Watch the interview with the authors
Watch the video presentation of this article
As the treatment for chronic hepatitis C virus (HCV) infection has improved over the last 2 decades, the number of patients for whom therapy fails has declined substantially. However, more than half of patients with HCV genotype 1 infections fail to achieve a sustained virological response (SVR) to pegylated interferon (PEG‐IFN) and ribavirin (RBV).1, 2, 3, 4 Subjects for whom combination therapy fails are a heterogeneous group and include individuals who experience virological breakthrough (detectable HCV RNA in serum during therapy after the achievement of an initial response) or virological relapse (the reappearance of HCV RNA in serum after the discontinuation of treatment and the achievement of an end of‐treatment response) as well as individuals who fail to achieve an initial virological response [i.e., partial responders (≥2‐log IU/mL decline in HCV RNA from the baseline to treatment week 12 but detectable HCV RNA at week 24) and null responders (≤2‐log IU/mL reduction in HCV RNA from the baseline to treatment week 12; Fig. 1].
Figure 1.
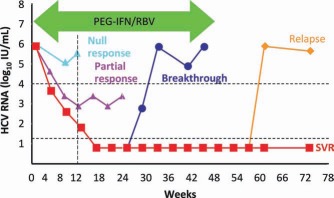
Virological responses during and after therapy for chronic HCV. Relapse refers to the reappearance of HCV RNA in serum after treatment is discontinued and an end‐of‐treatment response is documented. A nonresponse may be partial (>2‐log decline in HCV RNA by week 12 but still positive results at week 24) or null (<2‐log decline in HCV RNA by week 12).
Abbreviations.
DAA, direct‐acting antiviral; FDA, Food and Drug Administration; HCV, hepatitis C virus; PEG‐IFN, pegylated interferon; RBV, ribavirin; RGT, response‐guided triple‐drug therapy; SOC, standard of care; SVR, sustained virological response.
The reasons for treatment failure are not well understood. Resistance to interferon is believed to be an important cause. Specific polymorphisms of the interleukin‐28b gene probably explain 50% or more of the resistance to PEG‐IFN, but other host and viral factors are likely involved.5, 6, 7 Poor compliance with the prescribed regimen and adverse events requiring a dose reduction or discontinuation of therapy also contribute to treatment failure.8, 9 The latter two causes may be amenable to interventions, and successful retreatment with PEG‐IFN and RBV may be permitted.
Until recently, retreatment options were limited for persons for whom a PEG‐IFN/RBV regimen failed. Studies evaluating retreatment with PEG‐IFN and RBV yielded SVR rates of only 6% to 9% in partial and null responders and 33% in prior relapsers with an HCV genotype 1 infection.10, 11 In comparison with standard therapy, a higher dose of PEG‐IFN as induction therapy had no effect on either the end‐of‐treatment response rate or the SVR rate.10 However, extending therapy to 72 weeks resulted in a marginal increase in the SVR rate from 9% to 16%, primarily because of the prevention of virological relapse.10 Similarly, a different preparation of interferon, consensus interferon, was minimally effective.12 Three studies have evaluated the role of maintenance, low‐dose PEG‐IFN in prior nonresponders with advanced liver disease.3, 4, 5 All three studies showed no differences in clinical outcomes between treated and control subjects and suggested no benefit of maintenance, low‐dose PEG‐IFN in this group of patients. However, the Colchicine Versus PegIntron Long‐Term study and the Evaluation of PegIntron in Control of Hepatitis C Cirrhosis 3 study hinted at a lower rate of complications (particularly variceal bleeding) in patients with portal hypertension who were receiving colchicine or a placebo, respectively, instead of PEG‐IFN.14, 15
In May 2011, two new direct‐acting antiviral (DAA) agents belonging to a class of drugs known as protease inhibitors, boceprevir and telaprevir, were approved for use in combination with PEG‐IFN and RBV for both previously untreated and treatment‐experienced subjects.6, 7, 8, 9 In comparison with PEG‐IFN and RBV, these drugs led to 2‐ to 3‐fold increases in SVR rates for patients with previous treatment failures17, 19 (Figs. 2 and 3). Consequently, boceprevir or telaprevir in combination with PEG‐IFN and RBV now represent the new standard of care (SOC) for the retreatment of relapsers, prior partial responders, and null responders.17, 19
Figure 2.
