Summary
Invasive fungal disease (IFD) confers a substantial risk for morbidity and mortality to immunocompromised patients. Invasive aspergillosis (IA) is the most common IFD caused by moulds but the prevalence of other rare mould diseases, such as mucormycosis, hyalohyphomycosis and phaeohyphomycosis, may be increasing. Treatments are available for IA, but evidence to support efficacy and safety of antifungal agents for rare IFDs, or for IFDs in special patient populations, is limited or lacking. The VITAL trial was conducted to assess the efficacy and safety of isavuconazole for the treatment of patients with IA and renal impairment, or with IFDs caused by rare moulds, yeasts or dimorphic fungi. These patients stand to benefit most from a new treatment option but are unlikely to be included in a randomised, controlled trial. In this article, we review the challenges faced in the design and conduct of the VITAL trial. We also review the findings of VITAL, which included evidence of the efficacy and safety of isavuconazole. Finally, we consider the importance of trials such as VITAL to inform therapeutic decision making for clinicians faced with the challenge of treating patients with rare IFDs and as one paradigm of how to determine efficacy and safety of new drugs for rare and resistant infections without a suitable comparator.
Keywords: aspergillosis, cryptococcosis, dimorphic fungi, invasive fungal diseases, isavuconazole, mucormycosis, rare moulds, yeasts
1 ∣. INTRODUCTION
Invasive fungal diseases (IFDs) confer a substantial risk for morbidity and mortality, especially in patients who are immunocompromised, such as those being treated for haematological malignancies or those undergoing solid organ transplantation.1,2 Among IFDs caused by moulds, invasive aspergillosis (IA) is the most common; however, the prevalence of other rare mould infections, such as mucormycosis, hyalohyphomycosis and phaeohyphomycosis, may be increasing.3,4 Treatment options for IA include triazole antifungal agents (eg voriconazole or isavuconazole), amphotericin B formulations and echinocandins,5,6 but there is a scarcity of strong evidence to support any pharmacological treatment for many rare IFDs. Voriconazole is approved to treat fusariosis and scedosporiosis,7,8 and its use is featured in treatment guidelines,9 but susceptibility of those pathogens to triazole antifungal agents is not predictable and clinical failures remain common. The recommendation to treat mucormycosis with liposomal amphotericin B is based largely on animal studies10 and posaconazole has mainly been studied in the salvage setting.11,12 Amphotericin B formulations are also recommended for serious cryptococcal disease and other endemic mycoses;13-16 however, their intravenous-only formulations pose a challenge for extended treatment and use of these agents may be limited by nephrotoxicity.17,18 Various other monomicrobial and mixed fungal infections are sufficiently rare or refractory to tested antifungal agents such that no specific treatment recommendations exist.
The VITAL trial was designed and conducted to determine the efficacy and safety of isavuconazole (active moiety of the prodrug isavuconazonium sulphate) for the treatment of IA in patients with renal impairment, or in patients with IFDs caused by rare moulds, including Mucorales spp., yeasts or dimorphic fungi. This article provides an overview of the planning, performance, challenges and lessons of the VITAL trial, which has previously reported the efficacy and safety of isavuconazole for the treatment of mucormycosis,19 cryptococcosis and other endemic mycoses,20 other rare moulds and yeasts21 and mixed fungal infections.22
2 ∣. CHALLENGES IN THE DESIGN AND CONDUCT OF THE VITAL TRIAL
Compared with clinical trials of treatments for common diseases, trials for rare diseases pose some unique challenges. For example a systematic study showed that trials of rare conditions enrolled approximately half the number of patients, lasted approximately 50% longer, and were terminated at approximately twice the rate of trials for non-rare conditions.23 The design and conduct of the VITAL trial reflected the impact of these challenges.
Although clinical trials conducted in common diseases usually use a randomised controlled parallel group design, the low number of cases and the heterogeneity of underlying conditions and causative pathogens in patients with rare fungal diseases limits the ability to conduct a controlled trial versus a standard-of-care comparator within a reasonable length of time. An optimistic estimate for the time required to conduct such a study in patients with mucormycosis was more than 10 years,24 so studies of IFDs that are even more rare would be expected to take considerably longer. In addition, no standard-of-care treatments exist for many rare IFDs, and even when such a treatment may be available, differences in treatment administration (eg route, schedule) may preclude study blinding. Consequently, as with many trials of rare diseases,23 VITAL was designed as an open-label, non-comparator trial (ClinicalTrials.gov identifier, NCT00634049) (Figure 1).
