Abstract
Osteoclasts are large multinucleated cells exquisitely adapted to resorb bone matrix. Like other eukaryotes, osteoclasts possess an elaborate ensemble of intracellular organelles through which solutes, proteins and other macromolecules are trafficked to their target destinations via membrane-bound intermediaries. During bone resorption, membrane trafficking must be tightly regulated to sustain the structural and functional polarity of the osteoclasts’ membrane domains. Of these, the ruffled border (RB) is most characteristic, functioning as the osteoclasts' secretory apparatus. This highly convoluted organelle is classically considered to be formed by the targeted fusion of acidic vesicles with the bone-facing plasma membrane. Emerging findings disclose new evidence that the RB is far more complex than previously envisaged, possessing discrete subdomains that are serviced by several intersecting endocytic, secretory, transcytotic and autophagic pathways. Bone-resorbing osteoclasts therefore serve as a unique model system for studying polarized membrane trafficking. Recent advances in high-resolution microscopy together with the convergence of genetic and cell biological studies in humans and in mice have helped illuminate the major membrane trafficking pathways in osteoclasts and unmask the core molecular machinery that governs these distinct vesicle transport routes. Among these, small Rab GTPases, their binding partners and members of the endocytic sorting nexin family have emerged as critical regulators. This mini review summarizes our current understanding of membrane trafficking in osteoclasts, the key molecular participants, and discusses how these transport machinery may be exploited for the development of new therapies for metabolic disorders of bone-like osteoporosis.
Keywords: membrane trafficking, osteoclast, osteoporosis, Rab GTPases, secretory lysosomes, sorting nexins
Introduction
Throughout adult life, bone undergoes cyclical removal of old or damaged bone and replacement with a new bone to replenish the skeleton. This process, termed bone remodelling, is regulated by the complementary activities of bone-residing osteoblasts and osteoclasts [1,2]. Osteoblasts synthesize and secrete new bone matrix (i.e. osteoid) whereas osteoclasts are nature's provision for bone excavation (i.e. resorption). Imbalances in bone remodelling that favour a net increase in osteoclast numbers and/or activity leads to loss of bone mass as observed in patients with osteoporosis. Conversely, impairments in osteoclast differentiation or function are associated with sclerosing (i.e. high bone mass) conditions such as osteopetrosis [3].
By definition, osteoclasts are large multinucleated (>3 nuclei/cell) bone-resorbing cells. They are derived from the asynchronous fusion of mononuclear haematopoietic progenitors of the monocyte/macrophage lineage in response to two principal cytokines: (i) macrophage colony stimulating factor (M-CSF) and (ii) receptor activator of nuclear factor κ B ligand (RANKL) [4]. Upon attachment to bone osteoclasts adopt a polarized conformation via a series of cytoskeletal reorganizations which drive the segregation of the osteoclast surface membrane into four distinct domains: (i) the sealing zone (SZ); (ii) the ruffled border (RB); (iii) the basolateral domain (BD) and; (iv) the functional secretory domain (FSD) [5] (Figure 1). Of these, the RB functions as the osteoclasts bone-resorbing organelle. Formation and maintenance of the RB are dependent on the polarized trafficking of acidic vesicles to the bone-apposed plasmalemma. The fusion and incorporation of these carrier vesicles, in turn, furnishes the RB membrane with machinery necessary to solubilize bone mineral and digest organic bone matrix. Following degradation, bone matrix is engulfed by endocytic mechanisms at the RB and then transported apico-basolaterally to the opposing FSD where it is expelled into the extracellular circulation. To meet these intense membrane trafficking demands, osteoclasts acquire sets of structurally and functionally diverse trafficking protein families during RANKL-driven differentiation. Of these small Rab GTPases, their regulators and members sorting nexin family are now widely recognized as core components of osteoclasts bone-resorbing machinery.
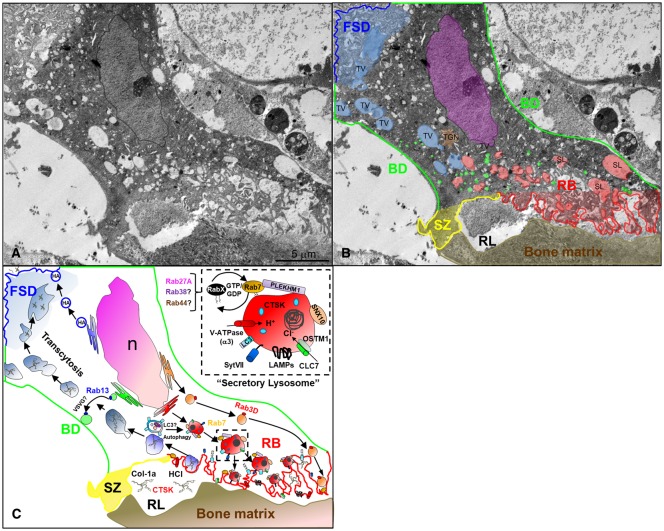
Figure 1. Intracellular roadmap of polarized membrane trafficking in bone-resorbing osteoclasts.
(A) Transmission electron micrograph of a cross-section taken through a bone-resorbing osteoclast in situ from a 5-day-old mouse femur. (B) The osteoclast plasma membrane is segregated into four distinct subdomains: the functional secretory domain (FSD, blue), the basolateral domain (BD, green), the sealing zone (SZ, yellow) and the ruffled border (RB, red). Key intracellular organelles are highlighted. TV, transcytotic vesicle; SL, secretory lysosome; TGN, trans-Golgi network; RL, resorptive lacunae. Note that the pseudo-coloured organelles represent arbitrary author interpretations based on the size, morphology, position and intraluminal content of the compartments in the absence of intracellular markers. (C) The major vesicular transport routes in osteoclasts superimposed with key molecular participants. The ruffled border membrane is formed by the polarized trafficking and fusion of secretory lysosomes with the bone-apposed plasma membrane. The final fusion step is regulated by synaptotagmin 7 (SytVII) and expels cathepsin K (CTSK) and acid into the resorption lacunae for bone digestion. Secretory lysosome trafficking is regulated by Rab7 and its effector PLEKHM1 (Pleckstrin Homology And RUN Domain Containing M1), possibly in conjunction with other LRO-associated Rabs (RabX), i.e. Rab27A, Rab38 and Rab44 that may be recruited through GTP/GDP-dependent ‘Rab-transitions’. At the same time, LC3 (Microtubule-associated protein 1A/1B-light chain 3) is recruited to the ruffled border, possibly via the fusion of autophagosomes/phagosomes with secretory lysosomes, in a process analogous to LC3-associated phagocytosis (LAP). The post-TGN-secretory vesicle trafficking route operated by Rab3D also services the ruffled border membrane but is distinct from lysosomes. During bone resorption degraded bone matrix including collagen-1a (Col-1a) is internalized at the ruffled border and transported apico-basolaterally to the FSD by transcytosis. The FSD serves as an exist site for degraded bone matrix and is distinguished from the basolateral domain via the post-Golgi trafficking of haemagglutinin (HA) and vesicular stomatitis virus G-protein (VSG-G), respectively, the latter potentially regulated by Rab13. The dashed boxed inset highlights the molecular anatomy of a secretory lysosome and its essential nanomachinery including SNX10 (Sorting nexin 10), the α3 subunit of the Vacuolar-type H+ ATPase (V-ATPase) proton pump, chloride ion channel 7 α subunit CLC-7 and its transmembrane coregulatory protein OSTM1 (osteopetrosis-associated transmembrane protein 1). N, nucleus.
