self-management.
This reciprocal inhibitory relationship between these two opposing brain networks has been supported in several studies using functional magnetic resonance imaging (fMRI) (Mars et al., 2012; Spunt & Lieberman, 2013; Tavares, Lawrence, & Barnard, 2008). Since health information tends to vary in the degree to which it is analytic or empathic, the ability of an individual to differentiate analytic processing from emotion/empathic processing (indicated by activation of the respective brain networks) may influence the likelihood of their use of both types of information for optimal self-management. This premise is supported by the work of Jack and colleagues (Jack, Boyatzis et al., 2013, 2014) that showed that personalized coaching containing both empathic and analytic content, resulted in more goal setting and action taking by individuals. In particular, a dose-effect was found on brain regions associated with the Empathy Network.
Summary and Hypotheses.
Thus, our brain signature for optimal ability to self-manage chronic conditions is task differentiation, which we define as the ability to differentiate analytic processing from emotion/empathic processing in response to different types of health information (analytic and emotional/empathic). This differentiation is measured using an fMRI paradigm developed by our team. We are examining task differentiation associated with two large-scale neural networks—the Analytic Network and the Empathy Network—in response to the two types of health information. The magnitude of the task differentiation in each of these networks when the information content is analytic (Analytic > Empathy) or emotional (Empathy Network > Analytic Network) are two of our markers for optimal ability to engage in self-management activities. The task differentiation values are at a continuous level of measurement, with higher values representing better task differentiation. A third marker is activation of the vmPFC, in which we expect to have high activation in response to emotional information and minimal activation in response to analytic information.
Methods
Population, Sample Description, and Enrollment Procedures.
We have combined the data from two of our center pilot studies for this report. Both of these studies were designed based on our center Model of Brain-Behavior Connections in Self-management (Figure 1). Therefore, study participants were individuals living with a chronic condition who agreed to participate in a study testing self-management interventions. One of the studies consisted of 28 participants who were diagnosed with HIV+; the other study comprised 24 participants with prehypertension. General criteria for participant inclusion in the studies included: (a) being an adult (18 years of age or older); (b) having a score on the Montreal Cognitive Assessment (MoCA) of ≥20 (Lin, O’Connor, Rossom, Perdue, & Eckstrom, 2013; Nasreddine et al., 2005; Rossetti, Lacritz, Cullum, & Weiner, 2011); and (c) without contraindications to undergoing fMRI (e.g., body metals, claustrophobia). (There also were additional study-specific criteria, such as confirmation of the respective diagnoses and diagnosis-related medication and treatment contraindications.) Both pilot studies consisted of convenience samples in which participants were enrolled consecutively from community settings, and provided written informed consent for participation. Demographic data were obtained by self-report in a baseline interview and followed by baseline brain imaging using fMRI (done in a separate research visit). All data reported herein are from baseline measures. Table 1 provides a description of the combined sample. Participants were predominantly low-income, African American, and single. There were no statistically significant differences in demographic characteristics between the two study samples. All procedures were approved by the institutional review board at University Hospitals of Cleavland.
TABLE 1.
Characteristics of the Sample
| Characteristic | Statistic | Sample (N = 52) |
|---|---|---|
| Age (years) | M | 52.0 |
| (SD) | (11.48) | |
| Range | 24.0–76.0 | |
| Gender | ||
| Male | n | 28 |
| (%) | (53.8) | |
| Female | n | 23 |
| (%) | (44.2) | |
| Transgender | n | 1 |
| (%) | (1.9) | |
| Race (Black)a | n | 51 |
| (%) | (98.1) | |
| Marital status | ||
| Single | n | 34 |
| (%) | (69.4) | |
| Married | n | 1 |
| (%) | (2.0) | |
| Divorced | n | 10 |
| (%) | (20.4) | |
| Other | n | 4 |
| (%) | (8.2) | |
| Education | ||
| Less than HS | n | 15 |
| (%) | (30.6) | |
| Finished HS | n | 13 |
| (%) | (26.5) | |
| Some College | n | 17 |
| (%) | (24.6) | |
| Finished College | n | 4 |
| (%) | (8.2) | |
| Household Income | ||
| <$200/month | n | 8 |
| (%) | (16.6) | |
| $200–599/month | n | 1 |
| (%) | (2.1) | |
| $600–799/month | n | 24 |
| (%) | (50.0) | |
| $800–999/month | n | 6 |
| (%) | (12.5) | |
| >$1,000/month | n | 9 |
| (%) | (18.8) | |
| Brain markers | ||
| Analytic Network (TPN) | M | 0.19 |
| (SD) | (0.18) | |
| Range | −0.12–0.84 | |
| Empathic Network (DMN) | M | 0.12 |
| (SD) | (0.24) | |
| Range | −0.80–0.53 | |
| vmPFC | M | 0.15 |
| (SD) | (0.31) | |
| Range | −0.50–1.09 |
Note. SD = Standard Deviation; HS = High school; TPN = Task Positive Network; DMN = Default Mode Network; vmPFC = Ventromedial Prefrontal Cortex.