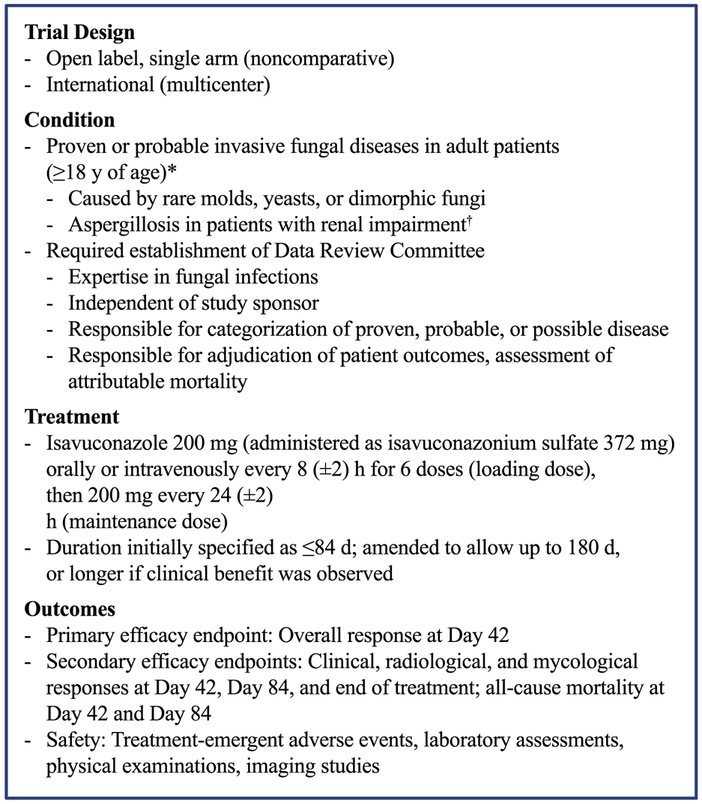
FIGURE 1.
Summary of methods and patients in the VITAL trial. *For patients enrolled with possible invasive aspergillosis, diagnostic tests to confirm the invasive aspergillosis as probable or proven were completed within 7 d after the first administration of study drug. †Could include patients on dialysis
From a sponsor’s logistical perspective, low patient numbers make finding and retaining appropriate study sites challenging. As a result, these trials are often conducted in selected medical centres across wide geographical areas. However, challenges in organisation, management and costs of trials increase as a function of the number of study sites, irrespective of whether those sites actually enrol eligible patients. To balance these competing factors, the study was planned to be conducted at approximately 150 centres globally.
Conducting trials of rare conditions in selected medical centres across a wide geographical area is usually associated with variation in standard-of-care and regional expertise that might influence and complicate diagnosis and assessment of outcome measures. To address this issue, the VITAL trial included a Data Review Committee (DRC). The DRC consisted of three experts in the field of fungal infections in immunocompromised hosts, and was independent of the trial sponsor and the study investigators. The DRC was established to adjudicate the diagnosis and categorisation of each patient’s IFD at enrolment, and to evaluate study endpoints.
A key aim of the VITAL trial was to determine the efficacy and safety of isavuconazole in adult patients (≥18 years, with or without renal impairment) with proven or culture-positive probable IFD, caused by Mucorales, rare moulds and yeasts or dimorphic fungi. The DRC-assessed appropriate inclusion of patients using the criteria initially established in 2002 by the European Organisation for Research and Treatment of Cancer/National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG).25 Revisions to the EORTC/MSG criteria for possible, probable and proven IFD were published in 200826 (ie after the initiation of the VITAL trial), and the study protocol was amended to include those revised definitions. Patients enrolled in the VITAL trial required primary therapy at enrolment; patients with clear documentation of disease progression or failure to improve clinically despite receiving at least 7 days of prior standard antifungal regimen were also enrolled. Patients initially assessed with possible IFD for whom criteria for probable or proven IFD were not met within 7 days after initiation of study drug were excluded from efficacy analyses. The VITAL trial included only patients aged 18 years or older because safety, efficacy and pharmacokinetics of isavuconazole had not been assessed in paediatric patients. Although isavuconazole may shorten the QT interval (without any obvious untoward effects),27 patients were also required to have a rate-corrected QTc interval of <500 ms. Concurrent treatments with strong inhibitors or inducers of cytochrome P450 enzymes were not allowed due to the potential for drug-drug interactions, although such interactions may be less prominent with isavuconazole compared with some other triazole antifungal agents.28 To prevent obscuring any study-treatment effects, other non-study systemic antifungal treatments were not permitted from the first dose of isavuconazole through the last follow-up visit. Isavuconazole is administered as water-soluble prodrug, whereas for IA and most IFD, all available parenteral treatment options at the time of the VITAL study were contraindicated in patients with renal impairment. For patients with possible, probable or proven IA,26 an estimated creatinine clearance (CrCl) of <50 mL/min (Cockcroft-Gault formula) was originally required; a later amendment to reflect clinical practice guidelines defined renal impairment as an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 (patients enrolled prior to the amendment were not excluded).29
Determining appropriate endpoints and the timeline for assessments is a key challenge of clinical studies conducted in rare conditions. Refractory and rare IFDs occur in a select group of patients with underlying conditions that in turn confer a high risk of treatment failure. Therefore, any measure of successful outcome in IFDs within these patients can be confounded by complications of the underlying disease, making attributable mortality and morbidity difficult to quantify.30 There have been substantial efforts to provide guidelines to assess outcomes, in which mycological, radiological and clinical outcomes are measured.30 However, these recommendations are imprecise and may be complicated by differences in disease sites. Current consensus guideline recommendations for evaluating treatment responses in patients with IA indicate that the primary endpoint should be assessed at 6 weeks of treatment (ie 42 days), and a secondary endpoint should include an assessment at 12 weeks of treatment (ie, 84 days).30 However, the optimal timing for assessments of responses in more rare IFDs is less clear.