Herein, we provide a contemporary overview of the major intracellular membrane trafficking pathways that contribute to osteoclast polarization and function. In particular, we emphasize the importance of the endomembrane system, secretory lysosomes and autophagic pathways, all which converge at the RB and contribute to its formation, maintenance and identity. Finally, we discuss how machineries that regulate these transport pathways may be targeted for next-generation anti-resorptive therapies to treat disorders of bone metabolism.
Membrane trafficking in osteoclasts
Like other eukaryotic cells, the intracellular anatomy of osteoclasts can be compartmentalized into sets of membrane-delimited organelles through which proteins, solutes and other macromolecules are transported via small carrier vesicles. This process, collectively known as membrane trafficking can be operationally divided in endocytic, secretory and transcytotic pathways. Compared with other polarized cell systems (e.g. epithelial cells) that possess well-delineated apical and basolateral membrane transport routes, the nature and number of vesicle trafficking pathways governing osteoclast polarity are incompletely understood.
Much of our contemporary understanding of polarized membrane trafficking in osteoclasts has been garnered from pioneering studies in the 1990s from the laboratories of Kalervo Väänänen and Michael Horton. Using enveloped viral glycoproteins and lectins as tools Väänänen and his colleagues were the first to discover that the basolateral surface of bone-resorbing osteoclasts was discontinuous and divided into discrete membrane domains [6]. Unexpectedly, they found that the influenza haemagglutinin, which is apically targeted in epithelial cells, was trafficked to a restricted area at the top of the basal surface of osteoclasts (i.e. the FSD), while vesicular stomatitis virus G-protein (VSVG), which is basolaterally targeted in epithelia, extended across the rest of the basolateral membrane. Neither of these viral glycoproteins trafficked to the RB nor sealing zone, implying that these membrane domains were compositionally and functionally distinct. Soon after, studies by the same group [7], together with independent work arising from Steve Nesbitt and Michael Horton [8] demonstrated the utility of this denoted ‘functional secretory domain’. By monitoring the intracellular fate of ingested fluorescently labelled bone, these parallel studies showed that the FSD served as an exit site for the degraded bone matrix, thus unmasking the existence of a transcytotic route connecting the RB to the basolateral membrane (Figures 1 and 2). In the same year, seminal studies by Palokangas et al. [9] employing a suite of endocytic tracers, not only confirmed that the RB shared characteristics with late endosomes but provided evidence that it is serviced by multiple endocytic pathways.
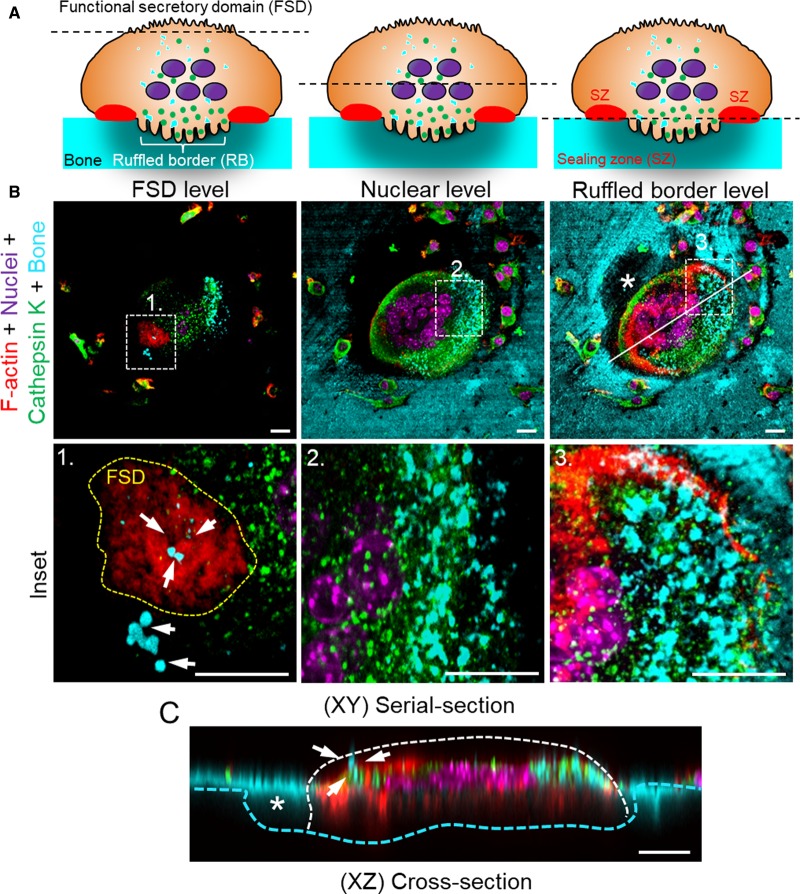
Figure 2. Apicobasolateral transcytosis of degraded bone matrix from the ruffled border to the FSD in osteoclasts.
(A) Cartoon depicting a cross-section of a polarized bone-resorbing osteoclast to orientate the respective imaging planes. (B,C) Fluorescent confocal images of a bone-resorbing osteoclast derived from mouse bone marrow monocytes cultured in the presence of M-CSF and RANKL. Osteoclasts were cultured on devitalized bovine bone discs labelled with fluorescently conjugated bisphosphonates (cyan), fixed with 4% paraformaldehyde and then stained with specific markers against cathepsin K (green), F-actin (Rhodamine-conjugated Phalloidin, red) and nuclei (Hoechst 33256, magenta). Images were obtained using a confocal microscope (Nikon A1) equipped with a 60× oil objective (NA = 1.4). Images represent serial confocal planes of individual pseudo-coloured fluorescent channels merged together. XY = top view (B), XZ = side view (C). Representative views of a bone-resorbing osteoclast are taken from the top (FSD), mid-point (nuclear level) and bottom (ruffled border/bone surface). Insets correspond to the magnification of boxed regions 1–3. Yellow dashed line in Inset 1 demarcates the FSD. White arrows denote degraded bone matrix delivered apciobasolaterally from the ruffled border to the FSD, presumably via transcytotic carrier vesicles. Solid white line indicates the orientation of XZ plane shown in (C). Asterisks correspond to the trailing resorptive pit. Scale bar = 10 µm.
In more recent years, several laboratories have made salient contributions towards our contemporary understanding of membrane trafficking in osteoclasts including, but not limited to, work by Mulari et al. [10] that uncovered the existence of two endo-exocytic subdomains within the RB: the peripheral vesicle ‘fusion’ zone and the central degraded matrix ‘uptake’ zone; studies by DeSelm and co-workers that linked the autophagy pathway to the RB [11], endocytic tracing studies by Stenbeck and Horton [12] and; the works of the Zhao and Coxon laboratories that have defined many of the molecular participants that direct vesicular transport in osteoclasts including Rab GTPases, Rab effectors and SNARE proteins [13,14]. Together, these studies have provided the blueprints for our currently accepted intracellular roadmap of polarized membrane trafficking in osteoclasts (Figure 1).
Membrane trafficking at the RB: a paradigm for regulated lysosomal secretion
Of the membrane domains established upon osteoclast polarity, the RB is most conspicuous. This highly convoluted collation of membrane in-folds not only distinguishes osteoclasts from other multinuclear macrophage descendants (e.g. foreign-body giant cells) but also signifies their resorptive status. The RB is apically orientated and abuts the bone surface where it functions as the osteoclasts secretory organelle. It serves as an exit site for protons (H+) and chloride ions (Cl−), which acidify the underlying resorptive space. Generation of a low pH environment (pH ∼ 4.5) is required for the dissolution of the mineral component (inorganic phase) of bone (i.e. hydroxyapatite) and the activation of lysosomal hydrolases (e.g. cathepsin K) that are released synchronously at the RB to digest the exposed organic matrix (i.e. collagen type1a).