Other race was White.
Imaging Protocol and Procedures.
We developed an fMRI paradigm (sequentially presented stimuli designed to produce a cognitive task) to assess brain activity while participants were exposed to different types of self-management health information. Relevant to this report is information that was distinctly analytic or emotional/empathic in nature. This information was delivered in a series of video clips (with audio) while the participant underwent an fMRI scan. The analytic video clips were fact-focused and consisted of animations of anatomical and physiological explanations of health and disease processes (e.g., how the lungs work, the immune process). The emotional/empathic videos consisted of short stories of families’ experiences in coping with and managing chronic conditions (e.g., how I felt when I was diagnosed with HIV). The videos were selected from health information on the internet. Over a scanning period, participants had four runs (exposures to the fMRI protocol) lasting 7 minutes and 40 seconds during which they were exposed to 16 video clips each of analytic and emotional/empathic information. Each video clip was 23 seconds in length, interspersed with equal length “rest” videos in which subjects viewed a red fixation cross on a black background. Each participant received the same protocol. Videos were projected onto a screen attached to the MRI head coil and were viewed by subjects through a mirror. The video conditions were presented using Eprime software; the magnetic resonance imaging data were obtained with a Siemens 3T Skyra scanner. A T1-weighted magnetization-prepared rapid-acquisition gradient-echo structural sequence (MP-RAGE) was completed first, followed by four T2-weighted functional task runs. Further information on the imaging data acquisition protocol is described in detail elsewhere (Jones, Wright, I, P, Fresco, & Veinot, in press).
Data Analysis.
All imaging data were preprocessed using the Washington University, St. Louis program, fidl. A general linear model with assumed hemodynamic response functions (HRF) was used to estimate the average magnitude of each participant’s response to the video conditions. Voxel-based estimates of response magnitude in the prespecified brain regions of interest were calculated. Greater detail of the preprocessing and analysis of the imaging data is described elsewhere (Jones et al., in press). Values were derived for each participant for each of the three task differentiation indices: the Analytic Network, the Empathy Network, and the vmPFC. Summary statistics (mean, standard deviation, range) were computed for the sociodemographic variables and the task differentiation indices. Distributions of the task differentiation scores were studied using histograms, box plots and tests for normality. Thus, assuming normal distribution, we used Pearson’s product moment correlation to assess associations among the task differentiation scores. All statistical analyses were performed using Stata 15.0 software.
Results
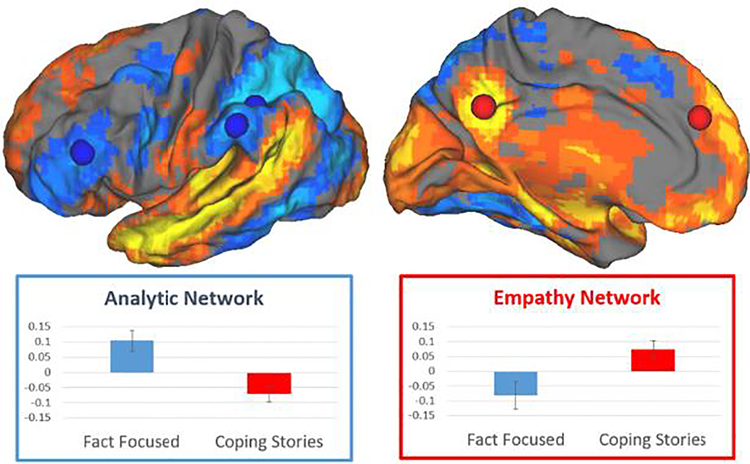
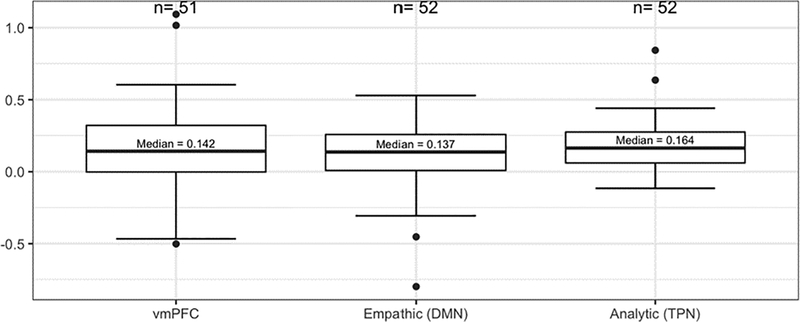
A major finding is the confirmation of the ability of the fMRI task protocol developed by our team to obtain measures of brain activation (task differentiation) in response to different types of health information. First, as shown in Figure 2, our protocol was successful to assess activation of different areas of the brain in response to the different types of information presented in our fMRI protocol (video clips with audio containing either fact-focused or emotion-focused health information). We found that the brain networks activated were consistent with our a priori selected regions of interest. Analytic information (fact-focused) stimulated activation in the Analytic Network, and emotional/empathic information (emotion-focused) stimulated activation in the Empathy Network and the