Abstract
Background
About 10% of patients admitted to a chest pain unit (CPU) exhibit atrial fibrillation (AF).
Hypothesis
To determine whether calcium scores (CS) are superior over common risk scores for coronary artery disease (CAD) in patients presenting with atypical chest pain, newly diagnosed AF, and intermediate pretest probability for CAD within the CPU.
Methods
In 73 subjects, CS was related to the following risk scores: Global Registry of Acute Coronary Events (GRACE) score, including a new model of a frequency‐normalized approach; Thrombolysis In Myocardial Infarction score; European Society of Cardiology Systematic Coronary Risk Evaluation (SCORE); Framingham risk score; and Prospective Cardiovascular Münster Study score. Revascularization rates during index stay were assessed.
Results
Median CS was 77 (interquartile range, 1–270), with higher values in men and the left anterior descending artery. Only the modified GRACE (ρ = 0.27; P = 0.02) and the SCORE (ρ = 0.39; P < 0.005) were significantly correlated with CS, whereas the GRACE (τ = 0.21; P = 0.04) and modified GRACE (τ = 0.23; P = 0.02) scores were significantly correlated with percentile groups. Only the CS significantly discriminated between those with and without stenosis (P < 0.01).
Conclusions
Apart from modified GRACE score, overall correlations between risk scores and calcium burden, as well as revascularization rates during index stay, were low. By contrast, the determination of CS may be used as an additional surrogate marker in risk stratification in AF patients with intermediate pretest likelihood for CAD admitted to a CPU.
Introduction
It has been shown that the admission of patients presenting with acute chest pain of possible ischemic origin to specialized units provides optimized care, lower hazard ratios, cost‐effectiveness, and higher patient satisfaction.1, 2, 3 Especially in the United States and Germany, broad networks of chest pain units (CPUs) have been created during the last decade targeting quality‐of‐care criteria for prompt identification of low‐risk patients and treatment of patients with acute coronary syndromes (ACS).4 In addition to the classical ACS as the principal diagnosis, more than 50% of admissions to CPUs are also related to a variety of other entities, including about 10% of patients with new‐onset atrial fibrillation (AF).5 Atrial fibrillation is a very common cardiac arrhythmia, with as many as 2.3 million cases in the United States; there are robust data suggesting a coincidence of AF and coronary artery disease (CAD).6, 7, 8 By contrast, there are only limited data regarding optimal diagnostic strategies for patients with newly diagnosed AF and atypical symptoms of an ACS.9 A relatively low risk for myocardial ischemia of 2% to 5% has been assigned to patients admitted to an emergency department with AF, without further increase of the risk of acute myocardial infarction.10, 11 Determining the utility of coronary artery calcification (CAC) scanning for risk stratification in CAD has been a subject of intensive investigation. Scanning of CAC has emerged as a robust predictor for coronary events in subjects with an intermediate pretest probability for CAD. A higher CAC burden carries a greater risk for future CAD events and all‐cause mortality, thereby appropriately reclassifying a majority of patients into low‐ and high‐risk categories.8, 12, 13, 14, 15 However, adequate determination of pretest risk assessment of CAD is still a matter of discussion within the CPU process, favoring additional pathways, at least within the chest pain center accreditation process in the United States and Germany.4, 9, 16, 17 It is recommended that scoring systems should be used to identify patients at risk on the one hand, and patients at low risk, who can be discharged quickly and safely, on the other hand. We report here the first evaluation of several widely used risk‐assessment tools in the evaluation of patients presenting with acute atypical chest pain and intermediate pretest probability for CAD and newly diagnosed AF. The aim of our study was to analyze the correlation of CAC burden by cardiac computed tomography (CCT) with common risk scores for CAD as an indicator for suspected ACS in this subset of patients admitted to a German CPU.
Methods
This study was approved by the local institutional review board. We retrospectively included patients who presented to the CPU at our institution with a first diagnosis of AF, acute atypical chest pain, and a clinical impression of intermediate likelihood for CAD during a 2‐year‐period (October 2012 to September 2014). Patients received CCT imaging within the index stay. Atrial fibrillation was defined according to the current European Society of Guidelines (ESC) guidelines for the management of AF.18 At the time of CT image acquisition, all eligible patients had sinus rhythm following spontaneous, medical, or electrical cardioversion. Major exclusion criteria were history of known CAD, known AF, new diagnostic ischemic changes on the initial electrocardiogram, and initial troponin in excess of 99th percentile of the local assay with a relevant dynamic rise or fall suggestive for ischemia.
