Abstract
Recent trials reported that risk of atrial fibrillation (AF) is increased in patients using ivabradine compared with controls. We performed this meta‐analysis to investigate the risk of AF association with ivabradine treatment on the basis of data obtained from randomized controlled trials (RCTs). We searched PubMed, EMBASE, Scopus, and the Cochrane Library for RCTs that comprised >100 patients. The incidence of AF was assessed. We obtained data from European Medicines Agency (EMA) scientific reports for the RCTs in which the incidence of AF was not reported. We used trial sequential analysis (TSA) to provide information on when we had reached firm evidence of new AF based on a 15% relative risk increase (RRI) in ivabradine treatment. Three RCTs and 1 EMA overall oral safety set (OOSS) pooled analysis (included 5 RCTs) were included in the meta‐analysis (N = 40 437). The incidence of AF was 5.34% in patients using ivabradine and 4.56% in placebo. There was significantly higher incidence of AF (24% RRI) in the ivabradine group when compared with placebo before (RR: 1.24, 95% confidence interval: 1.08‐1.42, P = 0.003, I 1980 = 53%) and after excluding OOSS (RR: 1.24, 95% confidence interval: 1.06‐1.44, P = 0.008). In the TSA, the cumulative z‐curve crossed both the traditional boundary (P = 0.05) and the trial sequential monitoring boundary, indicating firm evidence for ≥15% increase in ivabradine treatment when compared with placebo. Study results indicate that AF is more common in the ivabradine group (24% RRI) than in controls.
Keywords: atrial fibrillation, meta‐analysis, Ivabradine
1. INTRODUCTION
Ivabradine is a heart rate (HR)‐lowering drug and acts via specific and selective I f inhibition.1 Since 1980 it has been well known that resting HR is both a prognostic indicator and treatment target in coronary artery disease (CAD) and heart failure (HF).2, 3 Early clinical studies of ivabradine, such as the International Trial on the Treatment of Angina With Ivabradine vs Atenolol (INITIATIVE) study4 and the Morbidity‐Mortality Evaluation of the I f Inhibitor Ivabradine in Patients With Coronary Disease and Left Ventricular Dysfunction (BEAUTIFUL)5 trial, focused on its antianginal effects. It has been shown that selective reduction of HR improves coronary blood flow in ischemic myocardial area. Favorable effects have been more pronounced in patients with HR >70 bpm as determined in the prespecified subgroup analyses. Afterward, the Systolic Heart Failure Treatment With the I f Inhibitor Ivabradine Trial (SHIFT),6 which included HF patients, was conducted, and in this study it was determined that ivabradine reduced the adverse events in HF patients. In a meta‐analysis performed by Martin et al in 2014, it was determined that atrial fibrillation (AF) risk was significantly greater in patients using ivabradine.7 Recently, the Study Assessing the Morbidity‐Mortality Benefits of the I f Inhibitor Ivabradine in Patients With Coronary Artery Disease (SIGNIFY),8 which is the largest randomized controlled trial (RCT) including CAD patients without HF, was published, and positive effects of ivabradine were not observed in this patient group. Furthermore, it was determined that frequency of AF and bradycardia were significantly higher in the ivabradine arm when compared with placebo. In SIGNIFY subgroup analyses, which were published later, it was claimed that neither AF nor bradycardia were related to adverse events.9
Debates about increased AF risk in patients using ivabradine still continue. Therefore, we aimed to perform a meta‐analysis to assess the risk of AF in patients using ivabradine on the basis of data obtained from all double‐blind RCTs.
2. METHODS
We followed the preferred reporting items for systematic reviews and meta‐analyses guidelines to report our findings.10
2.1. Eligibility Criteria
The study's eligibility criteria were as follows: double‐blind RCTs that (1) compared ivabradine with placebo, (2) included the incidence of AF during follow‐up, and (3) had ≥50 patients in each group. We did not exclude trials where AF incidence was not reported in the published manuscript, but attempted to identify that data wherever possible.