Taking into account all the limited guidance available, the primary study endpoint for the VITAL trial was DRC-assessed overall response at Day 42; secondary endpoints included assessments of overall, clinical, radiological and mycological responses at Day 42, Day 84 and end of treatment (EOT), all-cause mortality at Days 42 and 84 and safety. Initially, the maximum treatment duration was set at 84 days. However, because no recommendations regarding duration of therapy exist for many rare IFDs, subsequent amendments extended the maximum duration to 180 days, and >180 days in cases where the investigator determined that the patient was deriving clinical benefit.
Overall response was defined as success (complete or partial) or failure (stable or progression) and was a composite of DRC-assessed clinical, mycological and radiological responses. Assessment of overall response was made using the following guidelines:
Complete: Resolution of all clinical symptoms and physical findings associated with IFD; resolution of radiological abnormalities and presumed or documented eradication.
Partial: Resolution of at least some clinical symptoms and physical findings associated with IFD; improvement of radiological abnormalities; and presumed or documented eradication.
Stable: Minor or no change in clinical symptoms, physical findings and radiological abnormalities associated with IFD, but no evidence of progression based on clinical, radiological and mycological criteria.
Progression: Evidence of progression based on clinical, radiological or mycological criteria (persistence or presumed persistence). Worsening or new clinical symptoms, physical findings or radiological abnormalities associated with IFD, or the requirement for alternative systemic antifungal treatment.
The DRC used the following guidelines for the response determination:
Clinical success required complete or partial resolution of all, some or none of the attributable clinical symptoms and physical findings; stable or worsening symptoms and findings were ruled as failures.
Mycological success required eradication (negative follow-up culture[s]/histology or cytology available) or presumed eradication (documentation missing but symptoms/signs disappeared); presumed persistence (documentation missing but symptoms/signs continue) or persistence by continued positive culture or histopathology, were considered failures.
Radiological success required at least a 25% improvement from baseline at 42 days or at least a 50% improvement from baseline at 84 days; patients not meeting these criteria, or those with no post-baseline radiology available that showed baseline evidence of radiological disease or without radiology available at baseline, were ruled as failures.
Categorisation of outcome data is difficult in studies of rare IFDs. Radiographs often do not change rapidly. The requirement for radiological resolution to interpret treatment success of some rare IFDs as “complete” presents another challenge. For example, imaging of pulmonary mucormycosis frequently shows cavitation resulting from localised tissue necrosis.31 In some cases, resolution of the infection is accompanied by thinning of the cavity wall without an appreciable reduction in cavity size. In such cases, radiological success, and therefore treatment success, may be underestimated. Mycological criteria may be difficult to assess due to the frequent lack of follow-up cultures and validated biomarkers. Under the criteria set out in the VITAL study protocol, the DRC often had to categorise a patient as “stable,” which was classified as an outcome failure, although disease stabilisation in a high-risk patient (which might include minor clinical improvement) may be considered a success in normal clinical practice. Although all-cause mortality is a much more defined and objective outcome than other measures of response, the underlying disease state of the patient is often very severe, and death is often not attributable to IFD. Thus, this outcome cannot fully capture the antifungal treatment effect.
Inclusion of a control group for the VITAL trial was not feasible given the limitations discussed above, and the low numbers of patients in any study of rare diseases can limit the ability to develop any statistical power to form robust conclusions. However, in the absence of a control group, historical data can sometimes be used for studies of rare diseases, in accordance with guidance provided by the US Food and Drug Administration.32 In the case of mucormycosis, a sufficient number of patients were enrolled in the VITAL trial to allow a subsequent case-matched analysis using mortality outcomes data from the FungiScope: Global Emerging Fungal Infection Registry (ClinicalTrials.gov identifier: NCT01731353),33 as described in more detail elsewhere.19
3 ∣. OVERVIEW OF RESULTS FROM THE VITAL TRIAL
The VITAL trial began enrolment on April 22, 2008. The study was put on hold on January 23, 2009 to allow for completion of in vivo genotoxicity studies of a newly identified isavuconazole synthesis by-product. Those studies were completed and supported the resumption of enrolment. In February 2010, while the trial was on hold, sponsorship was transferred from Basilea Pharmaceutica International Ltd. to Astellas Pharma Global Development, Inc. The trial was reopened for enrolment by Astellas on April 3, 2011 (Figure 2).
FIGURE 2.
