Watch the interview with the authors
Watch the video presentation of this article
Abbreviations.
- BOC
boceprevir
- DAA
direct‐acting antiviral
- eRVR
extended rapid virological response
- HCV
hepatitis C virus
- IFN
interferon
- NANB
non‐A, non‐B
- PEG
pegylated interferon
- PI
protease inhibitor
- RBV
ribavirin
- RGT
response‐guided therapy
- RVR
rapid virological response
- SVR
sustained virological response
- tiw
3 times a week
- TVR
telaprevir.
In the early 1960s, when only hepatitis types A and B were recognized, treatment was focused on the acute illness and consisted of strict bed rest, a nutritious diet, and the judicious use of medications.1, 2 Corticosteroid treatment was attempted and reduced serum bilirubin levels, but it probably promoted the evolution of acute hepatitis B to chronic hepatitis B.3, 4 The search for drugs was impelled by the discovery of hepatitis B virus, which permitted the accurate identification of chronic hepatitis B.5 Numerous drugs were evaluated, but many had little positive effect or actually caused harm.4, 6 Among them, interferon (IFN) appeared to be the most effective.7
In the mid‐1970s, after hepatitis A virus had been identified, non‐A, non‐B (NANB) hepatitis was recognized8; it was originally believed to be inconsequential but was later documented to be a mostly progressive disease that often advanced silently to cirrhosis and even cancer.9 Thus, together with efforts to identify the causative agent, attention was turned to seeking drug treatments that might impede this inexorable advance.
Acyclovir, one of the first antiviral agents to be evaluated, failed to show a positive effect.10 The apparent success of IFN for hepatitis B, however, encouraged a pilot study at the National Institutes of Health in 1986: recombinant IFNα was used to treat patients with NANB hepatitis even before hepatitis C virus (HCV) was identified as the cause of the disease.11 Encouraging results from this study prompted several mid‐sized controlled trials with IFN for NANB hepatitis,12, 13 and its partial effectiveness was confirmed. IFNα alone, dosed at 3 MU 3 times a week (tiw) for 24 weeks, induced an end‐of‐treatment response in approximately one‐third of patients; most, however, relapsed, and this left a sustained virological response (SVR) rate of approximately 6% (Fig. 1).12, 13 Several studies followed in the 1990s with the aim of establishing the appropriate dose and the necessary treatment length. Increasing the treatment length to 48 weeks raised SVR rates to approximately 16%. Meanwhile, ribavirin (RBV), when it was evaluated as monotherapy, was found to lower alanine aminotransferase levels but not HCV RNA levels.14 However, when it was added to IFNα, RBV raised SVR rates to approximately 34% after 24 weeks of treatment and to approximately 42% after 48 weeks.15, 16 The achievement of SVR was later regarded as a virological cure because almost all who achieved SVR remained aviremic a decade or more later with an excellent long‐term prognosis (Fig. 2).17–19 The next step was enhancing the half‐life of IFN via pegylation, which improved the virological response rates and reduced the injection frequency. Trials using weekly injections of long‐acting pegylated interferon (PEG) alone for 48 weeks induced a positive SVR rate of approximately 39%, and this rate increased to approximately 54% to 56% when PEG was coupled with RBV for 48 weeks (Fig. 1).20–22 Host factors that were associated with a good response were young age, female sex, non–African American heritage, and low fibrosis levels.23–25
Figure 1.
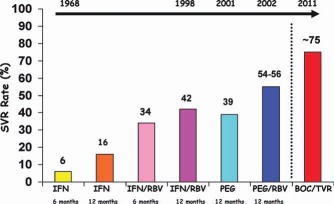
SVR rates for patients with HCV infections (genotypes 1–3) according to the treatment regimens and durations.
Figure 2.
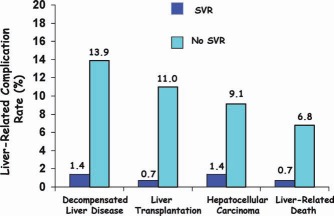
Frequency of liver‐related complications after treatment with PEG and RBV for patients with SVR and patients without SVR.