2.2. Information Sources and Searching
We searched the MEDLINE, Scopus, EMBASE, and Cochrane Library for RCTs published up to January 2016 in the English language and in humans. Also, European Medicines Agency (EMA) scientific discussion as evidence of licensing was searched for data that were not published in the original trial or unpublished trials. In addition, to find any potential eligible studies, we performed a manual search by checking all the references of RCTs, meta‐analyses, and reviews. A computerized search using the terms “ivabradine” and “randomized controlled trial” was made for any indication. All searches were conducted by 2 authors (IHT and ST).
2.3. Selection and Quality Assessment of Randomized Controlled Trials
Two authors (IHT and ST) independently assessed study eligibility and risk of bias and extracted data. Disagreements were resolved by consensus. The risk of bias was assessed by recommendation of the Cochrane Collaboration: sequence generation of allocation; allocation concealment; blinding of participants, staff, and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. Trials with high or unclear risk for bias for any 1 of the first 3 components were considered as at high risk of bias; otherwise, they were considered as low risk of bias.
2.4. Outcome Measure
The primary endpoint of our study was the incidence of AF during follow‐up.
2.5. Trial Sequential Analysis
We applied trial sequential analysis (TSA) to all RCTs included in the meta‐analysis. Trial sequential analysis was performed according to the monitoring boundaries approach for the incidence of AF.11, 12 Trial sequential analysis is a statistical method that combines a prior information size calculation for a meta‐analysis with adaptation of monitoring boundaries to evaluate the accumulating evidence.13 Our assumptions included 2‐sided testing, type 1 error = 5%, power = 80%. We chose a 15% relative risk increase (RRI) for the incidence of AF. The main result of TSA was expressed through a cumulative z‐curve graph; the boundaries in this graph for concluding superiority or inferiority or futility were determined according to the O'Brien‐Fleming α spending function. All calculations were carried out using a specific statistical software, TSA version 0.9 beta (User Manual for TSA, Copenhagen Trial Unit 2011; http://www.ctu.dk/tsa).
To assess the magnitude of difference is of clinical importance; we calculated absolute risk reduction/increase (ARR/ARI), relative risk reduction/increase (RRR/RRI), and number needed to treat/harm (NNT/NNH). The ARR/ARI, RRR/RRI, and NNT/NNH were calculated as defined previously.14, 15 Clinical importance was among the criteria defined as ARR/ARI ≥5%, RRR/RRI ≥15%, and NNT <50; and statistical significance was defined as P < 0.05.16
2.6. Statistical Analysis
Summary risk ratio (RR) and 95% confidence interval (CI) were calculated between ivabradine and control regarding the incidence of AF using fixed‐ and random‐effects models. The random‐effects model was indicated in outcomes with significant heterogeneity (I 2 > 25%). In others, the fixed‐effects model was used. The Q together with the resulting degrees of freedom (df), τ2, and I 2 statistic were used to evaluate heterogeneity. Furthermore, we investigated possible reasons for heterogeneity using a meta‐regression, evaluating the impact of prespecified covariates such as age, sex, diabetes mellitus, hypertension (HTN), baseline HR, baseline ejection fraction (EF), previous CAD and stroke, study indication (angina or HF), and ivabradine dose (2.5–10 mg vs >10 mg) on the incidence of AF. Sensitivity analysis was performed by excluding trials one at a time to assess the contribution of each study to the pooled estimates. The EMA–overall oral safety set (OOSS) were excluded and sensitivity analyses were repeated. Statistical significance was defined as P < 0.05 (2‐tailed tests). Statistical analysis was performed with RevMan 5.3 software (the Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark).