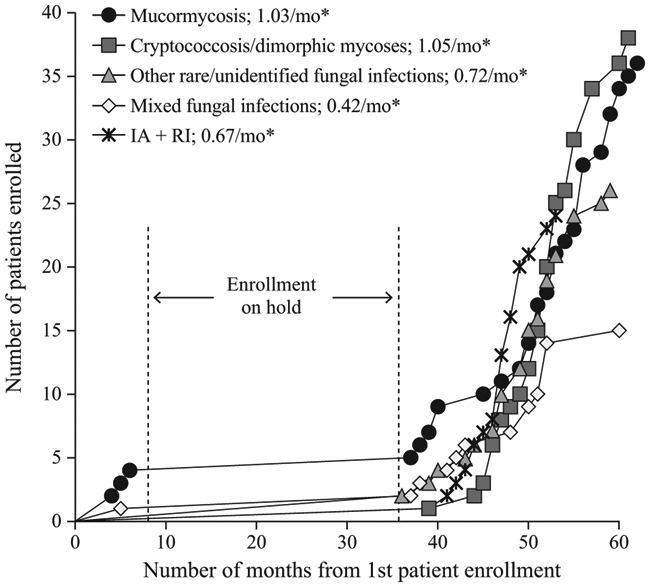
Enrolment of patients over time in the VITAL trial. IA, invasive aspergillosis; RI, renal impairment. *Enrolment rates calculated as number of patients divided by 36-month enrolment duration (63 mo from study start to last patient enrolled minus 27 mo while trial was on hold)
3.1 ∣. Baseline characteristics and treatment duration
Between August 25, 2008 and June 21, 2013, 149 patients consented to participate in the trial and 146 received at least one dose of isavuconazole. Six patients were assessed by the DRC as having possible or no IFD and were excluded. Thus the modified intent- to-treat (mITT) population (patients with DRC-confirmed IFD who received at least one dose of isavuconazole) was composed of 140 patients with DRC-confirmed, qualifying IFDs (5 enrolled prior to hold, 135 after resuming the trial). Patients were enrolled from 34 study centres in Belgium, Brazil, Germany, India, Israel, Lebanon, Mexico, Russia, South Korea, Thailand and the United States. The trial population included 37 patients with mucormycosis only, 38 patients with IFD caused by Cryptococcus spp. or dimorphic fungi, 26 patients with IFD caused by other rare moulds or yeasts, 15 patients with IFD caused by multiple (mixed) fungal species and 24 patients with IA and renal impairment at enrolment. Patients with IFD caused by multiple fungal species were not specifically targeted for enrolment but were allowed to participate at the discretion of the investigator(s) (see accompanying article22). After the protocol amendment redefining renal impairment on the basis of eGFR instead of CrCl (see previous section), 4 of the 24 patients initially categorised as having renal impairment were omitted from that analysis population (Table 1).
TABLE 1.
Baseline characteristics of patients with proven/probable invasive fungal disease in the VITAL trial
| Parameter | Mucormycosis19 n = 37 |
Cryptococcosis and dimorphic mycoses20 n = 38 |
Emerging fungal infections21 n = 26 |
Mixed fungal infections22 n = 15 |
Invasive aspergillosis with renal impairment n = 20 |
Invasive aspergillosis with renal impairment n = 4 |
|---|---|---|---|---|---|---|
| Median age, years | 50 | 45 | 50.5 | 54 | 61 | 38.5 |
| Male, n (%) | 30 (81.1) | 27 (71.1) | 16 (61.5) | 8 (53.3) | 12 (60.0) | 3 (75.0) |
| Race, n (%) | ||||||
| White | 25 (67.6) | 25 (65.8) | 19 (73.1) | 12 (80.0) | 17 (85.0) | 4 (100) |
| Black or African American | 4 (10.8) | 3 (7.9) | 2 (7.7) | 1 (6.7) | 0 | 0 |
| Other | 8 (21.6) | 10 (26.3) | 5 (19.2) | 2 (13.3) | 3 (25.0) | 0 |
| Neutropaenic, n (%) | 10 (27.0) | 0 | 10 (45.5) | 6 (40.0) | 5 (25.0) | 3 (75.0) |
| Allogeneic BMT, n (%) | 13 (35.1) | 0 | 3 (11.5) | 0 | 7 (35.0) | 2 (50.0) |
| Uncontrolled malignancy, n (%) | 18 (48.6) | 2 (5.3) | 10 (38.5) | 5 (33.3) | 5 (25.0) | 2 (50.0) |
| Haematological malignancy, n (%) | 22 (59.5) | 1 (2.6) | 14 (53.8) | 7 (46.7) | 11 (55.0) | 3 (75.0) |
| Corticosteroid use, n (%) | 10 (27.0) | 4 (10.5) | 4 (15.4) | 4 (26.7) | 12 (60.0) | 1 (25.0) |
BMT, bone marrow transplant.