On this background, 3 registration trials were conducted using 2 different products: peginterferon alfa‐2a, which was given weekly in a fixed SQ dose of 180 μg and ribavirin tablets daily, the dose of which were adjusted for weight (1,000 mg for <75 kg; 1,200 mg for >75 kg given daily), and peginterferon alfa‐2b, 1.5 μg/kg SQ given weekly with a fixed dose of ribavirin (800 mg daily) (Figures 3, 4, 5).26–28 Three important findings emerged from these trials:
-
1
SVR rates were higher for patients infected with HCV genotype 2 or 3 (76%‐82%) versus patients with genotype 1 (42%‐46%; Figs. 3 and 4).
-
2
SVR rates were higher (78%) when the baseline viral loads were lower (HCV RNA ≤ 2 × 106 IU/ml), and they were lower (42%) when the baseline viral loads were higher (HCV RNA > 2 × 106; Fig. 4).
-
3
Genotype 2/3 patients could be treated for 24 weeks, whereas patients with genotype 1 infections required treatment for 48 weeks (Fig. 5).
Figure 3.
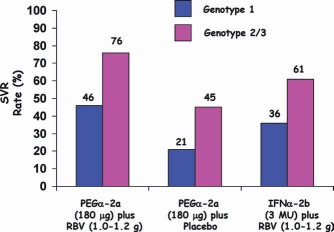
SVR rates with PEGα‐2a or IFNα‐2b and RBV according to the genotype.27
Figure 4.
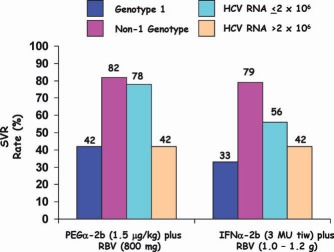
SVR rates with PEGα‐2b and RBV therapy for 48 weeks according to the genotype and the viral concentration.26
Figure 5.
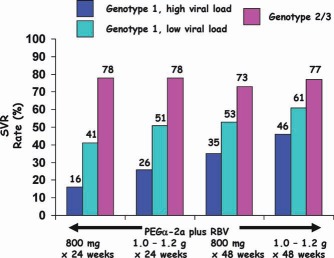
SVR rates for recipients of PEGα‐2a and RBV (two different doses) for 24 or 48 weeks.28
Moreover, the improvement in the SVR rate with the addition of RBV was primarily the result of a reduced relapse rate; there was only a small direct antiviral effect (Fig. 6).
Figure 6.
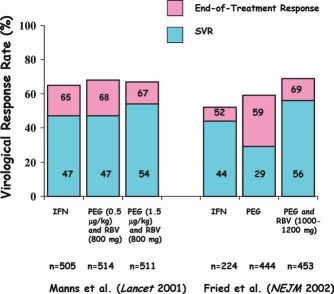
Virological responses to IFN, PEG, and RBV in two US registration trials demonstrating that RBV reduces virological relapse.26, 27
Subsequent efforts focused on improving response rates among patients naive to treatment through increases in the treatment dose or duration, and there was partial success.29–32 More useful was the creation of definitions for virological responses, including the end‐of‐treatment response, SVR, breakthrough, relapse, nonresponse, and null response. Definitions for predicting early virological responses were particularly useful for shortening or curtailing treatment. Negative HCV RNA results in treatment week 4 [i.e., a rapid virological response (RVR)] allowed the treatment to be shortened for patients with genotype 2/3 or genotype 1 with a low viral load33–35; negative HCV RNA results or reduced HCV RNA levels (at least 2‐log) at week 12 (an early virological response) predicted the likelihood of SVR.36, 37
The treatment effectiveness varied in retreated partial responders and nonresponders and in difficult‐to‐treat groups and other originally untreated groups: children; patients with acute HCV, human immunodeficiency virus coinfections, or kidney disease; patients who received liver or solid organ transplants; patients with compensated or decompensated cirrhosis; patients with psychiatric disorders; African Americans; and active injection drug users.38–64 One large trial of nonresponders failed to show efficacy with the extension of PEG treatment to 3.5 years.41
As our knowledge of the structure, function, lifecycle, and pathogenesis of HCV increased, intense investigations were undertaken in the past decade to develop therapies directed at the virus itself because of lingering subpar response rates of HCV genotype 1 to PEG/RBV treatment.65 Protease inhibitors (PIs) are the first of these direct‐acting antivirals (DAAs) to show promise: they inhibit the nonstructural 3/4A protease of HCV genotype 1 and dramatically decrease HCV RNA levels.66, 67 However, when they are used as monotherapy, resistance mutations develop rapidly; these mutations significantly decline when PIs are combined with PEG and RBV.68 International phase 2 and 3 trials of two PIs, telaprevir (TVR) and boceprevir (BOC), which were evaluated for their safety and efficacy in treating HCV genotype 1 disease, demonstrated the following69–75 (Figs. 7, 8, 9):
-
4
Both led to rapid, sustained declines in HCV RNA in treatment‐naive patients with HCV genotype 1.