The long-accepted model for RB formation involves the fusion of intracellular acidic vesicles with the bone-apposed plasma membrane en masse [15]. Unlike most mammalian cell types that possess conventional lysosomes, osteoclasts (and other haematopoietic lineage cells, e.g. melanocytes) have evolved specialized lysosome-related organelles (LROs) [16], termed ‘secretory lysosomes’, that undergo regulated exocytosis, i.e. capable of fusing with the plasma membrane in response to external stimuli.
Secretory lysosomes represent the major storehouse and activation sites of acidic hydrolases such as cathepsin K (Figures 1 and 2), the most abundant collagenase expressed in osteoclasts [17]. Upon delivery to the trans-Golgi network (TGN), newly synthesized cathepsin K is sorted via mannose-6-phosphate receptors into secretory lysosomes that are destined for fusion with the RB membrane [18,19]. The precise trigger eliciting secretory lysosome exocytosis remains incompletely understood but the final fusion step requires calcium and is regulated by the calcium-sensing SNARE fusiogen synaptotagmin VII [20]. Fusion and incorporation of secretory lysosomes enrich the RB membrane with cholesterol [21]. At the same time equips it the RB with sets of nanoscale biomachinery that are indispensable for bone resorption. These include: (i) active macromolecule membrane transporters (most notably V-ATPase proton pumps) [22] and ion channels (CLC-7) required for acidification and the maintenance of electroneutrality across the RB membrane; (ii) lysosomal membrane proteins such as OSTM1 (osteopetrosis-associated transmembrane protein 1) and LAMP1/2 (lysosome-associated membrane protein); (iii) members of membrane trafficking protein families (e.g. small Rab GTPases and Sorting Nexins (SNX) described in detail below) and; (iv) components of the autophagy pathway. Hence, the lipid and protein composition of the RB shares close analogies with late endosomal and lysosomal membranes [10,18,23]. Together with the underlying resorptive lacunae, the RB is considered a ‘giant extracellular lysosome’ [23] and serves as a useful paradigm for regulated lysosomal secretion. It is unsurprising therefore that most of the known forms osteoclast-rich osteopetrosis (>70%) in humans are associated with mutations in genes encoding proteins that function in either the (i) trafficking (e.g. SNX10), (ii) maturation (e.g. PHEKHM1) and/or (ii) function (e.g. TCIRG1, CLCN7, OSTM1) of secretory lysosomes [24,25].
Autophagy in osteoclasts
The lysosomal network has traditionally been viewed as the default pathway for the degradation of extracellular proteins and receptors but also intersects with accessory pathways including those which regulate ‘self-catabolism’ i.e. autophagy. While the term ‘autophagy’ encompasses a broad class of intracellular digestion processes, in its simplest form, autophagy can be considered a dynamic process that comprises three sequential steps: (i) formation of autophagosomes, (ii) the fusion of autophagosomes with lysosomes and (iii) degradation [26]. The entrapped cargo materials within autolysosomes are degraded by hydrolases, most notably, cathepsins [27]. In recent years, the importance of the autophagy (i.e. macroautophagy) during osteoclastic bone resorption and skeletal disease has been increasingly appreciated [28–30]. Indeed, several key components of the autophagic conjugation cascade (including ATG5, ATG7, ATG4B, LC3 and PLEKHM1) have been implicated in osteoclast secretory action and the maturation of the RB during bone resorption [12]. In particular, ATG5 has been shown to be a prerequisite for the targeting of lipidated LC3, Rab7, cathepsin K and LAMP1 to the RB membrane. Similarly, loss of ATG5 leads to deregulated autophagic flux in osteoclasts, impaired bone resorption and elevated bone mass in mice [12]. More recently, LC3 has been shown to traffic to the RB through an autophagy-independent process akin to LC3-associated phagocytosis (LAP), in which LC3 is acquired by phagosomes (instead of autophagosomes) which converge with lysosomes at the RB [31]. Accordingly, LC3 targeting to the RB and bone resorptive function was unaltered in osteoclasts derived from an autophagy-deficient mouse model (i.e. FIP200-null: focal adhesion kinase family interacting protein of 200 kD) implying that a process analogous to LAP may contribute to RB formation [31]. At present, the molecular link for this LAP-RB convergence in osteoclasts remains unknown but is potentially mediated by the Rab7-effector protein PLEKHM1 (Pleckstrin Homology And RUN Domain Containing M1), an endolysosomal adaptor protein housing an LC3-binding domain that has been implicated in both autophagy and osteopetrosis (described in detail below). Regardless of the exact molecular mechanism, these recent findings further highlight the intimate relationship that exists between lysosomal and autophagic pathways at the osteoclast RB membrane.
Rab GTPases and their regulators in osteoclasts
Of the molecular participants known to orchestrate membrane trafficking in eukaryotes, members of the small Rab (ras-like in rat brain) GTPase superfamily are best recognized [32,33]. Over 70 Rab proteins (including isoforms) have been identified in mammalian cells, each localized to a distinct subcellular compartment. Organelle selectivity and specificity are encoded within the far C-terminal of each Rab. This region contains cysteines (usually two) that undergo post-translational modification (i.e. isoprenylation) resulting in the addition of geranylgeranyl (GGPP) groups, requisite for membrane binding. Rabs are thus considered ‘deciphers of organelle identity’, with each Rab regulating at a distinct stage of membrane trafficking along the endocytic and secretory pathways. Like other ras-related GTPases, Rabs function as molecular switches, oscillating between ‘GTP’ (active) and ‘GDP’ (inactive) bound-conformations. This functional GTPase cycle is governed by cytosolic factors that facilitate (i) GTP/GDP exchange (Rab GEFs); (ii) GTP-hydrolysis (Rab GAPs) and/or (iii) Rab retrieval (Rab GDI) and coincides with Rab membrane association/dissociation. Upon activation, GTP-Rabs recruit effector molecules from the cytosol to organelle membrane where they are organized within discrete micro-domains (‘Rab-domains’). Rab effectors constitute a highly pleiotropic group of structurally and functionally diverse proteins and include multiprotein scaffolding adaptors, cytoskeletal motor proteins and tethering/docking complexes, each required for a specific stage of membrane transport ranging from vesicle budding to membrane fusion.
Osteoclasts express more than half the complement of mammalian Rab proteins [34,35,36]. Despite their abundance and well-established roles in other cell systems, very few Rabs have been functionally characterized in osteoclasts. Rab7, a late endosomal/lysosomal resident, is best recognized in osteoclasts and ranks among the most intensively studied member of all Rab GTPases. Rab7 localizes to secretory lysosomes and redistributes to the RB membrane during bone resorption [37]. Accordingly, down-regulation of Rab7 expression impairs osteoclast polarization, cathepsin K secretion and bone resorption in vitro. The complement of Rab GEFs, GAPs and effectors through which Rab7 operates during bone resorption has only just begun to emerge. To date, only two GTP-dependent Rab7 effectors have been characterized in osteoclasts, namely Rac1 and PLEKHM1. Rac1, a small GTPase of the Rho family, was identified during a bacterial two-hybrid screen for Rab7-binding partners [38]. This association was validated by recombinant protein–protein interaction studies which showed that Rac1 preferentially bound Rab7 in its GTP-bound state. In addition, Rab7 and Rac1 colocalized, albeit partially, at the RB. Based on these findings the authors speculated that Rab7–Rac1 interaction may mediate late endosomal transport between microtubules and microfilaments during RB formation. While the precise structural and functional implications of this interaction are currently lacking, it is noteworthy that conditional deletion of either Rac [39] or Rab7 [40] in osteoclasts corresponds with osteopetrosis and osteoclast dysfunction. Moreover, the recent observation that the Rac1 effector (ARMUS/TBC1D2A) moonlights as a Rab7 RabGAP offers a physical basis through which GTPase inter-switching in osteoclasts might occur [41,42].