vmPFC. Additionally, correlations among the task differentiation scores were consistent with our expectations regarding their relationships. Significant negative correlations between task differentiation scores of the Analytic Network and both the Empathy Network and the vmPFC were found (Table 2), supporting the hypothesized reciprocal inhibitory relationships among them. Also, as expected, a positive correlation was found between the Empathy Network and the vmPFC. Next, because our brain markers are new, it is important to characterize their variability from individual to individual. An examination of the distributions of each of the three task differentiation scores (Figure 3) indicated good variability in each of the three task differentiation scores.
FIGURE 2.
Different types of health information processed in different ways in the brain. At the top, lateral and medial views of the brain illustrate different brain areas engaged by analytic Fact Focused health information (blue), and empathic Coping Stories about dealing with chronic illness (orange/yellow). At the bottom, graphs show how these conditions also suppress parts of the other brain network.
TABLE 2.
Pearson’s Product-Moment Correlations Among Brain Markers
Note:
First number in each cell represents the sample size used for computing the correlation;
Second number is the correlation coefficient between the two markers;
Third number is the p-value for test of significance of the correlation.
FIGURE 3.
Distribution of Brain Markers.
We also were able to establish the feasibility of obtaining brain imaging data from persons with chronic conditions. First, there were no adverse events in either of these two pilot studies of persons with chronic conditions. Our sample consists of a wide age range, with a considerable number of subjects who were over 65 years of age. With the exception of one individual who requested to be removed from the scanner, participants tolerated the procedure well, and many reported the experience as interesting and enjoyable. A study challenge in obtaining good imaging data was keeping participants awake while in the scanner. Some participants came directly from work and became so relaxed while in the scanner that they fell asleep.
Discussion
The design and delivery of personalized interventions to support self-management has the potential to greatly improve the health and quality of life of persons with chronic conditions. The provision of health information is a common intervention for self-management; however, health information is usually only minimally tailored, with previous tailoring approaches being limited primarily to cultural and developmental factors (Ryan & Sawin, 2009). A “deeper dive” to increase our understanding of how to improve the tailoring of health information may be the identification of biologic factors on which to tailor, such as how the brain processes different types of information and how this varies among individuals.
In this report, we provide preliminary findings to demonstrate the feasibility and potential usefulness of a specific brain marker, task differentiation associated with two neural networks (the Analytic Network and the Empathy Network) in response to different types of health information (analytic and emotion/empathic) and the potential relevance to precision science. We have shown that our fMRI protocol distinguishes brain responses to both analytic and emotional information in the hypothesized brain regions of interest. Also, the dispersion of our scores among participants indicates that individuals vary in their inclination towards these different ways of thinking. This may influence how well they receive and act on different types of health information. The primary reports of our main effects (i.e., how different brain areas are recruited by different types of health information and subsequently affect self-management behaviors) are forthcoming.