-
5
Both shortened the duration of therapy without sacrificing SVR in patients achieving RVR.
-
6
Both led to minimal relapse and development of resistance mutations.
-
7
Both had efficacy in prior relapsers and partial responders to PEG and RBV.
-
8
Both had improved (albeit lesser) efficacy in patients with cirrhosis and in African Americans.
Figure 7.
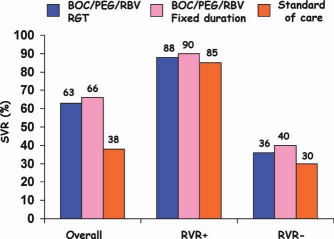
Overall SVR rates and SVR rates based on RVR [i.e., undetectable HCV RNA at week 8 (week 4 of triple therapy)] for treatment‐naive patients with HCV genotype 1 infections. Abbreviation: RGT, response‐guided therapy.73
Figure 8.
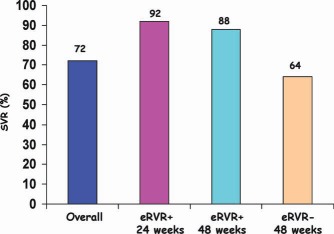
SVR rates for treatment‐naive patients with chronic HCV genotype 1 infections who received triple therapy (TVR, PEG, and RBV) for 24 or 48 weeks according to the achievement or nonachievement of extended rapid virological response (eRVR; i.e., undetectable HCV RNA at weeks 4 and 12).75
Figure 9.
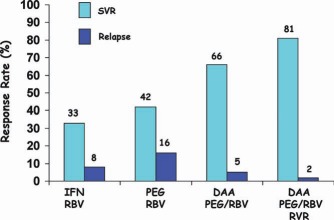
SVR and relapse rates according to the treatment regimen. The DAAs were BOC and TVR.
The PIs induced SVR rates in patients with genotype 1 infections similar to the rates achieved by patients with genotype 2/3 infections who were treated with PEG and RBV alone (Figs. 10 and 11). Triple therapy is now regarded as the standard of care for patients with HCV genotype 1 infections,76 although it is very likely that future DAAs will be effective and safe without the need for added PEG and RBV.
Figure 10.
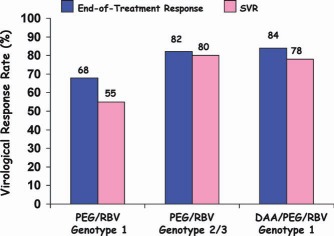
End‐of‐treatment and SVR rates for treatment‐naive patients with genotype 1 or genotype 2/3 HCV infections who received standard‐of‐care therapy (PEG) versus treatment‐naive patients with genotype 1 infections who were treated with DAAs (BOC and TVR).
Figure 11.
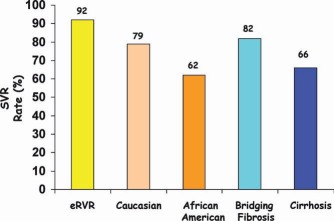
SVR rates for treatment‐naive patients with chronic HCV genotype 1 infections who were treated with PIs according to the achievement of extended rapid virological response (eRVR) and racial and histological characteristics.
Potential conflict of interest: Nothing to report.