PLEKHM1, by comparison, is a multi-domain containing cytosolic protein that functions as a molecular adaptor to integrate endolysosomes with the autophagy pathway [43,44]. It possesses a RUN domain and two pleckstrin homology (PH1/2) domains, the former an established interaction moiety of ras-like GTPases. Accordingly, PLEKHM1 is recruited directly by GTP-Rab7 to late endosomes and lysosomes [45,46]. Mutations in PLEKHM1 correspond with an intermediate form of osteopetrosis in humans and underscore the osteoporotic phenotype observed in the naturally occurring incisor absence (ia) rats Plekhm1ia/ia [45,47]. Osteoclasts derived from patients harbouring mutations in PLEKHM1 fail to develop mature RBs, exhibit impaired cathepsin K secretion and have a reduced capacity to resorb bone [45]. This phenotype is recapitulated in mice conditionally or globally lacking Plekhm1 [48]. Here, deletion of Plekhm1 correlates with bone resorption defects and altered lysosomal distribution in osteoclasts owing to a loss of connectivity between lysosomes and microtubules. Moreover, PLEKHM1 acts as a molecular platform upon which microtubule-associated proteins FAM98A, NDEL1 and LIS1 assemble a molecular complex that links lysosomes to dynein/dynactin and the underlying cytoskeleton, all whilst under the aegis of Rab7 [48,49].
Whereas the vesicular transport route governed by Rab7 in osteoclasts is now well defined, the intracellular trafficking pathway regulated by Rab3D is less so. Rab3D is a non-neuronal member of the Rab3 subfamily (Rab3A,-B,-C and -D) of exocytic-related GTPases which are expressed in osteoclasts [50,51] and other secretion competent cells [52]. In osteoclasts, Rab3D localizes to a subset of post-TGN vesicles that are required to sustain membrane equilibrium at the RB [51]. In keeping with this position, inhibition of Rab3D activity by either genetic ablation or expression of a dominant-negative mutant (N135I) impairs osteoclast bone resorption. Moreover, mice lacking Rab3D develop osteosclerosis [51]. Although the precise cargo trafficked by Rab3D-bearing vesicles remains unclear, like Rab7, their directionality is coupled to microtubules, in this instance, via the GTP-dependent recruitment of Tctex-1, a light chain of the minus-end directed dynein motor complex [53,54].
Outside of Rab7 and Rab3D, the functional contribution of other osteoclast Rabs remains surprisingly scant. Nonetheless, many Rab proteins have recently emerged whose expression is up-regulated in during RANKL-induced osteoclast differentiation and thus are predicted to modulate osteoclast formation and/or function. For example, Rab27A, which occupies LROs in other haematopoietic cells (e.g. melanosomes in melanocytes and platelet dense granules), has been recently implicated in osteoclast differentiation and lysosomal function [55]. By combining siRNA knockdown studies in RAW264.7 macrophages with osteoclasts derived from ashen mice, which possess a naturally occurring mutation in RAB27A, the authors demonstrated that Rab27A modulates the trafficking of key surface receptors that drive both multinucleation (i.e. c-fms and RANK) and lysosome-associated functions required for osteoclast polarization and resorption. Curiously, these findings diverge from the osteoclast phenotype observed from gunmetal (gm/gm) mice that bear a mutation in the catalytic subunit of Rab geranylgeranyl transferase (RGGT), which results in widespread Rab prenylation deficiency but primarily targets Rab27A [56]. In this setting, osteoclast formation and polarization are normal but gm/gm osteoclasts exhibit reduced bone resorptive capacity in vitro [36]. The exact reason for this discrepancy is unclear but may reflect differences in the genetic strains of the mice bearing the respective mutations (i.e. ashen:C3H/HeSnJ vs gm/gm: C57BL/6J) or point to differences in the residual levels of Rab27A in each model. More recently, inactive GDP-Rab27A has been shown to be recruited to secretory lysosomes via a direct association with the a3 subunit of the V-ATPase complex [57]. This finding is somewhat unexpected when considering that Rab27A does not undergo the classical REP1 and/or GDI-mediated membrane delivery [58] and remains largely constitutively active on membranes during vesicle trafficking [59,60]. Notwithstanding, a similar phenomenon was observed for GDP-bound Rab7 in the same study [57]. Based on these findings, the authors posit a model whereby the V-ATPase proton pump directs secretory lysosome trafficking via recruitment of Rab7 and Rab27A. Future studies will be required, however, to determine the structural basis for this association as well as the identity of the putative RabGEF/GAP catalysing this ‘Rab-transition’.
Rab38 is another LRO-associated GTPase whose expression in osteoclasts is dependent on the RANKL-induced NFATc1 transactivation [61]. Despite this, characterization of chocolate mice (Rab38cht/ch) which house a naturally Gly to Val substitution in the GTP-binding pocket of Rab38 failed to yield any overt bone phenotypes. Reflecting this, in vitro osteoclast formation and function is unaltered in Rab38cht/ch mice implying an accessory or redundant role for this GTPase. Similarly, the function of Rab13, another transcript that is up-regulated during osteoclast differentiation remains unknown [35]. Rab13 localizes to small carrier vesicles distinct from early or recycling endosomes but positioned between the TGN and basolateral membrane domain of bone-resorbing osteoclasts. Together with Rab8, Rab13 interacts with small transmembrane endospanins [62]; however, the physiological relevance of this interaction in osteoclast remains to be elucidated. Lastly, Rab44, a large atypical Rab GTPase has been recently identified in RAW-D derived osteoclast-like cells [63]. Rab44 is up-regulated during osteoclast differentiation, localizes to the Golgi and lysosomes and prompts early endosome enlargement upon exogenous expression. By combining siRNA knockdown studies together with a series of structural deletion mutants, the authors showed that Rab44 modulates osteoclast differentiation, acting as a negative regulator via the modulation of intracellular calcium and, in turn, influencing NFATc1 activation.
Sorting nexins in osteoclasts: the emerging role of SNX10
Sorting nexins (SNXs) are a family of structurally and functionally diverse proteins (34 members in total) unified by the presence of a PX (phox-homology) domain, responsible for membrane lipid attachment [64]. In particular, SNX10 binds to phosphoinositide-3-phosphate (PI(3)P)-enriched endosomes and has recently emerged as a crucial regulator of osteoclast function. SNX10 expression is robustly induced in response to RANKL-signalling and is a critical component of the osteoclasts bone resorptive machinery [65]. This is exemplified in humans with patients harbouring mutations in SNX10 manifesting severe Autosomal Recessive Osteopetrosis attributed to osteoclast dysfunction [66–71]. Osteoclasts derived from these patients exhibit defective RBs and fail to resorb bone [70]. This is phenocopied in mice conditionally lacking SNX10 in osteoclasts but deviates in germline-deficient animals where osteopetrosis is superimposed with rickets ‘osteopetrorickets’, thus extending SNX10 function to gastrointestinal calcium absorption [71]. Despite its established importance, the exact localization and function of SNX10 in osteoclasts remain ill-defined. Based on analogies with studies performed in zebrafish and immortalized HeLa cells [72], SNX10 is thought to localize to endosomes and has been speculated to facilitate in the post-TGN sorting and/or trafficking of V-ATPases to the RB [66]. More recently, the function of SNX10 has been expanded to the trafficking and secretion of MMP9 [73] and chaperone-mediated autophagy [74]. Precisely how SNX10 modulates these diverse function(s) in osteoclasts warrants further investigation.