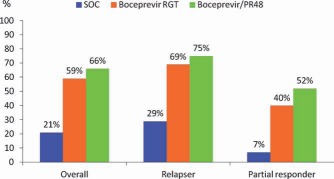
Boceprevir plus PEG‐IFN/RBV: overall SVR rates and SVR rates according to prior responses in treatment‐experienced subjects. Overall, the SVR rates were significantly higher for patients receiving a boceprevir‐containing regimen versus patients receiving PEG‐IFN and RBV (59% and 66% versus 21%). The response was dependent on the prior response, with prior relapsers responding better than prior partial responders. Adapted with permission from New England Journal of Medicine.17 RGT means response guided therapy. PR48 means pegylated interferon and ribavirin taken for 48 weeks.
Figure 3.
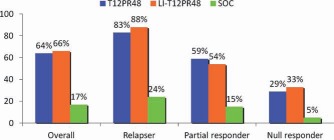
Telaprevir plus PEG‐IFN/RBV: overall SVR rates and SVR rates according to prior responses in treatment‐experienced subjects. The SVR rates were significantly higher for patients receiving telaprevir with or without a lead‐in phase. The SVR rates were not improved with a lead‐in phase. There was a gradient in the response, with the highest SVR rates achieved by relapsers and partial responders and the lowest rates achieved by null responders. T12PR48: telaprevir × 12 weeks, pegylated interferon and ribavirin × 48 weeks; LI means ‘lead‐in’ phase using pegylated interferon and ribavirin; SOC is ‘standard of care’ i.e., pegylated interferon and ribavirin.
The Retreatment With HCV Serine Protease Inhibitor Boceprevir and Peginterferon/Rebetol 2 study, also known as RESPOND‐2, (a phase 3 boceprevir trial) enrolled relapsers and partial responders; previous null responders were excluded.17 The study design began with a 4‐week lead‐in phase of PEG‐IFN and RBV for all subjects, after which the subjects were stratified to one of three study arms:
-
1.
Response‐guided triple‐drug therapy (RGT). The duration of PEG‐IFN and RBV was tailored to the HCV RNA response after 4 and 8 weeks of triple therapy; all subjects in this arm received 32 weeks of boceprevir plus PEG‐IFN and RBV and completed therapy at week 36 if HCV RNA was undetectable at weeks 8 and 12. Slow responders, that is, those for whom HCV RNA was detectable at week 8 but undetectable at week 12, received another 12 weeks of PEG‐IFN and RBV alone after week 36 for a total duration of 48 weeks.
-
2.
Fixed‐duration triple therapy for 44 weeks.
-
3.
SOC therapy. This comprised PEG‐IFN and RBV plus a placebo for 48 weeks.
The study × demonstrated that SVR rates were significantly higher among subjects receiving a boceprevir‐containing regimen versus patients receiving SOC therapy: 59% in the RGT arm and 66% in the fixed‐duration arm versus 21% in the SOC arm (Fig. 2). Successful treatment was more common in prior relapsers (69%‐75%) than prior partial responders (40%‐52%); the response was lower in patients with cirrhosis, particularly in the RGT arm. Anemia, dry skin, dysgeusia, and rashes were reported more commonly by subjects who received boceprevir versus subjects who received SOC. On the basis of this phase 3 trial, the Food and Drug Administration (FDA) approved the regimen shown in Fig. 4. Because of low response rates in patients with cirrhosis receiving RGT,17 the FDA has recommended that this group of patients receive lead‐in therapy and then triple therapy for a fixed duration of 44 weeks.20
Figure 4.
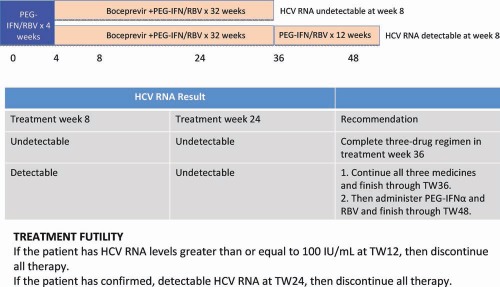
Boceprevir plus PEG‐IFN/RBV for previous partial responders and relapsers: the FDA‐approved regimen. Subjects should start treatment with PEG‐IFN and RBV for 4 weeks; after this, boceprevir (800 mg three times a day with food) is given. The duration of boceprevir use depends on the response to treatment at weeks 8 and 24. If HCV RNA is undetectable at weeks 8 and 24, patients should receive 32 weeks of triple therapy. If a patient is slow to respond and has detectable HCV RNA at week 8 but HCV RNA is undetectable at week 24, then triple therapy should be given for 32 weeks and should be followed by another 12 weeks of PEG‐IFN and RBV. TW, treatment week.