References
- Seeff LB. Diagnosis, therapy, and prognosis of viral hepatitis In: Zakim D, Boyer TD, eds. Hepatology: A Textbook of Liver Disease. Philadelphia, PA: WB Saunders; 1982: 911–977. [Google Scholar]
- Chalmers TC, Reynolds WE, Eckhardt RD, Cigarroa JG, Reifenstein WE, Smith CW, et al. Treatment of acute infectious hepatitis in the armed forces: advantages of ad lib bed rest and early reconditioning. JAMA 1955; 159: 1431–1434. [DOI] [PubMed] [Google Scholar]
- Huber TE, Wiley AT. Cortisone in the treatment of infectious hepatitis. Ann Intern Med 1955; 42: 1011–1025. [DOI] [PubMed] [Google Scholar]
- Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 2001; 120: 1009–1022. [DOI] [PubMed] [Google Scholar]
- Blumberg BS, Gerstley BJ, Hungerford DA, London WT, Sutnick AI. A serum antigen (Australia antigen) in Down's syndrome, leukemia, and hepatitis. Ann Intern Med 1967; 66: 924–931. [DOI] [PubMed] [Google Scholar]
- Ferenci P. Historical treatment of chronic hepatitis B and C. Gut 1993; 34(suppl): S69‐S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg HB, Pollard RB, Litwick LI, Gregory PB, Robinson WS, Merigan TC. Effect of human leukocyte interferon on hepatitis B virus infection in patients with chronic active hepatitis. N Engl J Med 1976; 295: 517–522. [DOI] [PubMed] [Google Scholar]
- Feinstone SM, Kapikian AZ, Puecell RH, Alter HJ, Holland PV. Transfusion‐associated hepatitis not due to viral hepatitis type A or B. N Engl J Med 1975; 292: 767–770. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis 2005; 9: 383–398. [DOI] [PubMed] [Google Scholar]
- Pappas SC, Hoofnagle JH, Young, N , Straus SE, Jones EA. Treatment of chronic non‐A, non‐B hepatitis with acyclovir: a pilot study. J Med Virol 1985; 15: 1–9. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH, Mullen KD, Jones DB, Rustgi V, Di Bisceglie A , Peters M, et al. Treatment of chronic non‐A, non‐B hepatitis with recombinant human alpha interferon. N Engl J Med 1986; 315: 1575–1578. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie A , Martin P, Kassianedes C, Lisker‐Melman M, Murray L, Waggoner J, et al. Recombinant interferon alpha therapy for chronic hepatitis C: a randomized, double‐blind, placebo controlled trial. N Engl J Med 1908; 321: 1506–1510. [DOI] [PubMed] [Google Scholar]
- Davis GL, Balart L, Schiff E, Lindsay K, Bodenheimer HC Jr, Perrillo RP, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Intervention Therapy Group. N Engl J Med 1989; 321: 1501–1506. [DOI] [PubMed] [Google Scholar]
- Dusheiko G, Main J, Thomas H, Reichard O, Lee C, Dillon A, et al. Ribavirin treatment for patients with chronic hepatitis C: results of a placebo‐controlled trial. J Hepatol 1996; 25: 591–598. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa‐2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med 1998; 339: 1485–1492. [DOI] [PubMed] [Google Scholar]
- Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group. Lancet 1998; 352: 1426–1432. [DOI] [PubMed] [Google Scholar]
- Maylin S, Martinot‐Peignoux M, Moucari R, Boyer N, Ripault MP, Cazal‐Hatem D, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology 2008; 135: 821–829. [DOI] [PubMed] [Google Scholar]
- Swain MG, Lai MY, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, et al. A sustained virological response is durable in patients with chronic hepatitis C treated with peginterferon alpha‐2a and ribavirin. Gastroenterology 2010; 139: 1593–1601. [DOI] [PubMed] [Google Scholar]
- Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C: a cure and so much more. Clin Infect Dis 2011; 52: 889–900. [DOI] [PubMed] [Google Scholar]
- Reddy RK, Wright TL, Pockros PJ, Shiffman M, Everson G, Reindollar R, et al. Efficacy and safety of pegylated (40‐kd) interferon alpha‐2a compared with interferon apha‐2a in noncirrhotic patients with chronic hepatitis C. Hepatology 2001; 33: 433–438. [DOI] [PubMed] [Google Scholar]
- Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, et al. Peginterferon alfa‐2a in patients with chronic hepatitis C. N Engl J Med 2000; 343: 1666–1673. [DOI] [PubMed] [Google Scholar]