Imaging
All scans were performed in supine position in mid‐inspiration breathhold. The CCT scans were performed using a 128‐slice scanner (Aquilion CX; Toshiba Medical Systems, Tokyo, Japan). The CCTs were acquired in a single‐slice axial scan from the base to the apex with an image acquisition time of 350 ms. Slice thickness was 3 mm. Prospective electrocardiogram triggering was performed at 80% of the R‐R interval. The reconstructed image data were transferred to the computer workstation for postprocessing. All analyses were performed by a single experienced reader (F.B.) using a Vitrea workstation (Toshiba Medical Systems, Tokyo, Japan). The CAC score was determined according to the methods of Agatston et al5 and expressed in Hounsfield units. At least 4 contiguous pixels with a density of ≥130 Hounsfield units were used to define an area of CAC.19 Total CAC scores comprising all calcified coronary lesions were computed. Percentile ranking was performed on the basis of the German population‐based Heinz Nixdorf Recall (HNR) study.20
Risk Scores
The following risk scores were retrospectively assessed as previously described: Global Registry of Acute Coronary Events (GRACE) in‐hospital mortality score, Thrombolysis In Myocardial Infarction (TIMI) risk score, the ESC Systematic Coronary Risk Evaluation (SCORE), Framingham risk score, and Prospective Cardiovascular Münster Study (PROCAM) score.21, 22, 23, 24, 25, 26, 27 Additionally, we also analyzed a modified GRACE score excluding heart rate, as we anticipated that an increased heart rate in AF overestimates the traditional GRACE score.
Major Adverse Cardiac and Cerebrovascular Events During Index Stay
Additionally, major adverse cardiac and cerebrovascular events as well as revascularization rates in terms of percutaneous coronary intervention (PCI) or coronary artery bypass graft placement (CABG) during index stay were evaluated.
Statistical Analysis
Continuous variables were graphically assessed for normality. As the assumption of normality could not be maintained for any variable, descriptive data are reported as percentage or as median (interquartile range [IQR]). Percentile ranking was performed on the basis of the German population‐based HNR study.20 Correlations were assessed using the Pearson correlation on transformed Agatston scores using the transformation f(x) = log(x + 1) or the nonparametric Kendall rank correlation test on other variables; differences in CAC scores were assessed using the Wilcoxon rank sum test; and basic demographics were assessed using the Wilcoxon rank‐sum test or Fisher exact test where appropriate. P values <0.05 were considered significant. Tests were not corrected for multiple comparisons. All statistical analyses were performed using the R Language and Environment for Statistical Computing, version 3.1.2 (R Foundation, Vienna, Austria).
Results
Patient Demographics
A total of 618 patients were screened for study participation. Of those, 73 patients (41% male; median age, 71 years [IQR, 62–78 years]) fulfilled the inclusion criteria. Typical symptoms at admission included atypical angina pectoris (95%), palpitations (81%), dyspnea (55%), and/or agitation (21%). Conventional risk factors were present as follows: smoking, 8%; arterial hypertension, 78%; positive family history, 30%; hyperlipidemia, 52%; diabetes, 5%; and body mass index >25 kg/m2, 70%. Basic demographic data stratified by CAC score are given in Table 1. Patients without coronary calcification were significantly younger, and those with high CAC burden were significantly more often male.
Table 1.
Overview of Cohort Demographics Stratified by CAC Score
| Basic Demographics | CAC Burden | P Across Groups | ||
|---|---|---|---|---|
| CS = 0 (n = 18) | CS ≤ 400 (n = 40) | CS > 400 (n = 15) | ||
| Male sex | 39 | 30 | 73 | 0.01 |
| Age, y | 62 (57–69) | 75 (64–81) | 72 (68–76) | 0.004 |
| Smoking | 11 | 10 | — | 0.61 |
| HTN | 72 | 78 | 87 | 0.64 |
| Hyperlipidemia | 50 | 58 | 47 | 0.73 |
| BMI, kg/m2 | 28 (26–30) | 26 (24–29) | 29 (25–38) | 0.20 |
| DM | 6 | 2 | 13 | 0.15 |
| CAD family history | 44 | 28 | 20 | 0.32 |
| HR, bpm | 122 (106–139) | 124 (92–136) | 101 (81–121) | 0.24 |
| SBP, mm Hg | 125 (116–130) | 135 (124–150) | 130 (130–145) | 0.17 |
| Impaired LVEF (<55%) | 11 | 20 | 13 | 0.77 |
| CHA2DS2‐VASc | 3 (1–3) | 3 (2–4) | 2 (2–4) | 0.25 |
Abbreviations: BMI, body mass index; CAC, coronary artery calcification; CAD, coronary artery disease; CHA2DS2‐VASc, congestive heart failure, HTN, age ≥75 y, DM, stroke/TIA, vascular disease, age 65–74 y, sex category (women); CS, calcium score; DM, diabetes mellitus; HR, heart rate at admission; HTN, hypertension; IQR, interquartile range; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure at admission; TIA, transient ischemic attack.