Among patients with mucormycosis only,19 baseline characteristics were largely consistent with the epidemiologic characteristics of patients with mucormycosis identified in an analysis of a European registry.34 For example the underlying risk factor for IFD in most patients was haematological malignancy, most patients had pulmonary disease (with or without dissemination to other organs) and the most frequent pathogens were Rhizopus spp. and Mucor spp. Data regarding risk factors and disease sites for cryptococcosis and dimorphic mycoses are more scant, but the frequent localisation of cryptococcosis in the lungs and central nervous system of many patients in the VITAL trial20 was consistent with available data.35 Haematological malignancies were rare in patients with cryptococcosis and dimorphic mycoses (1 of 38 patients). The most common underlying condition in these patients was diabetes mellitus (5 of 38 patients) and no underlying condition was identified in half of this patient group. Data regarding risk factors for infections with other fungal species reported in the accompanying articles21,22 are also comparatively limited. A recent analysis of data from the literature found that the most common underlying risk factor for fusariosis was haematological malignancy,36 which was consistent with the most common underlying risk factors observed in the VITAL trial in patients with fusariosis alone.21 All other pathogens in the VITAL trial were too rare to allow any meaningful comparisons with existing data. Among the patients with IA in the VITAL trial, those with renal impairment tended to be older than those without renal impairment. They also included smaller proportions of patients with neutropenia, allogeneic bone marrow transplant, uncontrolled malignancy and haematological malignancy, and included a larger proportion with corticosteroid use.
The duration of treatment for each of the groups of patients with rare IFDs assessed in the VITAL trial was substantially longer than observed for treatment of IA in the SECURE trial (isavuconazole group, median 45 days37). In patients from VITAL with IA, the median duration of treatment was 54 days for those with renal impairment and 204 days for those without renal impairment. The median durations of treatment for patients with mucormycosis, cryptococcosis and dimorphic mycoses, other emerging fungal infections, and mixed fungal infections were 84 days, 180 days, 114.5 days and 97 days respectively. Some patients with rare and mixed fungal infections had a particularly protracted treatment regimen. Maximum durations of treatment for patients with mucormycosis, cryptococcosis and dimorphic mycoses, other emerging fungal infections, mixed fungal infections and IA with or without renal impairment were 882 days, 331 days, 496 days, 544 days, 174 days and 343 days, respectively. Two patients with mucormycosis, one patient with histoplasmosis, one patient with a mixed fungal infection (aspergillosis and mucormycosis) and one patient with IA without renal impairment continued treatment past the end of the study. These data suggest that the optimal treatment duration for many rare IFDs may be substantially longer than for IA.
3.2 ∣. Efficacy
Among the different patient groups, rates of treatment success (overall response) at Day 42 ranged from 10.8% to 50.0%, and confirmed survival at Day 42 ranged from 62.2% to 100% (Table 2). In the analysis of patients with only mucormycosis, 21 patients who received isavuconazole as primary treatment were matched with 33 case controls from the FungiScope registry.19 Higher proportions of patients from the VITAL trial had proven mucormycosis and severe disease compared with the amphotericin B-treated controls (86% vs 61%, and 57% vs 39%, respectively). Despite that, the rates of survival in patients from the VITAL trial were similar in both crude and weighted analyses (67% vs 61%, and 67% vs 59%, respectively). Outcomes among patients with cryptococcosis or dimorphic mycoses were also notable,20 as the rates of treatment success were within the range of those observed in previous studies assessing the efficacy of other triazole antifungal agents to treat subsets of these IFDs (38.9% to 94.4%38-43). Among patients with other emerging fungal infections, more than half of evaluable patients responded by EOT.21 The overall success rate at EOT was considerably less for patients with mixed fungal infections; still, two-thirds of patients in this group were confirmed alive at the end of the study.22 In patients with IA, the rate of treatment success overall at EOT (8/24 [33.3%]) was similar to that observed with isavuconazole in patients with proven and probable IFD in the SECURE trial (35%37), and was highest in the subgroup of patients without renal impairment (66.7%). The rates of all-cause mortality in patients with and without renal impairment were lower at both Day 42 (15% and 0% respectively) and Day 84 (25% for both) than observed with isavuconazole in the SECURE trial (20% at Day 42; 30% at Day 8437).
TABLE 2.
Treatment outcomes for patients with proven/probable invasive fungal disease in the VITAL trial
| Parameter, n (%) |
Mucormycosis19 n = 37 |
Cryptococcosis and dimorphic mycoses20 n = 38 |
Emerging fungal infections21 n = 26 |
Mixed fungal infections22 n = 15 |
Invasive aspergillosis with renal impairment n = 20 |
Invasive aspergillosis without renal impairment n = 4 |
|---|---|---|---|---|---|---|
| Overall response, Day 42 | 4 (10.8) | 15 (39.5) | 11 (42.3) | 2 (13.3) | 5 (25.0) | 2 (50.0) |
| Overall response, Day 84 | 7 (18.9) | 16 (42.1) | 10 (38.5) | 2 (13.3) | 6 (30.0) | 1 (25.0) |
| Overall response, end of treatment | 11 (31.4)a | 24 (64.9)b | 15 (57.7) | 2 (14.3)b | 6 (30.0) | 2 (66.7)b |
| All-cause mortality, Day 42 | 14 (37.8) | 3 (7.9) | 2 (7.7) | 2 (13.3) | 3 (15.0) | 0 |
| All-cause mortality, Day 84 | 16 (43.2) | 3 (7.9) | 4 (15.4) | 4 (26.7) | 5 (25.0) | 1 (25.0) |
All-cause mortality data include patients lost to follow-up.