- Lindsay KL, Trepo C, Heinges T, Shiffman ML, Gordon SC, Hoefs JC, et al. A randomized double‐blind trial comparing pegylated interferon alfa‐2b to interferon alfa‐2b as initial treatment for chronic hepatitis C. Hepatology 2001. 34: 395–403. [DOI] [PubMed] [Google Scholar]
- Pawlotsky JM, Roudot‐Thoraval F, Bastie A, Darthuy F, Remire J, Metreau JM, et al. Factors effecting treatment responses to interferon‐alpha in chronic hepatitis C. J Infect Dis 1996; 174: 1–7. [DOI] [PubMed] [Google Scholar]
- Davis GL, Lau JY. Factors predictive of a beneficial response to therapy of hepatitis C. Hepatology 1997; 26(suppl 1): 122S‐127S. [DOI] [PubMed] [Google Scholar]
- Layden‐Almer JE, Ribeiro RM, Wiley T, Perelson AS, Layden TJ. Viral dynamics and response differences in HCV‐infected African American and white patients treated with IFN and ribavirin. Hepatology 2003; 37: 1343–1350. [DOI] [PubMed] [Google Scholar]
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358: 958–965. [DOI] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975–982. [DOI] [PubMed] [Google Scholar]
- Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al.; for PEGASYS International Study Group . Peginterferon‐alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140: 346–355. [DOI] [PubMed] [Google Scholar]
- Buti M, Sanchez Avila F, Lurie Y, Staglis C, Valdes A, Martell M, et al. Viral kinetics in genotype 1 chronic hepatitis C patients during therapy with 2 different doses of peginterferon alfa‐2b plus ribavirin. Hepatology 2002; 35: 930–936. [DOI] [PubMed] [Google Scholar]
- Lindahl K, Stahle L, Bruchfeld A, Schvarez R. High‐dose ribavirin in combination with standard dose peginterferon for treatment of patients with chronic hepatitis C. Hepatology 2005; 41: 275–279. [DOI] [PubMed] [Google Scholar]
- Bert T, von Wagner M , Nasser S, Sarazin C, Heintges T, Gerlach T, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon‐alfa‐2a plus ribavirin. Gastroenterology 2006; 130: 1086–1097. [DOI] [PubMed] [Google Scholar]
- Pearlman BL, Ehleben C, Saifee S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis C genotype 1‐infected slow responders. Hepatology 2007; 46: 1688–1694. [DOI] [PubMed] [Google Scholar]
- Jensen DM, Morgan RT, Marcellin P, Pockros PJ, Reddy KR, Hadziyannis SJ, et al. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha‐2a (40 kd)/ribavirin therapy. Hepatology 2006; 43: 954–960. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Fried MW, Shiffman ML, Smith CI, Marinos G, Goncales FL Jr, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa‐2a (40KD)/ribavirin. J Hepatol 2005; 43: 425–433. [DOI] [PubMed] [Google Scholar]
- Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Sola RM, et al. Peginterferon alfa‐2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med 2007; 357: 124–134. [DOI] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marions G, Goncales Jr, et al. Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975–982. [DOI] [PubMed] [Google Scholar]
- Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa‐2b plus ribavirin in patients with chronic hepatitis C. Hepatology 2003; 38: 645–652. [DOI] [PubMed] [Google Scholar]
- Jacobson IM, Gonzalez SA, Ahmed F, Lebovics E, Min AD, Bodenheimer HC Jr, et al. A randomized trial of pegylated interferon alpha‐2b plus ribavirin in the retreatment of chronic hepatitis C. Am J Gastroenterol 2005; 100: 2453–2462. [DOI] [PubMed] [Google Scholar]
- Cheruvattath R, Rosati MJ, Gautam M, Vargas HE, Rakela J, Balan V. Pegylated interferon and ribavirin failures: is retreatment an option? Dig Dis Sci 2007; 52: 732–736. [DOI] [PubMed] [Google Scholar]