Data are presented as % or median (IQR).
Calcium Score
Agatston scores ranged between 0 and 2726 (median, 77; IQR, 1–270). A CS of zero occurred in 24% of the patients (Figure 1). Detailed data on sex‐ and territory‐specific CAC burden are shown in Table 2.
Figure 1.
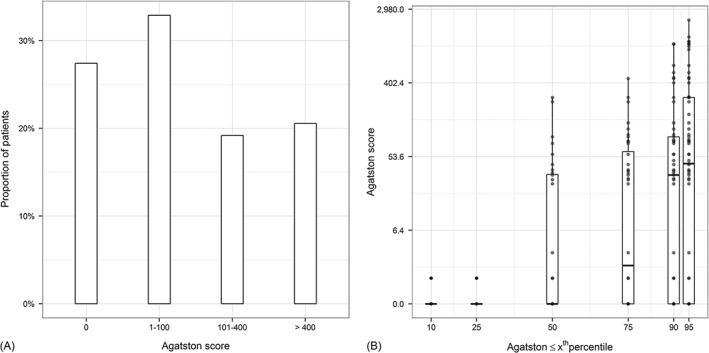
Bar diagram highlighting the distribution of (A) coronary calcium burden as well as (B) the distribution within the corresponding percentile ranking by age and sex.
Table 2.
Overview of Sex‐ and Territory‐Specific CAC Burden
| Territory | Overall, N = 73 | Sex | |
|---|---|---|---|
| F, n = 43 | M, n = 30 | ||
| MS | 0 (0–11) | 0 (0–0) | 2 (0–39) |
| LAD | 44 (0–136) | 34 (0–93) | 78 (0–432) |
| LCX | 0 (0–49) | 0 (0–6) | 0 (0–82) |
| RCA | 0 (0–48) | 0 (0–14) | 4 (0–165) |
Abbreviations: CAC, coronary artery calcification; F, female; IQR, interquartile range; LAD, left anterior descending artery; LCX, left circumflex artery; M, male; MS, main stem; RCA, right coronary artery.
Data are presented as median (IQR).
Risk Scoring
As visualized in Figure 2, TIMI risk scores, GRACE scores, and modified GRACE scores as a measure of the risk of short‐term major adverse cardiac events in confirmed ACS patients (Figure 2A) showed by trend a good correlation, whereas correlations between PROCAM, Framingham, and ESC SCORE scores as a measure of major adverse cardiac events and/or overall CAD risk in suspected ACS patients were low (Figure 2B). Table 3 illuminates the proportions concerning the different risk categories within the different scoring systems tested.
Figure 2.
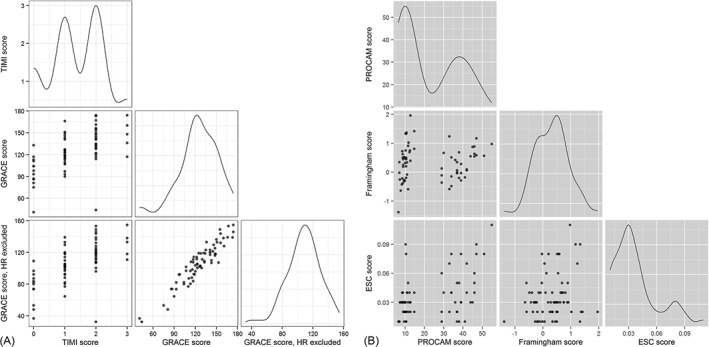
Correlations of the different scores tested, demonstrating (A) a good accordance between TIMI, GRACE, and modified GRACE scores. Deviations in (B) the correlation of PROCAM, FRS, and ESC SCORE mainly result from different design of the ESC SCORE and the bimodal distribution of the PROCAM score. Abbreviations: ESC SCORE, European Society of Cardiology Systematic Coronary Risk Evaluation; FRS, Framingham Risk Score; GRACE, Global Registry of Acute Coronary Events in‐hospital mortality score; HR, heart rate; PROCAM, Prospective Cardiovascular Münster score; TIMI, Thrombolysis In Myocardial Infarction.