Membrane trafficking machinery: targets for anti-osteoporosis therapy?
Current treatments for osteoclast-mediated diseases such as osteoporosis focus on reducing further bone loss using pharmacological bone mimetics such as bisphosphonates or anti-resorptive RANKL antibodies (Denosumab) [75]. Approved treatments to help build new bone (anabolics) such as parathyroid hormone are (i) expensive; (ii) require daily subcutaneous injection; and thus (iii) are currently restricted to patients with the most severe osteoporosis (i.e. sustained at least one osteoporosis-associated vertebral fracture). Despite several anti-resorptive drugs on the market, issues remain concerning compliance and unwanted side effects such as osteonecrosis (i.e. bone death) of the jaw or atypical sub-trochanteric femoral fractures following prolonged use [76]. Moreover, both bisphosphonates and anti-RANKL antibodies (Denosumab/Prolia) suppress osteoclast differentiation and survival. This leads to low turnover with low bone formation, as osteclasts help regulate bone remodelling by promoting recruitment of bone-forming osteoblasts (‘coupling’) [77]. Therefore, unravelling the molecular machinery that regulates membrane trafficking in osteoclasts offers unique opportunities to identify new targets for the development of next-generation anti-osteoporosis therapies that inhibit osteoclast activity while maintaining ‘coupling’ with osteoblasts, thereby preserving bone formation and quality. Indeed, Rab proteins (and other ras-related GTPases) are already well-established molecular targets of nitrogen-containing bisphosphonates (N-BPs) [78], which inhibit post-translational prenylation and, in turn, membrane attachment [79]. Within the last decade, significant progress has been made towards the development and characterization of N-BP analogues that selectively target Rabs such as phosphonocarboxylate, which blocks RGGT and inhibits bone resorption in vitro and in vivo [80,81]. Similar pharmacological interventions may be envisaged to target other membrane trafficking machinery. Regardless of the pharmacological strategy employed, development of future inhibitors targeting machinery or cargo related to secretory lysosomes must err on the side of caution following the discontinuation of the Merck & Co Inc. cathepsin K inhibitor Odanacatib, especially if the expression of the intended target extends beyond the osteoclast and the skeleton.
Conclusions and perspectives
Over the past few decades, significant advances in fluorescence microscopy together with the establishment of reproducible and reliable in vitro osteoclast culture methods have greatly accelerated our understanding of membrane trafficking in osteoclasts. Once considered meagre bone eaters, there is now rejuvenated interest and appreciation for these specialized polykaryons as a unique model system in which to study polarized membrane trafficking. Traditionally, most of our knowledge of the vesicular transport pathways that contribute to the formation of the osteoclast's functional membrane domains has been gleaned from static snapshots taken from in vitro settings. A future challenge remains, therefore, to illuminate and recapitulate these pathways in intact living systems where the dynamics and complexities can be monitored in real-time [82]. In particular, despite its unveiling more than two decades ago, our knowledge of the molecular participants governing the transcytosis of degraded bone matrix from the RB to the FSD remains in its infancy. This is, in part, due to the notorious technical limitations encountered when interrogating osteoclasts, i.e. they are not readily amenable to conventional manipulations such as transfection and must be studied on their native substrate in order to induce membrane polarity. Furthermore, the immense heterogeneity encountered when imaging bone-resorbing osteoclast in vitro makes it challenging to distinguish its discrete polarized membrane domains. Indeed, the existence and segregation of the FSD from the basolateral surface has long been contentious as no group other than that of Väänänen has, to our knowledge, replicated this observation. However, the FSD can be readily identified in bone-resorbing osteoclasts using fluorescently conjugated actin (Figure 2) or lectin-based stains (N. Pavlos et al., unpublished).
A final outstanding knowledge gap is how the vast volume of secretion activities through the osteoclastic RB and FSD may influence the behaviour of other cells within the bone marrow microenvironment (i.e. via intercellular ‘coupling’). For instance, the bone matrix embedded growth factor TGF-β may be released from the FSD in discrete quanta to stimulate osteoblast recruitment during bone remodelling. A similar scenario might be envisaged for the trafficking and release of other bone-tropic factors including insulin-like growth factors or cytokines secreted by mature osteoclasts (i.e. clastokines) such as Sphingosine-1-phosphate (S1P), collagen triple helix repeat containing 1 (Cthrc1), complement component 3a (C3a), BMP-6, Wnt10b and semaphorin4D (Sema4D) [83].
In closing, only by studying osteoclasts on bone and in four dimensions (i.e. three dimensions over time) can the true intricacy of these dynamic membrane networks be fully realized. The recent technological advances in super-resolution and electron microscopy coupled with the application of new fluorescent protein tags, organelle and bone-specific probes [84], biochemical organelle isolation methods and quantitative proteomics will undoubtedly yield unprecedented insights into the inner-workings of these enigmatic bone-resorbing polykaryons and unmask potential new drug targets for the treatment and alleviation of metabolic bone diseases.
Acknowledgements
We apologize to colleagues whose work could not be cited owing to space limitations. We thank members of Bone Biology and Disease Laboratory for their enthusiastic input and insightful discussions and Lisa Griffiths, Department of Health Western Australia (Electron Microscopy, Anatomical Pathology), for her technical assistance with electron microscopy used to generate Figure 1.
Abbreviations
- BD
basolateral domain
- FSD
functional secretory domain
- LAP
LC3-associated phagocytosis
- LRO
lysosome-related organelle
- M-CSF
macrophage colony stimulating factor
- N-BPs
nitrogen-containing bisphosphonates
- RANKL
receptor activator of nuclear factor κ B ligand
- RB
ruffled border
- RGGT
Rab geranylgeranyl transferase
- RL
resorptive lacunae
- SL
secretory lysosome
- SNX
sorting nexins
- SZ
sealing zone
- TGN
trans-Golgi network
- TV
transcytotic vesicle
- V-ATPase
vacuolar-type H+ ATPase
- VSG-G
vesicular stomatitis virus G-protein
- VSVG
vesicular stomatitis virus G-protein
Author Contribution
P.Y.N., A.B.R. and N.J.P. collectively contributed to the conceptualization, writing and editing of the manuscript and accompanying figures.
Funding
This work was supported, in part, by funding from the National Health and Medical Research Council of Australia (NHMRC) [APP1143921] to N.J.P. and Arthritis Australia to N.J.P. and P.Y.N.