- Cornberg M, Hadem J, Hermann E, Schuppert F, Schmidt HH, Reiser M, et al. Treatment with daily consensus interferon (CIFN) plus ribavirin in non‐responder patients with chronic hepatitis C: a randomized open‐label pilot study. J Hepatol 2006; 44: 291–301. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie AM , Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, et al. Prolonged therapy of advanced chronic hepatitis C with low‐dose peginterferon. N Engl J Med 2008; 359: 2429–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T, Inui A, Ohkawa T, Komastu H, Miyakawa Y, Onoue M. Response to interferon therapy in children with chronic hepatitis C. J Pediatr 1995; 127: 660–662. [DOI] [PubMed] [Google Scholar]
- Christensson B, Wiebe T, Akesson A, Widell A. Interferon‐alpha and ribavirin treatment of hepatitis C in children with malignancy in remission. Clin Infect Dis 2000; 30: 585–586. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Peralta RP, Kelly DA, Haber B, Molleston J, Murray KF, Jonas MM, et al. Interferon alfa‐2b in combination with ribavirin for the treatment of chronic hepatitis C in children: efficacy, safety, and pharmacokinetics. Hepatology 2005; 42: 1010–1018. [DOI] [PubMed] [Google Scholar]
- Wirth S, Lang T, Gehring S, Gerner P. Recombinant alfa‐interferon plus ribavirin therapy in children and adolescents with chronic hepatitis C. Hepatology 2002; 36: 1280–1284. [DOI] [PubMed] [Google Scholar]
- Wirth S, Pieper‐Boustani H, Lang T, Ballauff A, Kullmer U, Gerner P, et al. Peginterferon alfa‐2b plus ribavirin treatment in children and adolescents with chronic hepatitis C. Hepatology 2005; 41: 1013–1018. [DOI] [PubMed] [Google Scholar]
- Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, et al. Peginterferon alfa‐2a plus ribavirin versus interferon alfa‐2a plus ribavirin for chronic hepatitis C in HIV‐coinfected patients. Aids 2004; 351: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriani FJ, Rodriquez‐Torres M, Rockstroh JK, Lissen E, Gonzalez‐Garcia J, Lazzarin A, et al. Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection in HIV‐infected persons. N Engl J Med 2004; 351: 438–450. [DOI] [PubMed] [Google Scholar]
- Carrat F, Bani‐Sadr F, Pol S, Rosenthal E, Lunel‐Fabiani F, Benzekri A, et al. Pegylated interferon alfa‐2b vs standard interferon alfa‐2b, plus ribavirin, for chronic hepatitis C in HIV‐infected patients: a randomized controlled trial. JAMA 2004; 292: 2839–2848. [DOI] [PubMed] [Google Scholar]
- Soriano V, Bravo R, Garcia‐Samaniego J, Castilla J, Gonzalez J, Castro A, et al. Relapses of chronic hepatitis C in HIV‐infected patients who responded to interferon therapy. Hepatitis/HIV Spanish Study Group. Aids 1997; 11: 400–401. [PubMed] [Google Scholar]
- Bruchfeld A, Lindahl K, Stahle L, Soderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C‐associated renal disease and renal insufficiency. Nephrol Dial Transplant 2003; 18: 1573–1580. [DOI] [PubMed] [Google Scholar]
- Rendina M, Schena A, Castellaneta NM, Losito F, Amoruso AC, Stallone G, et al. The treatment for chronic hepatitis C with peginterferon alfa‐2a (40 kDa) plus ribavirin in haemodialysed patients awaiting renal transplant. J Hepatol 2007; 46: 768–774. [DOI] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) . KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2008; 73(suppl 109): S1‐S99. [DOI] [PubMed] [Google Scholar]
- Carriero D, Fabrizi F, Uriel AJ, Paark J, Martin P, Dieterich DT. Treatment of dialysis patients with chronic hepatitis C using pegylated interferon and low‐dose ribavirin. Int J Artif Organs 2008; 31: 295–302. [DOI] [PubMed] [Google Scholar]
- Fagiuoli S, Cooper DK, Zuhdi N. Hepatitis C status of heart transplant recipients. Clin Transplant 1998; 12: 5–10. [PubMed] [Google Scholar]
- Doucette KE, Weinkauf J, Sumner S, Ens K, Lien D. Treatment of hepatitis C in potential lung transplant candidates. Transplantation 2007; 83: 1652–1655. [DOI] [PubMed] [Google Scholar]
- Sugawaara Y, Makuuchi M, Matsui Y, Kishi Y, Akamatsu N, Kaneko J, et al. Preemptive therapy for hepatitis C virus after living‐donor liver transplantation: Transplantation 2004; 78: 1308–1311. [DOI] [PubMed] [Google Scholar]