3. RESULTS
Our initial search strategy identified 43 articles and 1 meta‐analysis. We excluded 29 trials that were not RCTs, not double blinded, that had no follow‐up or short duration of follow‐up, had duplicate data, and which were not written in English. The meta‐analysis performed by Martin et al7 was examined in detail in terms of references. Four studies in this meta‐analysis were not included in our study because the number of patients was <100.17, 18, 19, 20 Two studies were excluded because there were no data about AF.21, 22 Finally we included 8 RCTs in this meta‐analysis. Individual AF data were only reported in the SHIFT,6 BEAUTIFUL,5 and SIGNIFY trials.8 However, in the scientific discussion documents of EMA for ivabradine license, 5 RCTs were included as a single dataset in OOSS.23 In the OOSS, AF frequency during follow‐up was given as pooled (2 of these were published before,4, 24 but we could not determine whether the remaining 3 had been published or not) as a single study. In EMA‐OOSS pooled analyses, AF frequency in ivabradine 5 to 7.5 mg (n = 1650) and 10 mg (n = 1160), placebo (n = 313), amlodipine (n = 404), and atenolol (n = 408) arms were identified. Data about AF frequency were obtained from the original article in SIGNIFY8 and SHIFT6 trials, whereas the AF frequency data in the BEAUTIFUL study were obtained from the EMA website.25
Eight RCTs included 40 437 patients. When OOSS was excluded, the remaining patients numbered 36 501. Baseline characteristics are summarized in Table 1. The incidence of AF was 5.34% (n = 1126) in the ivabradine group and 4.56% (n = 885) in the placebo group. There was a significantly higher incidence of AF (24% RRI) in the ivabradine group when compared with placebo (RR: 1.24, 95% CI: 1.08‐1.42, P = 0.003; Figure 1A). There was a significant heterogeneity between trials (I 2 = 53%, τ2 = 0.01, Q [df: 3] = 6.3, and P = 0.10). The NNH, derived from pooled risk difference (0.77%), was 122 over a median of 2 years' follow‐up with ivabradine treatment. In the analysis performed after OOSS was excluded, AF frequency was still significantly higher in the ivabradine group (RR: 1.24, 95% CI: 1.06‐1.44, P = 0.008; Figure 1B). There was a significant heterogeneity between trials (I 2 = 68%, τ2 = 0.01, Q [df: 2] = 6.3, and P = 0.04). Similarly, the NNH derived from pooled risk difference (1.22%) was 82.
Table 1.
Baseline Characteristics of Included RCTs
| Trials | IVA, n | Control, n | Comparator | Indication | IVA Dose, mg | Age, y | EF, % | Previous CAD, % | Male Sex, % | Previous Stroke, % | HR, bpm | DM, % | HTN, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OOSS | 2811 | 1125 | Aten/Aml/placebo | Angina | 5–10 | NA | NA | 100 | NA | NA | NA | NA | NA |
| BEAUTIFUL | 5477 | 5430 | Placebo | HF + angina | 5–7.5 | 65.2 | 32.4 | 88 | 83 | 18 | 71.6 | 37 | 71 |
| SHIFT | 3232 | 3260 | Placebo | HF | 2.5–7.5 | 60.4 | 29 | 68 | 77 | 8 | 79.9 | 31 | 67 |
| SIGNIFY | 9550 | 9552 | Placebo | Angina | 10–20 | 65 | 56.5 | 100 | 72.5 | 6.6 | 77.2 | 43.1 | 86.2 |
Abbreviations: Aml, amlodipine; Aten, atenolol; BEAUTIFUL, Morbidity‐Mortality Evaluation of the I f Inhibitor Ivabradine in Patients With Coronary Disease and Left Ventricular Dysfunction; CAD, coronary artery disease; DM, diabetes mellitus; EF, ejection fraction; HF, heart failure; HR, heart rate; HTN, hypertension; IVA, ivabradine; NA, not available; OOSS, overall oral safety set; RCT, randomized controlled trial; SHIFT, Systolic Heart Failure Treatment With the I f Inhibitor Ivabradine Trial; SIGNIFY, Study Assessing the Morbidity‐Mortality Benefits of the I f Inhibitor Ivabradine in Patients With Coronary Artery Disease.
Figure 1.