Two patients continued treatment beyond the study period, excluded from denominator for calculation of percentage.
One patient continued treatment beyond the study period, excluded from denominator for calculation of percentage.
3.3 ∣. Safety and tolerability
Among all 146 patients who received at least one dose of isavuconazole in the VITAL trial, 139 patients (95.2%) experienced ≥1 treatment-emergent adverse event (TEAE), 60 patients (41.1%) experienced ≥1 TEAEs considered by the investigator as probably, possibly, or remotely related to isavuconazole treatment, 89 patients (61.0%) experienced ≥1 serious TEAEs and in 13 patients (8.9%) TEAEs led to permanent discontinuation of isavuconazole treatment. Analyses of TEAEs within each of the groups of patients with rare IFDs are shown in Table 3. Serious TEAEs were less frequent in the group of patients with cryptococcosis and dimorphic mycoses compared with all other groups. Most TEAEs involved the gastrointestinal tract, and the overall safety profile of isavuconazole in the VITAL trial did not differ substantially from that observed in the SECURE trial in patients with IFDs.37
TABLE 3.
Treatment-emergent adverse events (TEAEs) in patients with proven/probable invasive fungal disease in the VITAL trial
| TEAEs, n (%) |
Mucormycosis19 n = 37 |
Cryptococcosis and dimorphic mycoses20 n = 38 |
Emerging fungal infections21 n = 26 |
Mixed fungal infections22 n = 15 |
Invasive aspergillosisa n = 24 |
Total N = 140 |
|---|---|---|---|---|---|---|
| Any TEAE | 35 (94.6) | 33 (86.8) | 26 (100) | 15 (100) | 24 (100) | 133 (95.0) |
| Serious TEAEs | 28 (75.7) | 12 (31.6) | 15 (57.7) | 12 (80.0) | 19 (79.2) | 87 (62.1) |
| Most common TEAEs (MedDRA preferred term)b | ||||||
| Vomiting | 12 (32.4) | 5 (13.2) | 3 (11.5) | 2 (13.3) | 9 (37.5) | 31 (22.1) |
| Nausea | 10 (27.0) | 3 (7.9) | 6 (23.1) | 3 (20.0) | 8 (33.3) | 30 (21.4) |
| Diarrhoea | 10 (27.0) | 2 (5.3) | 6 (23.1) | 2 (13.3) | 5 (20.8) | 25 (17.9) |
| Pyrexia | 10 (27.0) | 0 | 4 (15.4) | 4 (26.7) | 5 (20.8) | 23 (16.4) |
| Headache | 6 (16.2) | 2 (5.3) | 3 (11.5) | 4 (26.7) | 7 (29.2) | 22 (15.7) |
| Peripheral oedema | 6 (16.2) | 0 | 5 (19.2) | 1 (6.7) | 3 (12.5) | 15 (10.7) |
| Constipation | 8 (21.6) | 2 (5.3) | 2 (7.7) | 2 (13.3) | 0 | 14 (10.0) |
| Hypokalaemia | 2 (5.4) | 2 (5.3) | 1 (3.8) | 2 (13.3) | 4 (16.7) | 11 (7.9) |
| Confusional state | 1 (2.7) | 2 (5.3) | 1 (3.8) | 2 (13.3) | 4 (16.7) | 10 (7.1) |
| Urinary tract infection | 2 (5.4) | 2 (5.3) | 4 (15.4) | 0 | 2 (8.3) | 10 (7.1) |
| Decreased appetite | 6 (16.2) | 0 | 1 (3.8) | 0 | 2 (8.3) | 9 (6.4) |
| Respiratory failure | 3 (8.1) | 0 | 5 (19.2) | 0 | 1 (4.2) | 9 (6.4) |
MedDRA, Medical Dictionary for Regulatory Activities, version 12.1.
Includes patients with and without renal impairment (n = 20 and n = 4 respectively).
Occurring in ≥15% of patients in any group.