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Martin T.J. and Sims N.A. (2005) Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol. Med. 11, 76–81 10.1016/j.molmed.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 2.Crockett J.C., Rogers M.J., Coxon F.P., Hocking L.J. and Helfrich M.H. (2011) Bone remodelling at a glance. J. Cell Sci. 124, 991–998 10.1242/jcs.063032 [DOI] [PubMed] [Google Scholar]
- 3.Novack D.V. and Teitelbaum S.L. (2008) The osteoclast: friend or foe? Annu. Rev. Pathol. 3, 457–484 10.1146/annurev.pathmechdis.3.121806.151431 [DOI] [PubMed] [Google Scholar]
- 4.Teitelbaum S.L. and Ross F.P. (2003) Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649 10.1038/nrg1122 [DOI] [PubMed] [Google Scholar]
- 5.Väänänen H.K., Zhao H., Mulari M. and Halleen J.M. (2000) The cell biology of osteoclast function. J. Cell Sci. 113, 377–381 PMID: [DOI] [PubMed] [Google Scholar]
- 6.Salo J., Metsikkö K., Palokangas H., Lehenkari P. and Väänänen H.K. (1996) Bone-resorbing osteoclasts reveal a dynamic division of basal plasma membrane into two different domains. J. Cell Sci. 109, 301–307 PMID: [DOI] [PubMed] [Google Scholar]
- 7.Salo J., Lehenkari P., Mulari M., Metsikko K. and Vaananen H.K. (1997) Removal of osteoclast bone resorption products by transcytosis. Science 276, 270–273 10.1126/science.276.5310.270 [DOI] [PubMed] [Google Scholar]
- 8.Nesbitt S.A. and Horton M.A. (1997) Trafficking of matrix collagens through bone-resorbing osteoclasts. Science 276, 266–269 10.1126/science.276.5310.266 [DOI] [PubMed] [Google Scholar]
- 9.Palokangas H., Mulari M. and Vaananen H.K. (1997) Endocytic pathway from the basal plasma membrane to the ruffled border membrane in bone-resorbing osteoclasts. J. Cell Sci. 110, 1767–1780 PMID: [DOI] [PubMed] [Google Scholar]
- 10.Mulari M.T., Zhao H., Lakkakorpi P.T. and Väänänen H.K. (2003) Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic 4, 113–125 10.1034/j.1600-0854.2003.40206.x [DOI] [PubMed] [Google Scholar]
- 11.DeSelm C.J., Miller B.C., Zou W., Beatty W.L., van Meel E., Takahata Y. et al. (2011) Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev. Cell 21, 966–974 10.1016/j.devcel.2011.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenbeck G. and Horton M.A. (2004) Endocytic trafficking in actively resorbing osteoclasts. J. Cell Sci. 117, 827–836 10.1242/jcs.00935 [DOI] [PubMed] [Google Scholar]
- 13.Coxon F.P. and Taylor A. (2008) Vesicular trafficking in osteoclasts. Semin. Cell Dev. Biol. 19, 424–433 10.1016/j.semcdb.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 14.Zhao H. (2012) Membrane trafficking in osteoblasts and osteoclasts: new avenues for understanding and treating skeletal diseases. Traffic 13, 1307–1314 10.1111/j.1600-0854.2012.01395.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaes G. (1965) Excretion of acid and of lysosomal hydrolytic enzymes during bone resorption induced in tissue culture by parathyroid extract. Exp. Cell Res. 39, 470–474 10.1016/0014-4827(65)90050-9 [DOI] [PubMed] [Google Scholar]
- 16.Luzio J.P., Hackmann Y., Dieckmann N.M. and Griffiths G.M. (2014) The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb. Perspect. Biol. 6, a016840 10.1101/cshperspect.a016840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czupalla C., Mansukoski H., Riedl T., Thiel D., Krause E. and Hoflack B. (2006) Proteomic analysis of lysosomal acid hydrolases secreted by osteoclasts: implications for lytic enzyme transport and bone metabolism. Mol. Cell. Proteomics 5, 134–143 10.1074/mcp.M500291-MCP200 [DOI] [PubMed] [Google Scholar]
- 18.Baron R., Neff L., Brown W., Courtoy P.J., Louvard D. and Farquhar M.G. (1988) Polarized secretion of lysosomal enzymes: co-distribution of cation-independent mannose-6-phosphate receptors and lysosomal enzymes along the osteoclast exocytic pathway. J. Cell Biol. 106, 1863–1872 10.1083/jcb.106.6.1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Meel E., Boonen M., Zhao H., Oorschot V., Ross F.P., Kornfeld S. et al. (2011) Disruption of the Man-6-P targeting pathway in mice impairs osteoclast secretory lysosome biogenesis. Traffic 12, 912–924 10.1111/j.1600-0854.2011.01203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H., Ito Y., Chappel J., Andrews N.W., Teitelbaum S.L. and Ross F.P. (2008) Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion. Dev. Cell 14, 914–925 10.1016/j.devcel.2008.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H. and Väänänen H.K. (2006) Pharmacological sequestration of intracellular cholesterol in late endosomes disrupts ruffled border formation in osteoclasts. J. Bone Miner. Res. 21, 456–465 10.1359/JBMR.051204 [DOI] [PubMed] [Google Scholar]
- 22.Qin A., Cheng T.S., Pavlos N.J., Lin Z., Dai K.R. and Zheng M.H. (2012) V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption. Int. J. Biochem. Cell Biol. 44, 1422–1435 10.1016/j.biocel.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 23.Väänänen H.K., Karhukorpi E.K., Sundquist K., Wallmark B., Roininen I., Hentunen T. et al. (1990) Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J. Cell Biol. 111, 1305–1311 10.1083/jcb.111.3.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobacchi C., Schulz A., Coxon F.P., Villa A. and Helfrich M.H. (2013) Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 9, 522–536 10.1038/nrendo.2013.137 [DOI] [PubMed] [Google Scholar]
- 25.Palagano E., Menale C., Sobacchi C. and Villa A. (2018) Genetics of osteopetrosis. Curr. Osteoporos. Rep. 16, 13–25 10.1007/s11914-018-0415-2 [DOI] [PubMed] [Google Scholar]
- 26.Klionsky D.J. (2018) Why do we need to regulate autophagy (and how can we do it)? A cartoon depiction. Autophagy 14, 1661–1664 10.1080/15548627.2018.1511218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizushima N. (2007) Autophagy: process and function. Genes Dev. 21, 2861–2873 10.1101/gad.1599207 [DOI] [PubMed] [Google Scholar]
- 28.Hocking L.J., Whitehouse C. and Helfrich M.H. (2012) Autophagy: a new player in skeletal maintenance? J. Bone Miner. Res. 27, 1439–1447 10.1002/jbmr.1668 [DOI] [PubMed] [Google Scholar]
- 29.Gelman A. and Elazar Z. (2011) Autophagic factors cut to the bone. Dev. Cell 21, 808–810 10.1016/j.devcel.2011.10.021 [DOI] [PubMed] [Google Scholar]
- 30.Roy M. and Roux S. (2018) Rab GTPases in osteoclastic endomembrane systems. BioMed. Res. Int. 2018, 4541538 10.1155/2018/4541538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran A., Coxon F.P., McDermott E., Ganley I., Odgren P.R., Martinez J. et al. (2016) The role of LC3 and autophagy in bone resorption by osteoclasts. Bone Abstracts 5, P195 10.1530/boneabs.5.P195 [DOI] [Google Scholar]