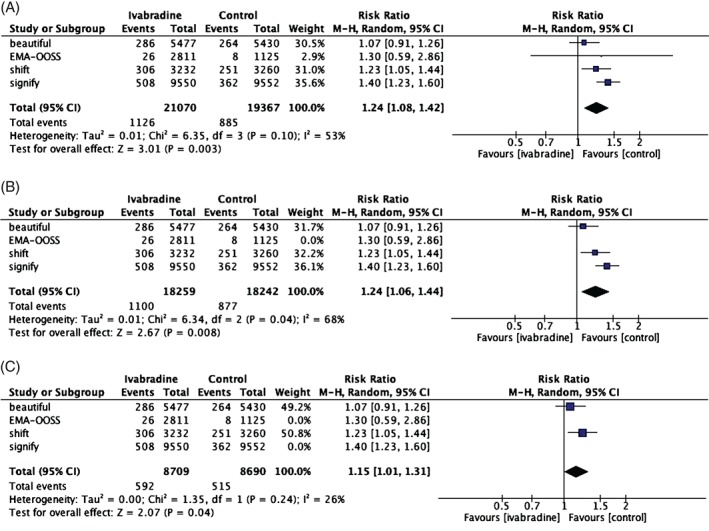
Forest plots of AF risk in (A) 8 RCTs; in (B) 3 RCTs, BEAUTIFUL, SHIFT, and SIGNIFY; and in (C) SHIFT and BEAUTIFUL. Abbreviations: AF, atrial fibrillation; BEAUTIFUL, Morbidity‐Mortality Evaluation of the I f Inhibitor Ivabradine in Patients With Coronary Disease and Left Ventricular Dysfunction; CI, confidence interval; df, degrees of freedom; EMA‐OOSS, European Medicines Agency–overall oral safety set; M‐H, Mantel‐Haenszel; RCT, randomized controlled trial; SHIFT, Systolic Heart Failure Treatment With the I f Inhibitor Ivabradine Trial; SIGNIFY, Study Assessing the Morbidity‐Mortality Benefits of the I f Inhibitor Ivabradine in Patients With Coronary Artery Disease.
When the SHIFT and BEAUTIFUL studies were both analyzed in the overall population (Figure 1C) and as previously reported in patients with HR >70 bpm,26 the AF incidence was found to be higher in the ivabradine group (RR: 1.15, 95% CI: 1.01‐1.31, P = 0.04 and RR: 1.25, 95% CI: 1.10‐1.42, respectively). In Table 2, we summarized clinical vs statistical importance for individual trials as well as from pairwise combinations to overall pooled combination.
Table 2.
Clinical Significance vs Statistical Significance
| Results | AF % (IVA) | AF % (Control) | RR (95% CI) | ARI | RRI, % | NNH, n | Clinically Significance? | Statistically Significance? |
|---|---|---|---|---|---|---|---|---|
| OOSS | 0.9 | 0.7 | 1.30 (0.59‐2.86) | 0.21 | 30 | 468 | N | N |
| BEAUTIFUL | 5.2 | 4.9 | 1.08 (0.91‐1.27) | 0.36 | 8 | 278 | N | N |
| SHIFT | 9.5 | 7.7 | 1.23 (1.05‐1.44) | 1.77 | 23 | 57 | Y | Y |
| SIGNIFY | 5.3 | 3.8 | 1.40 (1.23‐1.60) | 1.53 | 40 | 65 | Y | Y |
| BEAUTIFUL + SHIFT | 6.8 | 5.9 | 1.15 (1.01‐1.31) | 0.87 | 15 | 115 | Maybe | Y |
| BEAUTIFUL + SHIFT with HR >70 bpm | 8.4 | 6.7 | 1.26 (1.11‐1.43) | 1.72 | 26 | 58 | Y | Y |
| BEAUTIFUL + SHIFT + SIGNIFY | 6.0 | 4.8 | 1.24 (1.06‐1.44) | 1.22 | 24 | 82 | Y | Y |
| OOSS+ BEAUTIFUL + SHIFT + SIGNIFY | 5.3 | 4.6 | 1.24 (1.08‐1.42) | 0.77 | 24 | 129 | Y | Y |
Abbreviations: AF, atrial fibrillation; ARI, absolute risk increase; BEAUTIFUL, Morbidity‐Mortality Evaluation of the I f Inhibitor Ivabradine in Patients With Coronary Disease and Left Ventricular Dysfunction; CI, confidence interval; HR, heart rate; IVA, ivabradine; N, no; NNH, number needed to harm; OOSS, overall oral safety set; RR, relative risk; RRI, relative risk increase; SHIFT, Systolic Heart Failure Treatment With the I f Inhibitor Ivabradine Trial; SIGNIFY, Study Assessing the Morbidity‐Mortality Benefits of the I f Inhibitor Ivabradine in Patients With Coronary Artery Disease; Y, yes.