Table 3.
Comparison of Patients' Distribution to the Different Risk Categories Depending on Risk Score Used
| Score | Risk Category | ||
|---|---|---|---|
| Low | Intermediate | High | |
| TIMI | 0–2, 93% | 3–4, 7% | >5, 0% |
| GRACE | 1–108, 21% | 109–140, 45% | >140, 34% |
| Modified GRACE | 1–94.6, 27% | 94.7–122.7, 49% | >122.7, 23% |
| PROCAM | 0–37, 76% | 38–53, 23% | >53, 1% |
| FRS | 0–12, 98% | 13–15, 2% | >16, 0% |
| ESC SCORE | ≤1, 15% | 2–4, 62% | >5, 22% |
Abbreviations: ESC SCORE, European Society of Cardiology Systematic Coronary Risk Evaluation; FRS, Framingham Risk Score; GRACE, Global Registry of Acute Coronary Events 6‐month postdischarge score; PROCAM, Prospective Cardiovascular Münster; TIMI, Thrombolysis In Myocardial Infarction.
Correlation Between Calcium Scores and Risk Scores
Both absolute CS, as well as percentile ranking, were not randomly distributed but showed low positive correlations (correlations for absolute CS scores: TIMI score, ρ = 0.20; GRACE score, ρ = 0.22; modified GRACE score, ρ = 0.27; PROCAM score, ρ = 0.22; Framingham score, ρ = 0.08; ESC SCORE, ρ = 0.39 [Figure 3A–F]). Of these, only the correlations for the modified GRACE score and the ESC SCORE were significant (P = 0.02 and P < 0.005, respectively). Correlations for percentile groups (0–10th, 11th–25th, 26th–50th, 51st–75th, 76th–90th, 91st–95th, 96th–100th percentile; patients without CAC excluded) were as follows: TIMI score, τ = 0.07; GRACE score, τ = 0.21; modified GRACE score, τ = 0.23; PROCAM score, τ = −0.12; Framingham score, τ = −0.18; ESC SCORE, τ = 0 (Figure 4). Only the GRACE score (P = 0.04) and the modified GRACE score (P = 0.02) were significantly correlated with the percentile groups in this subset.
Figure 3.
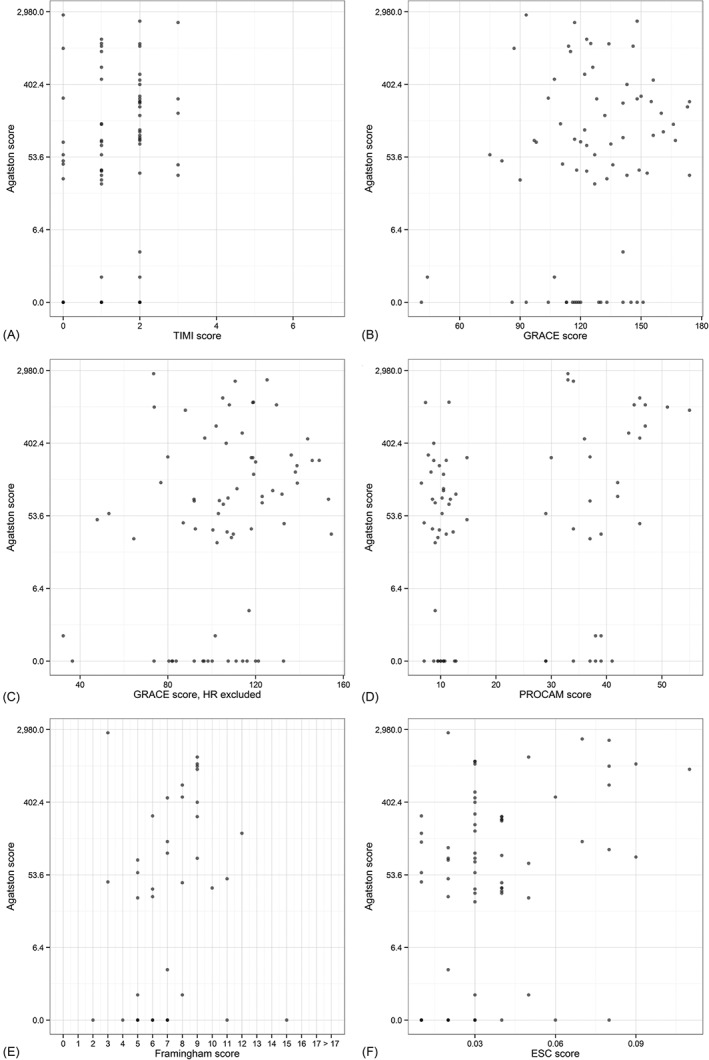
Visualization of the correlation between the CS and the (A) TIMI, (B) GRACE, (C) modified GRACE, (D) PROCAM, (E) FRS, and (F) ESC SCORE risk scores. Abbreviations: CS, calcium score; ESC SCORE, European Society of Cardiology Systematic Coronary Risk Evaluation; FRS, Framingham Risk Score; GRACE, Global Registry of Acute Coronary Events 6‐month postdischarge score; HR, heart rate; PROCAM, Prospective Cardiovascular Münster score; TIMI, Thrombolysis In Myocardial Infarction.