- 32.Stenmark H. (2009) Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- 33.Pfeffer S.R. (2017) Rab GTPases: master regulators that establish the secretory and endocytic pathways. Mol. Biol. Cell 28, 712–715 10.1091/mbc.e16-10-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao H., Ettala O. and Väänänen H.K. (2002) Intracellular membrane trafficking pathways in bone-resorbing osteoclasts revealed by cloning and subcellular localization studies of small GTP-binding rab proteins. Biochem. Biophys. Res. Commun. 293, 1060–1065 10.1016/S0006-291X(02)00326-1 [DOI] [PubMed] [Google Scholar]
- 35.Hirvonen M.J., Mulari M.T., Büki K.G., Vihko P., Härkönen P.L. and Väänänen H.K. (2012) Rab13 is upregulated during osteoclast differentiation and associates with small vesicles revealing polarized distribution in resorbing cells. J. Histochem. Cytochem. 60, 537–549 10.1369/0022155412448069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor A., Mules E.H., Seabra M.C., Helfrich M.H., Rogers M.J. and Coxon F.P. (2011) Impaired prenylation of Rab GTPases in the gunmetal mouse causes defects in bone cell function. Small GTPases 2, 131–142 10.4161/sgtp.2.3.16488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao H., Laitala-Leinonen T., Parikka V. and Väänänen H.K. (2001) Downregulation of small GTPase Rab7 impairs osteoclast polarization and bone resorption. J. Biol. Chem. 276, 39295–39302 10.1074/jbc.M010999200 [DOI] [PubMed] [Google Scholar]
- 38.Sun Y., Büki K.G., Ettala O., Vääräniemi J.P. and Väänänen H.K. (2005) Possible role of direct Rac1-Rab7 interaction in ruffled border formation of osteoclasts. J. Biol. Chem. 280, 32356–32361 10.1074/jbc.M414213200 [DOI] [PubMed] [Google Scholar]
- 39.Croke M., Ross F.P., Korhonen M., Williams D.A., Zou W. and Teitelbaum S.L. (2011) Rac deletion in osteoclasts causes severe osteopetrosis. J. Cell Sci. 124, 3811–3821 10.1242/jcs.086280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S., Vadim A., Arai A., Park N.H. and Kim R. (2017) Bisphosphonate inhibits bone resorption by blocking autophagy in osteoclasts. JBMR Supplemental Proceedings of the 2017 ASBMR Annual Scientific Meeting, Denver, CO [Google Scholar]
- 41.Carroll B., Mohd-Naim N., Maximiano F., Frasa M.A., McCormack J., Finelli M. et al. (2013) The TBC/RabGAP armus coordinates Rac1 and Rab7 functions during autophagy. Dev. Cell 25, 15–28 10.1016/j.devcel.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frasa M.A., Maximiano F.C., Smolarczyk K., Francis R.E., Betson M.E., Lozano E. et al. (2010) Armus is a Rac1 effector that inactivates Rab7 and regulates E-cadherin degradation. Curr. Biol. 20, 198–208 10.1016/j.cub.2009.12.053 [DOI] [PubMed] [Google Scholar]
- 43.McEwan D.G. and Dikic I. (2015) PLEKHM1: adapting to life at the lysosome. Autophagy 11, 720–722 10.1080/15548627.2015.1034419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rawet-Slobodkin M. and Elazar Z. (2015) PLEKHM1: a multiprotein adaptor for the endolysosomal system. Mol. Cell 57, 1–3 10.1016/j.molcel.2014.12.022 [DOI] [PubMed] [Google Scholar]
- 45.Van Wesenbeeck L., Odgren P.R., Coxon F.P., Frattini A., Moens P., Perdu B. et al. (2007) Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J. Clin. Invest. 117, 919–930 10.1172/JCI30328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabata K., Matsunaga K., Sakane A., Sasaki T., Noda T. and Yoshimori T. (2010) Rubicon and PLEKHM1 negatively regulate the endocytic/autophagic pathway via a novel Rab7-binding domain. Mol. Biol. Cell 21, 4162–4172 10.1091/mbc.e10-06-0495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Fattore A., Fornari R., Van Wesenbeeck L., de Freitas F., Timmermans J.P., Peruzzi B. et al. (2008) A new heterozygous mutation (R714C) of the osteopetrosis gene, pleckstrin homolog domain containing family M (with run domain) member 1 (PLEKHM1), impairs vesicular acidification and increases TRACP secretion in osteoclasts. J. Bone Miner. Res. 23, 380–391 10.1359/jbmr.071107 [DOI] [PubMed] [Google Scholar]
- 48.Fujiwara T., Ye S., Castro-Gomes T., Winchell C.G., Andrews N.W., Voth D.E. et al. (2016) PLEKHM1/DEF8/RAB7 complex regulates lysosome positioning and bone homeostasis. JCI Insight. 1, e86330 10.1172/jci.insight.86330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye S., Fowler T.W., Pavlos N.J., Ng P.Y., Liang K., Feng Y. et al. (2011) LIS1 regulates osteoclast formation and function through its interactions with dynein/dynactin and Plekhm1. PLoS ONE 6, e27285 10.1371/journal.pone.0027285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abu-Amer Y., Teitelbaum S.L., Chappel J.C., Schlesinger P. and Ross F.P. (1999) Expression and regulation of RAB3 proteins in osteoclasts and their precursors. J. Bone Miner. Res. 14, 1855–1860 10.1359/jbmr.1999.14.11.1855 [DOI] [PubMed] [Google Scholar]
- 51.Pavlos N.J., Xu J., Riedel D., Yeoh J.S., Teitelbaum S.L., Papadimitriou J.M. et al. (2005) Rab3d regulates a novel vesicular trafficking pathway that is required for osteoclastic bone resorption. Mol. Cell. Biol. 25, 5253–5269 10.1128/MCB.25.12.5253-5269.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Millar A.L., Pavios N.J., Xu J. and Zheng M.H. (2002) Rab3d: a regulator of exocytosis in non-neuronal cells. Histol. Histopathol. 17, 929–936 10.14670/HH-17.929 [DOI] [PubMed] [Google Scholar]
- 53.Ng P.Y., Cheng T.S., Zhao H., Ye S., Sm Ang E., Khor E.C. et al. (2013) Disruption of the dynein-dynactin complex unveils motor-specific functions in osteoclast formation and bone resorption. J. Bone Miner. Res. 28, 119–134 10.1002/jbmr.1725 [DOI] [PubMed] [Google Scholar]
- 54.Pavlos N.J., Cheng T.S., Qin A., Ng P.Y., Feng H.T., Ang E.S. et al. (2011) Tctex-1, a novel interaction partner of Rab3D, is required for osteoclastic bone resorption. Mol. Cell. Biol. 31, 1551–1564 10.1128/MCB.00834-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimada-Sugawara M., Sakai E., Okamoto K., Fukuda M., Izumi T., Yoshida N. et al. (2015) Rab27a regulates transport of cell surface receptors modulating multinucleation and lysosome-related organelles in osteoclasts. Sci. Rep. 5, 9620 10.1038/srep09620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Detter J.C., Zhang Q., Mules E.H., Novak E.K., Mishra V.S., Li W. et al. (2000) Rab geranylgeranyl transferase alpha mutation in the gunmetal mouse reduces Rab prenylation and platelet synthesis. Proc. Natl Acad. Sci. U.S.A. 97, 4144–4149 10.1073/pnas.080517697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto N., Sekiya M., Tohyama K., Ishiyama-Matsuura E., Sun-Wada G.H., Wada Y. et al. (2018) Essential role of the a3 isoform of V-ATPase in secretory lysosome trafficking via Rab7 recruitment. Sci. Rep. 8, 6701 10.1038/s41598-018-24918-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leung K.F., Baron R., Ali B.R., Magee A.I. and Seabra M.C. (2007) Rab GTPases containing a CAAX motif are processed post-geranylgeranylation by proteolysis and methylation. J. Biol. Chem. 282, 1487–1497 10.1074/jbc.M605557200 [DOI] [PubMed] [Google Scholar]