We did not assess small study effects and publication bias using funnel plot because the number of studies was <10.27 Sensitivity analysis indicated that none of the studies had a significant influential effect on the risk of AF and similar results to main findings, except that the SHIFT trial had a borderline significant effect on the risk of AF (P = 0.064).
After adjusting for baseline covariates (age, diabetes mellitus, HTN, baseline HR, baseline EF, previous CAD and stroke, study indications [angina or HF], and ivabradine doses [2.5–10 mg vs >10 mg]), we determined that previous CAD, baseline EF, study indications, ivabradine doses, previous stroke, and HTN might be the cause of heterogeneity for the development of AF during follow‐up.
In the TSA, the cumulative z‐curve crossed both the traditional boundary (P = 0.05) and the trial sequential monitoring boundary, indicating that there is firm evidence for ≥15% increase in ivabradine group when compared with placebo (Figure 2).
Figure 2.
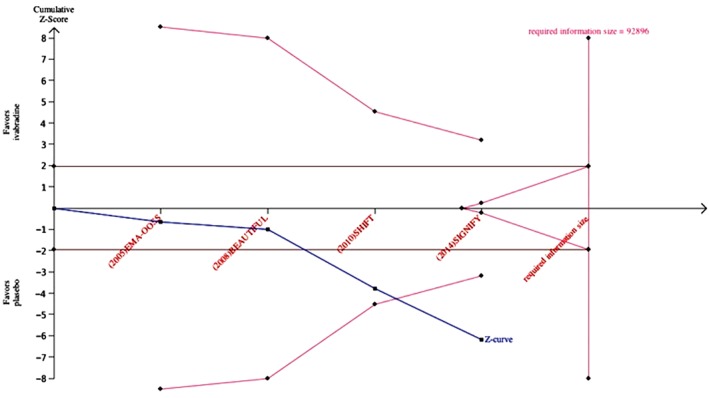
Trial sequential analysis evaluating the risk of AF in ivabradine treatment. The expected relative risk increase was assumed to be 15%. Abbreviations: AF, atrial fibrillation; BEAUTIFUL, Morbidity‐Mortality Evaluation of the I f Inhibitor Ivabradine in Patients With Coronary Disease and Left Ventricular Dysfunction; EMA‐OOSS, European Medicines Agency–overall oral safety set; SHIFT, Systolic Heart Failure Treatment With the I f Inhibitor Ivabradine Trial; SIGNIFY, Study Assessing the Morbidity‐Mortality Benefits of the I f Inhibitor Ivabradine in Patients With Coronary Artery Disease.
4. DISCUSSION
This meta‐analysis result showed that ivabradine treatment is associated with increased risk of AF with approximately 24% RRI. Also, TSA indicated that there was firm evidence for increased AF risk in ivabradine treatment.