Figure 4.
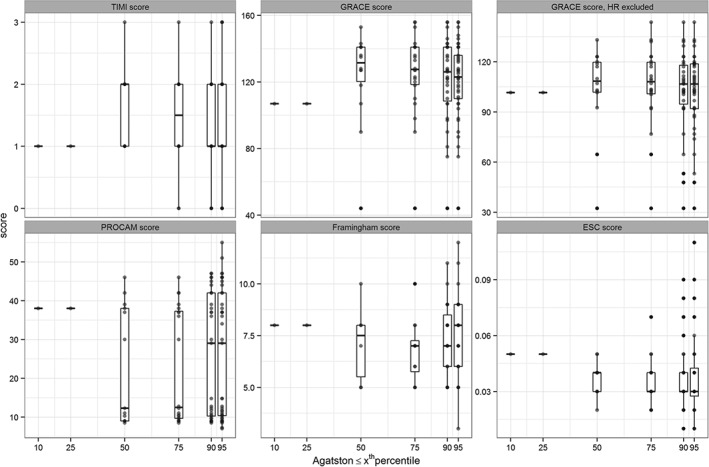
Visualization of the correlation between the percentile ranking according to the HNR study and the different risk scores. Patients without CAC are excluded from this analysis. CAC, coronary artery calcification; ESC SCORE, European Society of Cardiology Systematic Coronary Risk Evaluation; GRACE, Global Registry of Acute Coronary Events 6‐months post discharge score; HNR, Heinz Nixdorf Recall; HR, heart rate; PROCAM, Prospective Cardiovascular Münster score; TIMI, Thrombolysis In Myocardial Infarction.
Correlation Between Calcium Scores or Risk Scores and Stenotic Coronary Artery Disease
There were no major adverse cardiac and cerebrovascular events observed during index stay. Two individuals underwent PCI, and in 3 cases a CABG was performed. When comparing the different risk scores or CS with those patients receiving coronary intervention (either PCI or CABG), only the CS significantly discriminated between those with and without stenosis (P < 0.01). There was no significant association for the GRACE score (P = 0.90), modified GRACE score (P = 0.23), TIMI score (P = 0.28), ESC SCORE (P = 0.90), Framingham score (P = 0.31), or PROCAM score (P = 0.23).