4 ∣. LESSONS FROM THE VITAL TRIAL
Despite the challenges faced, the results of the VITAL trial provide evidence that an interventional trial to test a treatment for rare IFDs can be efficiently designed, conducted and communicated to the medical community. The VITAL trial has demonstrated efficacy of isavuconazole for the treatment of mucormycosis19 and suggests that isavuconazole is also effective in the treatment of infections caused by Cryptococcus spp. and dimorphic fungi.20 Although treatment successes were observed for some patients with IFDs caused by other rare fungal species21 and mixed fungal species,22 evidence for efficacy of isavuconazole in those groups is insufficient to allow any definitive conclusions, but form a basis that can be supplemented with data from future trials, case reports and database analyses. Evidence for efficacy of isavuconazole in patients with IA and renal impairment is also insufficient for any firm conclusions, but it suggests that there is no loss of efficacy or drug-specific safety concerns in those patients. Moreover, analysis of study data has allowed for the recruitment rates of several IFDs to be measured, which may help guide future studies of rare IFDs. The relatively low recruitment rates suggest that, as new broad-spectrum antifun-gals are developed, it may be advisable to start a clinical protocol for particular rare IFDs in early phase studies. In addition, the duration of treatment was extended to more than 180 days for a substantial number of patients who had been deemed by investigators to be deriving clinical benefit. Therefore, the optimal timing for assessing outcomes for treatments of rare IFDs still needs to be determined.
Assessing potential treatments for rare IFDs and in special patient populations will continue to pose a challenge for the foreseeable future. Nevertheless, it is crucial that such treatments for emerging IFDs and for IFDs in special patient populations are evaluated, even when there are multiple obstacles to the performance of robust studies. New drug classes with activity against azole-resistant fungal species are currently in clinical development (eg tetrazoles, pyrimidine synthesis inhibitors, glycosylphosphatidylinositol inhibitors44) and they are facing similar study-design challenges. It is hoped that future improvements and integration of biomarkers and radiography might be used to enhance assessment of the impact of antifungal agents in these patients. Until that time, carefully planned and executed, prospective trials such as the VITAL trial provide useful information to inform clinical decisions regarding the use of new antifungal agents and provide a reasonable estimate of their therapeutic outcome.
ACKNOWLEDGMENTS
This analysis was funded by Astellas Pharma, Inc. Isavuconazonium sulphate has been co-developed by Astellas Pharma Global Development, Inc. and Basilea Pharmaceutica International Ltd. Editorial assistance was provided by Ed Parr, PhD, CMPP, of Envision Scientific Solutions, funded by Astellas Pharma, Inc. We thank Neddie Zadeikis (formerly of Astellas Pharma Global Development, Inc.) for her contribution to the design and execution of the study. The authors are also grateful for the contributions of Nkechi Azie, formerly of Astellas Pharma Global Development, Inc., to the development of the manuscript. The authors are also grateful for the contributions of the investigators and staff who conducted the VITAL trial, and to the patients who volunteered for this study.
Funding information Astellas Pharma, Inc., Grant/Award Number: N/A
Footnotes
Markus Heep affiliation during the conduct of the study.
REFERENCES
- 1.Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48:265–273. [DOI] [PubMed] [Google Scholar]
- 2.Husain S, Silveira FP, Azie N, Franks B, Horn D. Epidemiological features of invasive mold infections among solid organ transplant recipients: PATH Alliance® registry analysis. Med Mycol. 2017;55:269–277. [DOI] [PubMed] [Google Scholar]
- 3.Araujo R, Oliveira M, Amorim A, Sampaio-Maia B. Unpredictable susceptibility of emerging clinical moulds to tri-azoles: review of the literature and upcoming challenges for mould identification. Eur J Clin Microbiol Infect Dis. 2015;34:1289–1301. [DOI] [PubMed] [Google Scholar]
- 4.Cornely OA, Cuenca-Estrella M, Meis JF, Ullmann AJ. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study Group (EFISG) and European Confederation of Medical Mycology (ECMM) 2013 joint guidelines on diagnosis and management of rare and emerging fungal diseases. Clin Microbiol Infect. 2014;20(Suppl 3):l–4. [DOI] [PubMed] [Google Scholar]
- 5.Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tissot F, Agrawal S, Pagano L, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2016;102:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Vfend (voriconazole) summary of product characteristics. 2014; http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000387/WC500049756.pdf. Accessed September 1, 2016.
- 8.Pfizer Inc. VFEND® (voriconazole) prescribing information. 2015; http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021266s038,021267s047,021630s028lbl.pdf. Accessed September 1, 2016.