The studies about ivabradine use in HF and angina started after 2000. Its use in angina pectoris and HF was approved by the EMA in 2005 and 2012, respectively. Increased AF risk related to ivabradine use as shown in the meta‐analysis by Martin et al7 in 2014, and in the SIGNIFY8 study published in the same year, has drawn attention. We included a total of 8 RCTs in our study. Among these, individual AF data were available only in the SHIFT,6 BEAUTIFUL,5 and SIGNIFY trials.8 However, in the EMA application for ivabradine license, 5 RCTs were included in the OOSS. In the OOSS, AF frequency during follow‐up was given as pooled; because of this, we considered the OOSS that included these 5 RCTs as a single study. Also, 2 of these were published before,4, 24 but we could not determine whether the remaining 3 had been published or not. The EMA‐OOSS analyzed as a single dataset is likely to result in an overestimation of within‐study variance and an underestimation of between‐study variance. In the meta‐analysis performed by Martin et al,7 BEAUTIFUL, SHIFT, and EMA‐OOSS were included and ivabradine treatment was shown to increase AF risk by 15% (RR: 1.15, 95% CI: 1.05‐1.26, P = 0.015). The number of patients in 4 RCTs was <10017, 18, 19, 20 and there were no data related to AF frequency; therefore, they were not included in our study (Martin et al7 indicated that they obtained the AF data of those studies via personal communication). On the other hand, in our own analysis, we included the SIGNIFY8 study, which was published after the meta‐analysis performed by Martin et al.7 Additionally, when we combined the SHIFT and BEAUTIFUL studies, which included patients with HF, their analysis demonstrated that the AF incidence was higher in the ivabradine group (RR: 1.15, 95% CI: 1.01‐1.31, P = 0.04). Also, Fox et al26 recently showed that in the pooled subgroup analysis of the SHIFT and BEAUTIFUL studies, there was a significantly increased risk of AF with ivabradine (RR: 1.25, 95% CI: 1.10‐1.42) in patients with HR >70 bpm. Finally, we performed a TSA and clinical vs statistical importance analysis in our meta‐analysis. In the TSA, we showed that there was firm evidence for a ≥15% increase in ivabradine treatment when compared with placebo. Also, pooled analysis of all study combinations in Table 2 showed that effect magnitude is meaningful both clinically and statistically.
Among studies included in our meta‐analysis, only the SHIFT study included patients with a past history of AF6; however, in the SIGNIFY and BEAUTIFUL studies, patients with NSR were indicated as an inclusion criteria.5, 8 There was no information about the history of AF in the EMA‐OOSS data.23 This may affect the AF incidence during follow‐up. Also, baseline characteristics of the studies may be one of the factors affecting the AF incidence during follow‐up. In the meta‐regression analysis, we determined that previous CAD, baseline EF, study indications, ivabradine doses, previous stroke, and HTN might be the cause of heterogeneity for the development of AF in follow‐up. For instance, the absence of HF patients and the high dose of ivabradine use in the SIGNIFY study may be related to the AF risk.8
There is only 1 trial with data about the effect of ivabradine‐related AF on the clinical outcomes.9 It was demonstrated strongly that AF was related to unfavorable clinical events in population‐based large clinical trials with long‐term follow‐up.28, 29 However, even though it was mentioned that there was no difference between patients with and without emergent AF regarding clinical outcomes in the SIGNIFY substudy,9 the fact that both the number of patients with emergent AF was relatively low and the follow‐up period was short might have masked the relation with clinical events.
There might be a few possible explanations regarding the increased risk of AF with ivabradine. First, when the baseline clinical characteristics of patients examined in detail in RCTs were included in this meta‐analysis, one might propose that such a group of patients is already prone to AF; however, the randomized design of the included trials makes this assumption weak. Evaluations listed in Table 2 demonstrate that increased AF risk is also clinically important. Another possible mechanism may be the mechanism claimed by Martin et al.7 As known, ivabradine inhibits I f channels coded by the HCN4 gene.30 Also, pulmonary venous myocardium,31 which is an important source in the initiation and maintenance of AF, contains high rates of I f channels.32 Genome‐wide association studies have identified associations between genetic variants in the region of the HCN4 gene.33, 34 This might be associated with the possibility that ivabradine treatment may increase AF risk.
4.1. Study Limitations
An important limitation of our study was that EMA‐OOSS was analyzed as a single dataset and there were no detailed data about the studies constituting this dataset. However, we repeated the pooled analyses and performed sensitivity analyses both including and excluding this dataset. In addition, because there were no comprehensive data demonstrating the relationship of newly developed AF during the follow‐up with clinical events, we could not analyze the relationship of newly developed AF with clinical events.