Discussion
The management of acute chest pain is still one of the hallmark entities for emergency physicians worldwide. The implementation of CPUs has improved the standard of care; however, timely and correct risk stratification of potential ACS patients remains challenging.9, 27 Several professional societies, including the German Cardiac Society (GCS) and the Society of Cardiovascular Patient Care (SCPC), recommend the regular use of risk scoring to identify those patients where inpatient admission might not be beneficial.4, 9, 16
In our study, common risk‐scoring systems were tested in patients with new‐onset AF (paroxysmal or persistent), atypical chest pain, and an intermediate pretest probability for CAD evaluated at a specialized CPU. Scoring included the TIMI risk score, GRACE score (including a modified GRACE score normalized for heart rate), PROCAM score, Framingham risk score, and ESC SCORE and was correlated to CAC burden measured by CCT. The Agatston score has been developed in an attempt to reflect a marker for the quantity and location of CAC within the coronary‐artery circulation and has attracted a considerable body of research and clinical attention with further imaging improvements over recent years.8, 12, 13, 14, 19 In contrast to cardiac stress testing, the determination of the CS aims to directly estimate coronary‐plaque severity, suggesting that increasing CS is associated with an increasing likelihood for obstructive lesions.28, 29 A CS score of zero is thought to virtually exclude relevant CAD, even in chest‐pain patients with a low to intermediate pretest probability demonstrating a high negative predictive value for ACS in this population.30, 31
Correlations between the different risk scores were low and best between TIMI, GRACE, and modified GRACE scores. Simultaneously, we found only limited correlations between each of the scores tested and the CAC burden in CCT. Only our model of a modified GRACE score showed statistically significant correlations with the CAC burden and percentile ranking. Prior studies investigating perfusion deficits in atrial branches of the coronary‐artery system demonstrated that CAD is an independent predictor for the development of AF, regardless of whether it originated from the left or right coronary system.32 By looking at the in‐hospital revascularization rates in our cohort, only the CS was found suitable to discriminate between those patients with and without an obstructive lesion. As a result, when proposing that CAC is a predictor for cardiac events and may also serve as an indicator for obstructive CAD, one might argue that the conventional risk scores may not be probate and/or sufficient in this AF subset presenting to a CPU. However, adequate scoring is crucial when looking at the CAD prevalence of up to 1% in the general population and a proportion of AF patients admitted to a CPU of up to 10%.7
So far, studies mainly focused on high‐sensitivity troponins or C‐reactive protein for optimizing risk stratification beyond the common risk scores have shown limited success.33, 34, 35 By contrast, our current data support the assumption that additional CCT evaluation may be beneficial and more accurate than conventional risk‐assessment tools and may improve the diagnostic workflow in this cohort.36 Even though overall CS percentile distribution in our cohort showed a relative accordance with the percentile distribution resulting from the unselected population‐based HNR study population, the quantification of CAC burden is particularly valuable in that respect that we found zero CS in about a quarter of the patients and high to very high CS in another quarter.20, 37
Although in our study coronary angiography as gold standard for determining obstructive CAD was not performed, based on the available studies we propose that a zero CS score may be useful to identify a subset of patients with AF and symptoms suggestive of ACS who may not require hospitalization or other advanced diagnostic testing. In patients with high (>400) or very high (>1000) CAC burden, the percentage of positive patients appears to be higher than anticipated and higher than previously reported within a population‐based approach, thereby favoring further inpatient care and potential invasive management in this subgroup identified as on a higher risk level than initially predicted.13 The fact that high CS was associated with a higher revascularization rate strongly supports that assumption. At the same time, patients may profit from atherosclerotic disease quantification allowing more intense risk‐factor modification. On the other hand, ruling out relevant CAD may also be helpful in choosing potential drugs for antiarrhythmic therapy, particularly when the use of class I antiarrhythmic drugs is considered.38, 39
Study Limitations
Data are based on a retrospective, single‐center, observational study without case–control matching with inherent limitations only. The study struggles with small sample size as a major limitation. Even though modern multislice volume scanners can handle imaging in AF patients, most centers with “common” CTs still struggle with a limited diagnostic accuracy due to irregular R‐R intervals. Therefore, we only included patients with first onset of AF with spontaneous or medical/electrical cardioversion, thus the large group of patients with persisting AF was excluded. With respect to scoring, retrospective assessment of the different scores was performed on basis of the documentation at admission only. It must be stressed that ultimately, regardless of the risk‐assessment tool used, risk stratification is a dynamic, ongoing process and should be reassessed periodically from initial presentation to discharge. With respect to revascularization, the level of significance may be altered by selection bias, as patients with high and very high CS were more likely to undergo invasive diagnostics. For the same reason, negative predictive values for a zero CS score cannot be provided. Additionally and most notably, the current study lacks long‐term follow‐up data, which would have provided more conclusive information on outcomes.