The REALIZE study, a phase 3 trial of telaprevir with PEG‐IFN and RBV in treatment‐experienced patients, compared 12 weeks of triple therapy with or without a 4‐week lead‐in phase of PEG‐IFN and RBV plus 36 or 32 weeks of PEG‐IFN and RBV, respectively, for a total treatment period of 48 weeks to SOC therapy for 48 weeks. A response‐guided approach was not investigated. The study included previous relapsers, partial responders, and null responders. SVR rates were significantly higher for patients receiving telaprevir with or without the lead‐in phase (64% and 66%) versus patients receiving SOC therapy (17%). The SVR rates were similar for the arms with and without a lead‐in strategy, and this demonstrated no advantage from a lead‐in phase. There was a gradient in the response based on prior response, with the highest SVR rates found in relapsers (followed by partial responders) and the lowest rates noted in null responders. These results serve to emphasize the importance of knowing the previous response to treatment when retreatment is being considered. Among previous relapsers, SVR rates with telaprevir plus PEG‐IFN and RBV were independent of the liver fibrosis stage. However, among partial and null responders, SVR rates declined with worsening fibrosis.19 Thus, the lowest response rate was observed for null responders with cirrhosis. Adverse events were more common in subjects receiving triple therapy containing telaprevir versus those receiving SOC, and they included fatigue, pruritus, rashes, nausea, anemia, anorectal symptoms, and diarrhea. On the basis of these results, the FDA approved the regimen shown in Fig. 5.20
Figure 5.
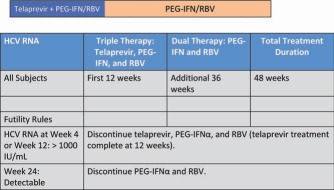
Telaprevir for partial and null responders: the FDA‐approved regimen. Partial and null responders should receive 12 weeks of triple therapy and then another 36 weeks of PEG‐IFN and RBV. Relapsers should be treated in the same way as treatment‐naive subjects and are eligible for response‐guided therapy. This regimen resulted in SVR rates of 83%, 59%, and 29% for relapsers, partial responders, and null responders, respectively.
A majority of patients who fail to achieve an SVR in response to triple therapy (including boceprevir or telaprevir) develop antiviral resistance.17, 19 Some of these viral variants may persist over the long term, but the clinical significance of resistance mutations is unclear at this time.
Whether a subject should be retreated now or wait for potentially better therapy in the future depends on many factors, including the individual's desire to be retreated, the reasons underlying the failure (e.g., inadequate drug dosing or side‐effect management), the severity of the underlying liver disease, the prior response to therapy, and the risk of disease progression over the next 3 to 5 years. More effective therapies that do not include interferon are likely to be available in the future. Indeed, a recent pilot trial compared a four‐drug regimen (PEG‐IFN, RBV, a protease inhibitor, and a nonstructural 5A inhibitor) to a combination of DAA agents without PEG‐IFN or RBV in previous null responders. This pilot trial reported an impressive 100% response rate with the four‐drug regimen, and 36% of nonresponders achieved an SVR after only 24 weeks of a combination of DAAs alone.21
There are currently no data on the management of individuals for whom a protease inhibitor–containing regimen has failed. Until such data become available, the implementation of futility rules and strict adherence to the drug regimen will be important to prevent the development of resistance and to optimize the chances for SVR while more effective and safer treatment is awaited.
In response to these registration studies, the guidelines committee of the American Association for the Study of Liver Diseases has approved the following guidelines for the use of antiviral therapy in treatment‐experienced patients with HCV genotype 1:22
-
1.
Retreatment with boceprevir or telaprevir, together with PEG‐IFNβ and weight‐based RBV, can be recommended for patients who experienced virological relapse or were partial responders after a previous course of treatment with standard interferon‐β or PEG‐IFNβ and/or RBV (class 1, level A).
-
2.
Retreatment with telaprevir, together with PEG‐IFNβ and weight‐based RBV, may be considered for patients who were previously null responders to a course of standard interferon‐β or PEG‐IFNβ and/or weight‐based RBV (class 2b, level B.)