We acknowledge that the use of brain imaging to measure task differentiation in response to health information is currently expensive, time-consuming, and burdensome for clinicians and patients. A future step in this program of research in precision health will be the development of a point-of-care tool that can be used by clinicians to classify how a particular individual responds to different types of health information. This ‘information processing assessment’ tool could be in the form of a brief, valid, and reliable set of questions that, when answered by a patient or family member, reveal information processing data corresponding to that obtained using neuroimaging. In other words, a simple questionnaire can potentially be developed that identifies a person’s brain information processing signature on which the type, dose, and timing of different types of health information can be personalized. In addition, our brain imaging findings could be further explored in conjunction with genomics in order to describe the cellular mechanisms underlying the neural circuitry of self-management behaviors (Uludağ, Uğurbil, & Berliner, 2015). This type of analysis is now made possible due to the recent advances and accessibility of genomic and translational neuroimaging methods. Linking the associations between brain signatures and genomics could also provide a more cost-effective way of analyzing this type of data and explain the underlying cellular mechanisms of brain function.
Our findings should be considered in light of several limitations. First, our sample size is small, thus, some findings may be spurious. We therefore report these results as preliminary in nature. As a larger sample is accrued by combining data across additional center studies, we will complete our planned analyses examining the associations between task differentiation and self-management processes (e.g., self-efficacy, self-regulation, garnering and receiving social support) and optimal performance of self-management behaviors, such as goal setting, symptom monitoring, healthy eating, physical activity, and medication taking. Since all of our studies are designed to assess the associations between neural processes and the effects of interventions containing different balances of analytic and emotional/empathic information, we will also be able to evaluate our overarching hypothesis that higher task differentiation in response to both analytic-focused and emotion-focused information processing will support optimal performance of self-management processes and behaviors and, in turn, achieve best health status outcomes.
Another limitation of this study is the need to further account for the possible influence of a larger number of confounding variables, such as cognitive functioning or underlying neuropathology that can potentially influence brain functioning (consequently altering our neuroimaging findings). Although a clinical measure of cognitive functioning (Lin et al., 2013; Rossetti et al., 2011) was used to screen subjects for participation in the study, this measure may not have been sufficiently sensitive to identify cognitive impairment that could potentially affect our neural processing indices. It is also possible that since we combined data from studies of participants with different chronic conditions, the underlying pathology of the different disease conditions or the neural effects of medications could influence the measures of brain functioning. Additionally, the self-relevance of the specific messages on the video clips (designed to elicit emotional or analytic processing) in our fMRI paradigm may differ among individuals with different chronic conditions, which could affect the validity of our imaging protocol. Understanding of the effect of the possible differences in the salience of the various video messages on individuals with different chronic illnesses might be further understood by coding (by independent coders) the characteristics of the video clips to understand their self-relevance. Last, we acknowledge the early phase of translational neuroimaging as a field of inquiry and the evolving precision in the measurement and interpretation of imaging data that could contribute to error in our findings. In this regard, our team is committed to examining hypotheses and brain regions of interest that are identified in an a priori manner. We are encouraged, however, that our early findings are consistent with emerging patterns in the literature regarding neural processing of different types of health messages (Falk et al., 2015; Jack, Boyatzis et al., 2013).
Our program of research has produced interesting questions that, if answered, can advance the science and practice of precision health. If we find that specific brain signatures, such as analytic and empathic task differentiation, are associated with optimal health information processing and self-management, further research can then better characterize phenotypes of these markers and suggest tailoring approaches for interventions. For example: Can we identify individuals most likely to benefit from a particular type of information? What dose of analytic versus emotional/empathic information is optimal to promote self-management? Is there an optimal order in the provision of information? Is the type, dose, and timing of information for self-management of chronic conditions different from self-management of an acute illness? What interventions can be used to improve neural processing of health information?
Conclusion
Precision health seeks to provide the “right intervention to the right patient at the right time.” In the goal to design more effective self-management interventions, more precision is sought in the identification of self-management intervention targets, intervention design, and intervention delivery. A unique characteristic of individuals on which to tailor self-management interventions is how their brains are able to process different types of health information. Preliminary findings from our studies indicate that brain biomarkers—specifically neural networks—have potential to explain and predict one’s ability to optimally process health information. The use of brain-behavior markers can substantially advance precision health in that they can help identify individuals at high risk for ineffective processing of health information as well as serve as patient-specific parameters on which to personalize the type, dose, and timing of health information. Brain signatures may be an important part of future self-management interventions.