Conclusion
All common risk scores used in the study showed relatively low correlations with the CAC burden, with best performance from a modified GRACE score with exclusion of heart rate. Only the CS significantly discriminated between patients with and without coronary stenosis. In that decisive point, all common risk scores, including both the GRACE and the modified GRACE score, failed in our cohort. In scope of the potential ischemic trigger of AF in patients with atypical chest pain and an intermediate pretest likelihood of CAD, our data lead to the suggestion that the determination of CAC burden serves as an independent surrogate marker in risk stratification and should be considered within routine diagnostic workup in this commonly encountered subset within the CPU concept. However, as the role of CAC screening is still a matter of debate and posthospitalization outcome data are not available, this clinical implication remains speculative at this time and may also be improved by adjunct novel biomarker assessment.40
Dr. Garvey received research support from and is an advisory board member for Philips Healthcare. This work is part of Liane Hinrichs' doctoral thesis.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Steurer J, Held U, Schmid D, et al. Clinical value of diagnostic instruments for ruling out acute coronary syndrome in patients with chest pain: a systematic review. Emerg Med J. 2010;27:896–902. [DOI] [PubMed] [Google Scholar]
- 2. Keller T, Post F, Tzikas S, et al. Improved outcome in acute coronary syndrome by establishing a chest pain unit. Clin Res Cardiol. 2010;99:149–155. [DOI] [PubMed] [Google Scholar]
- 3. Münzel T, Post F. The development of chest pain units in Germany. Eur Heart J. 2011;32:657–658. [PubMed] [Google Scholar]
- 4. Breuckmann F, Burt DR, Melching K, et al. Chest pain centers: a comparison of accreditation programs in Germany and the United States. Crit Pathw Cardiol. 2015;14:67–73. [DOI] [PubMed] [Google Scholar]
- 5. Schmitt J, Duray G, Gersh BJ, et al. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30:1038–1045. [DOI] [PubMed] [Google Scholar]
- 6. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 7. Illmann A, Riemer T, Erbel R, et al. Disease distribution and outcome in troponin‐positive patients with or without revascularization in a chest pain unit: results of the German CPU‐Registry. Clin Res Cardiol. 2014;103:29–40. [DOI] [PubMed] [Google Scholar]
- 8. Brown AM, Sease KL, Robey JL, et al. The risk for acute coronary syndrome associated with atrial fibrillation among ED patients with chest pain syndromes. Am J Emerg Med. 2007;25:523–528. [DOI] [PubMed] [Google Scholar]
- 9. Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 10. Zimetbaum PJ, Josephson ME, McDonald MJ, et al. Incidence and predictors of myocardial infarction among patients with atrial fibrillation. J Am Coll Cardiol. 2000;36:1223–1227. [DOI] [PubMed] [Google Scholar]
- 11. Friedman HZ, Weber‐Bornstein N, Deboe SF, et al. Cardiac care unit admission criteria for suspected acute myocardial infarction in new‐onset atrial fibrillation. Am J Cardiol. 1987;59:866–869. [DOI] [PubMed] [Google Scholar]
- 12. Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging. 2015;8:579–596. [DOI] [PubMed] [Google Scholar]
- 13. Schmermund A, Möhlenkamp S, Berenbein S, et al. Population‐based assessment of subclinical coronary atherosclerosis using electron‐beam computed tomography. Atherosclerosis. 2006;185:177–182. [DOI] [PubMed] [Google Scholar]
- 14. Oudkerk M, Stillman AE, Halliburton SS, et al. Coronary artery calcium screening: current status and recommendations from the European Society of Cardiac Radiology and North American Society for Cardiovascular Imaging. Int J Cardiovasc Imaging. 2008;24:645–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taylor AJ, Cerqueira M, Hodgson JM, et al. Appropriate use criteria for cardiac computed tomography: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525–e555. [DOI] [PubMed] [Google Scholar]
- 16. Breuckmann F, Post F, Giannitsis E, et al. Kriterien der Deutschen Gesellschaft für Kardiologie—Herz‐ und Kreislaufforschung für “Chest Pain Units” [article in German]. Kardiologe. 2008;2:389–394. [Google Scholar]
- 17. Peacock WF, Kontos MC, Amsterdam E, et al. Impact of Society of Cardiovascular Patient Care accreditation on quality: an ACTION Registry–Get With The Guidelines analysis. Crit Pathw Cardiol. 2013;12:116–120. [DOI] [PubMed] [Google Scholar]