- Gane EJ, Lo SK, Riordan SM, Portmann BC, Lau JY, Naoumov NV, et al. A randomized study comparing ribavirin and interferon alfa monotherapy for hepatitis C recurrence after liver transplantation. Hepatology 1998; 27: 1403–1407. [DOI] [PubMed] [Google Scholar]
- Hebling B, Jochum W, Stamenic I, Knopfli M, Cerny A, Borovicka J, et al. HCV‐related advanced fibrosis/cirrhosis: randomized controlled trial of pegylated interferon alpha‐2a and ribavirin. J Viral Hepat 2006; 13: 762–769. [DOI] [PubMed] [Google Scholar]
- Crippin JS, McCashland T, Terrault N, Sheiner P, Charlton MR. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus‐infected patients awaiting liver transplantation. Liver Transpl 2002; 8: 350–355. [DOI] [PubMed] [Google Scholar]
- Iaccobellis A, Siciliano M, Perri F, Annicchiarico BE, Leandro G, Caruso N, et al. Peginterferon alfa‐2b and ribavirin in patient with hepatitis C virus and decompensated cirrhosis: a controlled study. J Hepatol 2007; 46: 206–221. [DOI] [PubMed] [Google Scholar]
- Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley‐Lucas TE, Afdhal N, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology 2006; 131: 470–477. [DOI] [PubMed] [Google Scholar]
- Jeffers LJ, Cassidy W, Howell CD, Hu S, Reddy KR. Peginterferon alfa‐2a (40 kd) and ribavirin for black American patients with chronic HCV genotype 1. Hepatology 2004; 39: 1702–1708. [DOI] [PubMed] [Google Scholar]
- Rosen HR, Weston SJ, Im K, Yang H, Burton JR Jr, Erlich H; for Virahep C Study Group . Selective decrease in hepatitis C virus‐specific immunity among African Americans and outcome of anti‐viral therapy. Hepatology 2007; 46: 350–358. [DOI] [PubMed] [Google Scholar]
- Poenisch M, Bartenschlager R. New insights into structure and replication of the hepatitis C virus and clinical implications. Semin Liver Dis 2010; 30: 333–347. [DOI] [PubMed] [Google Scholar]
- Lin C, Kwoong AD, Perni RB. Discovery and development of VX‐950, a novel, covalent, and reversible inhibitor of hepatitis C virus NS3/4A serine protease. Infect Disord Drug Targets 2006; 6: 3–16. [DOI] [PubMed] [Google Scholar]
- Reesink HW, Zeusem S, Weegink CJ, Forestier N, van Vliet A , van de Wetering de Rooij J , et al. Rapid decline of viral RNA in hepatitis patients treated with VX‐950: a phase 1b, placebo‐controlled, randomized study. Gastroenterology 2006; 131: 997–1002. [DOI] [PubMed] [Google Scholar]
- Sarrazin C, Kieffer TL, Bartels D, Hanzelka B, Muh U, Martin W, et al. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 2007; 132: 1767–1777. [DOI] [PubMed] [Google Scholar]
- McHutchinson JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360: 1827–1838. [DOI] [PubMed] [Google Scholar]
- Hezode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, et al.; for PROVE2 Study Team . Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009; 360: 1839–1850. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, et al.; for PROVE3 Study Team . Telaprevir for previously treated chronic HCV infection. N Engl J Med 2010; 362: 1292–1303. [DOI] [PubMed] [Google Scholar]
- Kwo PY, Lawitz EJ, McCane J, Schiff ER, Vierling JM, Pound D, et al.; for SPRINT‐1 Investigators . Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa‐2b and ribavirin in treatment‐naive patients with genotype 1 hepatitis C infection (SPRINT‐1): an open‐label, randomised, multicentre phase 2 trial. Lancet 2010; 376: 705–716. [DOI] [PubMed] [Google Scholar]
- Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al.; for SPRINT‐2 Investigators . Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364: 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al.; for HCV RESPOND‐2 Investigators . N Engl J Med 2011; 364: 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman KE, Flamm SL, Afdahl NH, Nelson DR, Sulkowski MS, Everson GT, et al. Response‐guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med 2011; 365: 1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB; for American Association for Study of Liver Diseases . An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011; 54: 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]