Acknowledgements:
Research reported in this manuscript was supported by the National Institutes of Health, National Institute of Nursing Research, under Award Numbers P30NR015326 and T32 NR015433 and the National Center for Advancing Translational Sciences (NCATS) Award Number KL2TR000440, and The Ohio State University Discovery Themes-Chronic Brain Injury. The authors would like to thank Oluwatomisin Olayinka, RN, MPH, research assistant, Alexandra Jeanblanc, JD, MA, data analyst, Lingpeng Shan, MS, research assistant, and Jared Friedman, BA, research assistant for their assistance with data management and analysis. The neuroimaging work made use of the High-Performance Computing Resource in the Core Facility for Advanced Research Computing at Case Western Reserve University.
The authors would like to thank Oluwatomisin Olayinka, RN, MPH, research assistant, Alexandra Jeanblanc, JD, MA, data analyst, Lingpeng Shan, MS, research assistant, and Jared Friedman, BA, research assistant for their assistance with data management and analysis.
The neuroimaging work made use of the High-Performance Computing Resource in the Core Facility for Advanced Research Computing at Case Western Reserve University.
Research reported in this manuscript was supported by the National Institutes of Health, National Institute of Nursing Research, under Award Numbers P30NR015326 and T32 NR015433 and the National Center for Advancing Translational Sciences (NCATS) Award Number KL2TR000440, and The Ohio State University Discovery Themes-Chronic Brain Injury.
Footnotes
Conflict of Interest: The authors have indicated they have no potential conflicts of interest to disclose.
Ethical Conduct of Research: All procedures were approved by the Institutional Review Board at University Hospitals of Cleveland.
Clinical Trial Registration: Clinicaltrials.gov NCT02553291 and NCT02830529 (pilot studies from which these analyses were conducted)
Contributor Information
Shirley M. Moore, Edward J. and Louise Mellen Professor of Nursing, Case Western Reserve University, Cleveland, OH.
Carol M. Musil, Marvin E. and Ruth Durr Denekas Professor of Nursing, Case Western Reserve University, Cleveland, OH.
Anthony I. Jack, Case Western Reserve University, Cleveland, OH.
Megan L. Alder, Case Western Reserve University, Cleveland, OH.
David M. Fresco, Kent State University, Kent, OH.
Allison Webel, Case Western Reserve University, Cleveland, OH.
Kathy D. Wright, Chief Diversity Officer, The Ohio State University, Columbus, OH.
Abdus Sattar, Case Western Reserve University, Cleveland, OH.
Patricia Higgins, Case Western Reserve University, Cleveland, OH.
References
- Boyatzis RE, Rochford K, & Jack AI (2014). Antagonistic neural networks underlying differentiated leadership roles. Frontiers in Neuroscience, 8, 114. doi: 10.3389/fnhum.2014.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, & Varmus H (2015). A new initiative on precision medicine. New England Journal of Medicine, 372, 793–795. doi: 10.1056/NEJMp1500523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N, Bassett DS, & Falk EB (2017). Coherent activity between brain regions that code for value is linked to the malleability of human behavior. Scientific Reports, 7, 43250. doi: 10.1038/srep43250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, & Owen AM (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences, 23, 475–483. doi: 10.1016/S0166-2236(00)01633-7 [DOI] [PubMed] [Google Scholar]
- Falk EB, & Bassett DS (2017). Brain and social networks: Fundamental building blocks of human experience. Trends in Cognitive Sciences, 21, 674–690. doi: 10.1016/j.tics.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Mann T, Harrison B, & Lieberman MD (2010). Predicting persuasion-induced behavior change from the brain. Journal of Neuroscience, 30, 8421–8424. doi: 10.1523/jneurosci.0063-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, O’Donnell MB, Cascio CN, Tinney F, Kang Y, Lieberman MD, … Strecher VJ (2015). Self-affirmation alters the brain’s response to health messages and subsequent behavior change. Proceedings of the National Academy of Sciences of the United States of America, 112, 1977–1982. doi: 10.1073/pnas.1500247112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Jack AI, Rochford K, & Boyatzis RE (2015). Antagonistic neural networks underlying organizational behavior In Waldman DA, & Balthazard PA (Eds.), Organizational neuroscience: Monographs in leadership and management (pp. 115–141). Bingley, UK: Emerald Group Publishing. doi: 10.1108/S1479-357120150000007004 [DOI] [Google Scholar]