- 9.Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. 2014;20(Suppl 3):27–46. [DOI] [PubMed] [Google Scholar]
- 10.Cornely OA, Arikan-Akdagli S, Dannaoui E, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20(Suppl 3):5–26. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg RN, Mullane K, van Burik JA, et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother. 2006;50:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42:e61–e65. [DOI] [PubMed] [Google Scholar]
- 13.Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801–1812. [DOI] [PubMed] [Google Scholar]
- 14.Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217–1223. [DOI] [PubMed] [Google Scholar]
- 15.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825. [DOI] [PubMed] [Google Scholar]
- 17.Deray G Amphotericin B nephrotoxicity. J Antimicrob Chemother. 2002;49(Suppl 1):37–41. [DOI] [PubMed] [Google Scholar]
- 18.Wade RL, Chaudhari P, Natoli JL, Taylor RJ, Nathanson BH, Horn DL. Nephrotoxicity and other adverse events among inpatients receiving liposomal amphotericin B or amphotericin B lipid complex. Diagn Microbiol Infect Dis. 2013;76:361–367. [DOI] [PubMed] [Google Scholar]
- 19.Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828–837. [DOI] [PubMed] [Google Scholar]
- 20.Thompson GR, Rendon A, Ribeiro Dos Santos R, et al. Isavuconazole treatment of cryptococcosis and dimorphic mycoses. Clin Infect Dis. 2016;63:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornely OA, Mullane KM, Ostrosky-Zeichner L, et al. Isavuconazole for treatment of rare invasive fungal diseases. Mycoses. 2018; 10.1111/myc.12778. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Marty FM, Cornely OA, Mullane KM, et al. Isavuconazole for treatment of invasive fungal diseases caused by more than one fungal species. Mycoses. 2018. 10.1111/myc.12777. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Bell SA, Tudur Smith C. A comparison of interventional clinical trials in rare versus non-rare diseases: an analysis of ClinicalTrials.gov. Orphanet J Rare Dis. 2014;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornely OA, Maher RM, Marty FM, Lee M, Vehreschild JJ, Vehreschild MJ. The VITAL study: case control studies are hypothesis-generating - Authors’ reply. Lancet Infect Dis. 2016;16:886–887. [DOI] [PubMed] [Google Scholar]
- 25.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. [DOI] [PubMed] [Google Scholar]
- 26.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keirns J, Desai A, Kowalski D, et al. QT interval shortening with isavuconazole: in vitro and in vivo effects on cardiac repolarization. Clin Pharmacol Ther. 2017;101:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Townsend R, Dietz A, Hale C, et al. Pharmacokinetic evaluation of CYP3A4-mediated drug-drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethin-drone in healthy adults. Clin Pharmacol Drug Dev. 2017;6:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.KDIGO CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 30.Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAdams HP, Rosado de Christenson M, Strollo DC, Patz EF Jr. Pulmonary mucormycosis: radiologic findings in 32 cases. AJR Am J Roentgenol. 1997;168:1541–1548. [DOI] [PubMed] [Google Scholar]
- 32.US Food and Drug Administration. Guidance for industry: E10 choice of control group and related issues in clinical trials. 2001; http://www.fda.gov/downloads/drugs/guidancecomplianceregula-toryinformation/guidances/ucm073139.pdf. Accessed February 1, 2017.
- 33.Rüping MJ, Heinz WJ, Kindo AJ, et al. Forty-one recent cases of invasive zygomycosis from a global clinical registry. J Antimicrob Chemother. 2010;65:296–302. [DOI] [PubMed] [Google Scholar]
- 34.Skiada A, Pagano L, Groll A, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17:1859–1867. [DOI] [PubMed] [Google Scholar]
- 35.Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Hatmi AM, Hagen F, Menken SB, Meis JF, de Hoog GS. Global molecular epidemiology and genetic diversity of Fusarium, a significant emerging group of human opportunists from 1958 to 2015. Emerg Microbes Infect. 2016;5:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–769. [DOI] [PubMed] [Google Scholar]
- 38.Dismukes WE, Bradsher RW Jr, Cloud GC, et al. Itraconazole therapy for blastomycosis and histoplasmosis. NIAID Mycoses Study Group. Am J Med. 1992;93:489–497. [DOI] [PubMed] [Google Scholar]
- 39.Galgiani JN, Catanzaro A, Cloud GA, et al. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis: a randomized, double-blind trial. Ann Intern Med. 2000;133:676–686. [DOI] [PubMed] [Google Scholar]
- 40.Kim MM, Vikram HR, Kusne S, Seville MT, Blair JE. Treatment of refractory coccidioidomycosis with voriconazole or posaconazole. Clin Infect Dis. 2011;53:1060–1066. [DOI] [PubMed] [Google Scholar]
- 41.Perfect JR, Marr KA, Walsh TJ, et al. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003;36:1122–1131. [DOI] [PubMed] [Google Scholar]
- 42.Pitisuttithum P, Negroni R, Graybill JR, et al. Activity of posaconazole in the treatment of central nervous system fungal infections. J Antimicrob Chemother. 2005;56:745–755. [DOI] [PubMed] [Google Scholar]
- 43.Queiroz-Telles F, Goldani LZ, Schlamm HT, Goodrich JM, Espinel-Ingroff A, Shikanai-Yasuda MA. An open-label comparative pilot study of oral voriconazole and itraconazole for long-term treatment of paracoccidioidomycosis. Clin Infect Dis. 2007;45:1462–1469. [DOI] [PubMed] [Google Scholar]
- 44.McCarthy MW, Kontoyiannis DP, Cornely OA, Perfect JR, Walsh TJ. Novel agents and drug targets to meet the challenges of resistant fungi. J Infect Dis. 2017;216(suppl_3):S474–S483. [DOI] [PubMed] [Google Scholar]