- 59.Kondo H., Shirakawa R., Higashi T., Kawato M., Fukuda M., Kita T. et al. (2006) Constitutive GDP/GTP exchange and secretion-dependent GTP hydrolysis activity for Rab27 in platelets. J. Biol. Chem. 281, 28657–28665 10.1074/jbc.M603227200 [DOI] [PubMed] [Google Scholar]
- 60.Handley M.T., Haynes L.P. and Burgoyne R.D. (2007) Differential dynamics of Rab3A and Rab27A on secretory granules. J. Cell Sci. 120, 973–984 10.1242/jcs.03406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Charles J.F., Coury F., Sulyanto R., Sitara D., Wu J., Brady N. et al. (2012) The collection of NFATc1-dependent transcripts in the osteoclast includes numerous genes non-essential to physiologic bone resorption. Bone 51, 902–912 10.1016/j.bone.2012.08.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirvonen M.J., Buki K.G., Sun Y., Mulari M.T., Härkönen P.L. and Väänänen K.H. (2013) Novel interaction of Rab13 and Rab8 with endospanins. FEBS Open Bio 3, 83–88 10.1016/j.fob.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaguchi Y., Sakai E., Okamoto K., Kajiya H., Okabe K., Naito M. et al. (2018) Rab44, a novel large Rab GTPase, negatively regulates osteoclast differentiation by modulating intracellular calcium levels followed by NFATc1 activation. Cell. Mol. Life Sci. 75, 33–48 10.1007/s00018-017-2607-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teasdale R.D. and Collins B.M. (2012) Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: structures, functions and roles in disease. Biochem. J. 441, 39–59 10.1042/BJ20111226 [DOI] [PubMed] [Google Scholar]
- 65.Zhu C.H., Morse L.R. and Battaglino R.A. (2011) SNX10 is required for osteoclast formation and resorption activity. J. Cell. Biochem. 113, 1608–1615 10.1002/jcb.24029 [DOI] [PubMed] [Google Scholar]
- 66.Aker M., Rouvinski A., Hashavia S., Ta-Shma A., Shaag A., Zenvirt S. et al. (2012) An SNX10 mutation causes malignant osteopetrosis of infancy. J. Med. Genet. 49, 221–226 10.1136/jmedgenet-2011-100520 [DOI] [PubMed] [Google Scholar]
- 67.Mégarbané A., Pangrazio A., Villa A., Chouery E., Maarawi J., Sabbagh S. et al. (2013) Homozygous stop mutation in the SNX10 gene in a consanguineous Iraqi boy with osteopetrosis and corpus callosum hypoplasia. Eur. J. Med. Genet. 56, 32–35 10.1016/j.ejmg.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 68.Pangrazio A., Fasth A., Sbardellati A., Orchard P.J., Kasow K.A., Raza J. et al. (2013) SNX10 mutations define a subgroup of human autosomal recessive osteopetrosis with variable clinical severity. J. Bone Miner. Res. 28, 1041–1049 10.1002/jbmr.1849 [DOI] [PubMed] [Google Scholar]
- 69.Amirfiroozy A., Hamidieh A.A., Golchehre Z., Rezamand A., Yahyaei M., Beiranvandi F. et al. (2017) A novel mutation in SNX10 gene causes malignant infantile osteopetrosis. Avicenna J. Med. Biotechnol. 9, 205–208 PMID: [PMC free article] [PubMed] [Google Scholar]
- 70.Stattin E.L., Henning P., Klar J., McDermott E., Stecksen-Blicks C., Sandström P.E. et al. (2017) SNX10 gene mutation leading to osteopetrosis with dysfunctional osteoclasts. Sci. Rep. 7, 3012 10.1038/s41598-017-02533-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye L., Morse L.R., Zhang L., Sasaki H., Mills J.C., Odgren P.R. et al. (2015) Osteopetrorickets due to Snx10 deficiency in mice results from both failed osteoclast activity and loss of gastric acid-dependent calcium absorption. PLoS Genet. 11, e1005057 10.1371/journal.pgen.1005057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y., Wu B., Xu L., Li H., Xia J., Yin W. et al. (2012) A SNX10/V-ATPase pathway regulates ciliogenesis in vitro and in vivo. Cell Res. 22, 333–345 10.1038/cr.2011.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou C., Wang Y., Peng J., Li C., Liu P. and Shen X. (2017) SNX10 plays a critical role in MMP9 secretion via JNK-p38-ERK signaling pathway. J. Cell. Biochem. 118, 4664–4671 10.1002/jcb.26132 [DOI] [PubMed] [Google Scholar]
- 74.You Y., Li W.Z., Zhang S., Hu B., Li Y.X., Li H.D. et al. (2018) SNX10 mediates alcohol-induced liver injury and steatosis by regulating the activation of chaperone-mediated autophagy. J. Hepatol. 69, 129–141 10.1016/j.jhep.2018.01.038 [DOI] [PubMed] [Google Scholar]
- 75.Vaananen K. (2005) Mechanism of osteoclast mediated bone resorption–rationale for the design of new therapeutics. Adv. Drug Deliv. Rev. 57, 959–971 10.1016/j.addr.2004.12.018 [DOI] [PubMed] [Google Scholar]
- 76.Milat F. and Ebeling P.R. (2016) Osteoporosis treatment: a missed opportunity. Med. J. Aust. 205, 185–190 10.5694/mja16.00568 [DOI] [PubMed] [Google Scholar]
- 77.Teitelbaum S.L. (2016) Therapeutic implications of suppressing osteoclast formation versus function. Rheumatology (Oxford) 55, ii61–iii3 10.1093/rheumatology/kew350 [DOI] [PubMed] [Google Scholar]
- 78.Luckman S.P., Hughes D.E., Coxon F.P., Graham R., Russell G. and Rogers M.J. (1998) Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J. Bone Miner. Res. 13, 581–589 10.1359/jbmr.1998.13.4.581 [DOI] [PubMed] [Google Scholar]
- 79.Coxon F.P., Helfrich M.H., Van't Hof R., Sebti S., Ralston S.H., Hamilton A. et al. (2000) Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J. Bone Miner. Res. 15, 1467–1476 10.1359/jbmr.2000.15.8.1467 [DOI] [PubMed] [Google Scholar]
- 80.Coxon F.P., Ebetino F.H., Mules E.H., Seabra M.C., McKenna C.E. and Rogers M.J. (2005) Phosphonocarboxylate inhibitors of Rab geranylgeranyl transferase disrupt the prenylation and membrane localization of Rab proteins in osteoclasts in vitro and in vivo. Bone 37, 349–358 10.1016/j.bone.2005.04.021 [DOI] [PubMed] [Google Scholar]
- 81.Coxon F.P., Helfrich M.H., Larijani B., Muzylak M., Dunford J.E., Marshall D. et al. (2001) Identification of a novel phosphonocarboxylate inhibitor of Rab geranylgeranyl transferase that specifically prevents Rab prenylation in osteoclasts and macrophages. J. Biol. Chem. 276, 48213–48222 10.1074/jbc.M106473200 [DOI] [PubMed] [Google Scholar]
- 82.Soe K. and Delaisse J.M. (2017) Time-lapse reveals that osteoclasts can move across the bone surface while resorbing. J. Cell Sci. 130, 2026–2035 10.1242/jcs.202036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henriksen K., Karsdal M.A. and Martin T.J. (2014) Osteoclast-derived coupling factors in bone remodeling. Calcif. Tissue Int. 94, 88–97 10.1007/s00223-013-9741-7 [DOI] [PubMed] [Google Scholar]
- 84.Sun S., Błażewska K.M., Kadina A.P., Kashemirov B.A., Duan X., Triffitt J.T. et al. (2016) Fluorescent bisphosphonate and carboxyphosphonate probes: a versatile imaging toolkit for applications in bone biology and biomedicine. Bioconjug. Chem. 27, 329–340 10.1021/acs.bioconjchem.5b00369 [DOI] [PMC free article] [PubMed] [Google Scholar]