- Grey M, Knafl K, & McCorkle R (2006). A framework for the study of self- and family management of chronic conditions. Nursing Outlook, 54, 278–286. doi: 10.1016/j.outlook.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Insel TR, Landis SC, & Collins FS (2013). The NIH BRAIN initiative. Science, 340, 687–688. doi: 10.1126/science.1239276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack AI, Boyatzis RE, Khawaja MS, Passarelli AM, & Leckie RL (2013). Visioning in the brain: An fMRI study of inspirational coaching and mentoring. Social Neuroscience, 8, 369–384. doi: 10.1080/17470919.2013.808259 [DOI] [PubMed] [Google Scholar]
- Jack AI, Dawson AJ, Begany KL, Leckie RL, Barry KP, Ciccia AH, & Snyder AZ (2013). fMRI reveals reciprocal inhibition between social and physical cognitive domains. Neuroimage, 66, 385–401. doi: 10.1016/j.neuroimage.2012.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LM, Wright KDI, Jack AP, Friedman J, Fresco DM, & Veinot T (in press). The relationships between health information behavior and neural procesing in African-Americans with prehypertenson. Journal of the Association for Information Science and Technology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury MJ, Iademarco MF, & Riley WT (2016). Precision public health for the era of precision medicine. American Journal of Preventive Medicine, 50, 398–401. doi: 10.1016/j.amepre.2015.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, O’Connor E, Rossom RC, Perdue LA, & Eckstrom E (2013). Screening for cognitive impairment in older adults: A systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine, 159, 601–612. doi: 10.7326/0003-4819-159-9-201311050-00730 [DOI] [PubMed] [Google Scholar]
- Lorig KR, & Holman HR (2003). Self-management education: History, definition, outcomes, and mechanisms. Annals of Behavioral Medicine, 26, 1–7. doi: 10.1207/S15324796ABM2601_01 [DOI] [PubMed] [Google Scholar]
- Mars RB, Neubert F-X, Noonan MP, Sallet J, Toni I, & Rushworth MFS (2012). On the relationship between the “default mode network” and the “social brain.” Frontiers in Human Neuroscience, 6, 189. doi: 10.3389/fnhum.2012.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, … Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Rossetti HC, Lacritz LH, Cullum CM, & Weiner MF (2011). Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology, 77, 1272–1275. doi: 10.1212/WNL.0b013e318230208a [DOI] [PubMed] [Google Scholar]
- Ryan P, & Sawin KJ (2009). The individual and family self-management theory: Background and perspectives on context, process, and outcomes. Nursing Outlook, 57, 217–225. doi: 10.1016/j.outlook.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Fiez JA, Miezin FM, Raichle ME, & Petersen SE (1997). Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex. Journal of Cognitive Neuroscience, 9, 624–647. doi: 10.1162/jocn.1997.9.5.624 [DOI] [PubMed] [Google Scholar]
- Spunt RP, & Lieberman MD (2013). The busy social brain: Evidence for automaticity and control in the neural systems supporting social cognition and action understanding. Psychological Science, 24, 80–86. doi: 10.1177/0956797612450884 [DOI] [PubMed] [Google Scholar]
- Tavares P, Lawrence AD, & Barnard PJ (2008). Paying attention to social meaning: An fMRI study. Cerebral Cortex, 18, 1876–1885. doi: 10.1093/cercor/bhm212 [DOI] [PubMed] [Google Scholar]
- Tompson S, Lieberman MD, & Falk EB (2015). Grounding the neuroscience of behavior change in the sociocultural context. Current Opinion in Behavioral Sciences, 5, 58–63. doi: 10.1016/j.cobeha.2015.07.004 [DOI] [Google Scholar]
- Uludağ K, Uğurbil K, & Berliner L (Eds.). (2015). fMRI: From nuclear spins to brain functions. New York, NY: Springer. [Google Scholar]
- Vezich IS, Falk EB, & Lieberman MD (2016). Persuasion neuroscience: New potential to test dual-process theories In Harmon-Jones E & Inzlicht M (Eds.), Social neuroscience: Biological approaches to social psychology (pp. 34–58). New York, NY: Routledge. [Google Scholar]
- Whelan ME, Morgan PS, Sherar LB, Orme MW, & Esliger DW (2017). Can functional magnetic resonance imaging studies help with the optimization of health messaging for lifestyle behavior change? A systematic review. Preventive Medicine, 99, 185–196. doi: 10.1016/j.ypmed.2017.02.004 [DOI] [PubMed] [Google Scholar]