- 18. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) [published correction appears in Eur Heart J. 2011;32:1172]. Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 19. Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 20. Erbel R, Lehmann N, Churzidse S, et al. Progression of coronary artery calcification seems to be inevitable, but predictable—results of the Heinz Nixdorf Recall (HNR) study. Eur Heart J. 2014;35:2960–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med. 2003;163:2345–2353. [DOI] [PubMed] [Google Scholar]
- 22. Correia LC, Freitas R, Bittencourt AP, et al. Prognostic value of GRACE scores versus TIMI score in acute coronary syndromes [article in Portuguese]. Arq Bras Cardiol. 2010;94:613–619. [DOI] [PubMed] [Google Scholar]
- 23. Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten‐year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 24. Okwuosa TM, Greenland P, Ning H, et al. Distribution of coronary artery calcium scores by Framingham 10‐year risk strata in the MESA (Multi‐Ethnic Study of Atherosclerosis) potential implications for coronary risk assessment. J Am Coll Cardiol. 2011;57:1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Versteylen MO, Joosen IA, Shaw LJ, et al. Comparison of Framingham, PROCAM, SCORE, and Diamond Forrester to predict coronary atherosclerosis and cardiovascular events. J Nucl Cardiol. 2011;18:904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10‐year follow‐up of the prospective cardiovascular Münster (PROCAM) study [published correction appears in Circulation. 2002;105:900]. Circulation. 2002;105:310–315. [DOI] [PubMed] [Google Scholar]
- 27. Breuckmann F, Hochadel M, Darius H, et al. Guideline adherence and perspectives in the acute management of unstable angina—initial results from the German chest pain unit registry. J Cardiol. 2015;66:108–113. [DOI] [PubMed] [Google Scholar]
- 28. Chang SM, Nabi F, Xu J, et al. Value of CACS compared with ETT and myocardial perfusion imaging for predicting long‐term cardiac outcome in asymptomatic and symptomatic patients at low risk for coronary disease: clinical implications in a multimodality imaging world. JACC Cardiovasc Imaging. 2015;8:134–144. [DOI] [PubMed] [Google Scholar]
- 29. Ibrahim O, Oteh M, Anwar IR, et al. Calcium score of coronary artery stratifies the risk of obstructive coronary artery diseases. Clin Ter. 2013;164:391–395. [DOI] [PubMed] [Google Scholar]
- 30. Stillman AE, Oudkerk M, Ackerman M, et al. Use of multidetector computed tomography for the assessment of acute chest pain: a consensus statement of the North American Society of Cardiac Imaging and the European Society of Cardiac Radiology. Eur Radiol. 2007;17:2196–2207. [DOI] [PubMed] [Google Scholar]
- 31. Vogler N, Meyer M, Fink C, et al. Predictive value of zero calcium score and low‐end percentiles for the presence of significant coronary artery stenosis in stable patients with suspected coronary artery disease. Rofo. 2013;185:726–732. [DOI] [PubMed] [Google Scholar]
- 32. Alasady M, Abhayaratna WP, Leong DP, et al. Coronary artery disease affecting the atrial branches is an independent determinant of atrial fibrillation after myocardial infarction. Heart Rhythm. 2011;8:955–960. [DOI] [PubMed] [Google Scholar]
- 33. Filion KB, Agarwal SK, Ballantyne CM, et al. High‐sensitivity cardiac troponin T and the risk of incident atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2015;169:31.e3–38.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C‐reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population‐based cohort study. Lancet. 2011;378:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmermund A, Voigtländer T. Predictive ability of coronary artery calcium and CRP. Lancet. 2011;378:641–643. [DOI] [PubMed] [Google Scholar]
- 36. Valenti V, Ó Hartaigh B, Heo R, et al. A 15‐year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow‐up of 9715 individuals. JACC Cardiovasc Imaging. 2015;8:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Möhlenkamp S, Schmermund A, Lehmann N, et al; Heinz Nixdorf Recall Study Investigators. Subclinical coronary atherosclerosis and resting ECG abnormalities in an unselected general population. Atherosclerosis. 2008;196:786–794. [DOI] [PubMed] [Google Scholar]
- 38. Aliot E, Capucci A, Crijns HJ, et al. Twenty‐five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace. 2011;13:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Erbel R, Möhlenkamp S, Lehmann N, et al; Heinz Nixdorf Recall Study Investigators. Sex‐related cardiovascular risk stratification based on quantification of atherosclerosis and inflammation. Atherosclerosis. 2008;197:662–672. [DOI] [PubMed] [Google Scholar]
- 40. Singh M, Babayan Z, Harjai KJ, et al. Utilization patterns of single‐photon emission cardiac tomography myocardial perfusion imaging studies in a rural tertiary care setting. Clin Cardiol. 2014;37:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