-
3.
In the case of treatment‐experienced patients, response‐guided therapy with a boceprevir‐ or telaprevir‐based regimen can be considered for relapsers (class 2a, level B for boceprevir; class 2b, level C for telaprevir), may be considered for partial responders (class 2b, level B for boceprevir; class 3, level C for telaprevir), but cannot be recommended for null responders (class 3, level C).
-
4.
Patients retreated with boceprevir plus PEG‐IFNβ and RBV who continue to have detectable HCV RNA at levels > 100 IU at week 12 should be withdrawn from all therapy because of the high likelihood of developing antiviral resistance (class 1, level B).
-
5.
Patients retreated with telaprevir plus PEG‐IFNβ and RBV who continue to have detectable HCV RNA at levels > 1000 IU at week 4 or 12 should be withdrawn from all therapy because of the high likelihood of developing antiviral resistance (class 1, level B).
This research was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute (National Institutes of Health).
Potential conflict of interest: Nothing to report
References
- 1. Poynard T, Leroy V, Cohard M, Thevenot T, Mathurin P, Opolon P, et al. Meta‐analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology 1996;24:778–789. [DOI] [PubMed] [Google Scholar]
- 2. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958–965. [DOI] [PubMed] [Google Scholar]
- 3. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975–982. [DOI] [PubMed] [Google Scholar]
- 4. Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon‐alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140:346–355. [DOI] [PubMed] [Google Scholar]
- 5. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment‐induced viral clearance. Nature 2009;461:399–401. [DOI] [PubMed] [Google Scholar]
- 6. Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome‐wide association of IL28B with response to pegylated interferon‐alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009;41:1105–1109. [DOI] [PubMed] [Google Scholar]
- 7. Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome‐wide association study. Gastroenterology 2010;138:1338–1345. [DOI] [PubMed] [Google Scholar]
- 8. Shiffman ML, Ghany MG, Morgan TR, Wright EC, Everson GT, Lindsay KL, et al. Impact of reducing peginterferon alfa‐2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology 2007;132:103–112. [DOI] [PubMed] [Google Scholar]
- 9. McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype‐1‐infected patients with chronic hepatitis C. Gastroenterology 2002;123:1061–1069. [DOI] [PubMed] [Google Scholar]
- 10. Jensen DM, Marcellin P, Freilich B, Andreone P, Di Bisceglie A, Brandao‐Mello CE, et al. Re‐treatment of patients with chronic hepatitis C who do not respond to peginterferon‐alpha2b: a randomized trial. Ann Intern Med 2009;150:528–540. [DOI] [PubMed] [Google Scholar]
- 11. Poynard T, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, et al. Peginterferon alfa‐2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology 2009;136:1618–1628. [DOI] [PubMed] [Google Scholar]
- 12. Bacon BR, Shiffman ML, Mendes F, Ghalib R, Hassanein T, Morelli G, et al. Retreating chronic hepatitis C with daily interferon alfacon‐1/ribavirin after nonresponse to pegylated interferon/ribavirin: DIRECT results. Hepatology 2009;49:1838–1846. [DOI] [PubMed] [Google Scholar]
- 13. Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, et al. Prolonged therapy of advanced chronic hepatitis C with low‐dose peginterferon. N Engl J Med 2008;359:2429–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Afdhal NF, Levine R, Brown R Jr, Freilich B, O'Brien M, Brass C. Colchicine versus peg‐interferon alfa 2b long term therapy: results of the 4 year COPILOT trial. J Hepatol 2008;48:S4. [Google Scholar]
- 15. Bruix J, Poynard T, Colombo M, Schiff E, Reichen J, Burak K, et al. PegIntron maintenance therapy in cirrhotic (METAVIR F4) HCV patients who failed to respond to interferon/ribavirin (IR) therapy: final results of the EPIC3 cirrhosis maintenance trial. J Hepatol 2009;50:S22. [Google Scholar]
- 16. Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011;364:1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011;364:1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011;364:2405–2416. [DOI] [PubMed] [Google Scholar]
- 19. Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med 2011;364:2417–2428. [DOI] [PubMed] [Google Scholar]
- 20. Food and Drug Administration . Prescribing information for Incivek. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/201917lbl.pdf. Accessed January 2012.
- 21. Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 2012;366:216–224. [DOI] [PubMed] [Google Scholar]
- 22. Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011; 54:1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]