5. CONCLUSION
In this study, we showed that ivabradine treatment is associated with increased risk of AF.
Tanboğa İH, Topçu S, Aksakal E, Gulcu O, Aksakal E, Aksu U, Oduncu V, Ulusoy FR, Sevimli S, Kaymaz C. The Risk of Atrial Fibrillation With Ivabradine Treatment: A Meta‐analysis With Trial Sequential Analysis of More Than 40000 Patients, Clin Cardiol 2016, 39, 615–620. DOI: 10.1002/clc.22568
The authors have no funding, financial relationships, or conflicts of interest to disclose.
REFERENCES
- 1. DiFrancesco D. Funny channels in the control of cardiac rhythm and mode of action of selective blockers. Pharmacol Res. 2006;53:399–406. [DOI] [PubMed] [Google Scholar]
- 2. Dyer AR, Persky V, Stamler J, et al. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol. 1980;112:736–749. [DOI] [PubMed] [Google Scholar]
- 3. Kannel WB, Kannel C, Paffenbarger RS Jr, et al. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. [DOI] [PubMed] [Google Scholar]
- 4. Tardif JC, Ford I, Tendera M, et al; INITIATIVE Investigators . Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J . 2005;26:2529–2536. [DOI] [PubMed] [Google Scholar]
- 5. Fox K, Ford I, Steg PG, et al; BEAUTIFUL Investigators . Ivabradine for patients with stable coronary artery disease and left‐ventricular systolic dysfunction (BEAUTIFUL): a randomised, double‐blind, placebo‐controlled trial. Lancet . 2008;372:807–816. [DOI] [PubMed] [Google Scholar]
- 6. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study [published correction appears in Lancet. 2010;376:1988]. Lancet. 2010;376:875–885. [DOI] [PubMed] [Google Scholar]
- 7. Martin RI, Pogoryelova O, Koref MS, et al. Atrial fibrillation associated with ivabradine treatment: meta‐analysis of randomised controlled trials. Heart. 2014;100:1506–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fox K, Ford I, Steg PG, et al. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371:1091–1099. [DOI] [PubMed] [Google Scholar]
- 9. Fox K, Ford I, Steg PG, et al. Bradycardia and atrial fibrillation in patients with stable coronary artery disease treated with ivabradine: an analysis from the SIGNIFY study. Eur Heart J. 2015;36:3291–3296. [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 11. Brok J, Thorlund K, Wetterslev J, et al. Apparently conclusive meta‐analyses may be inconclusive—trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. Int J Epidemiol. 2009;38:287–298. [DOI] [PubMed] [Google Scholar]
- 12. Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. J Clin Epidemiol. 2008;61:64–75. [DOI] [PubMed] [Google Scholar]
- 13. Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses? Int J Epidemiol. 2009;38:276–286. [DOI] [PubMed] [Google Scholar]
- 14. Barratt A, Wyer PC, Hatala R, et al. Tips for learners of evidence‐based medicine: 1. Relative risk reduction, absolute risk reduction and number needed to treat. CMAJ. 2004;171:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diamond GA, Kaul S. Prior convictions: Bayesian approaches to the analysis and interpretation of clinical megatrials. J Am Coll Cardiol. 2004;43:1929–1939. [DOI] [PubMed] [Google Scholar]
- 16. Kaul S, Diamond GA. Trial and error: how to avoid commonly encountered limitations of published clinical trials. J Am Coll Cardiol. 2010;55:415–427. [DOI] [PubMed] [Google Scholar]
- 17. Nerla R, Di Franco A, Milo M, et al. Differential effects of heart rate reduction by atenolol or ivabradine on peripheral endothelial function in type 2 diabetic patients. Heart. 2012;98:1812–1816. [DOI] [PubMed] [Google Scholar]
- 18. Cappato R, Castelvecchio S, Ricci C, et al. Clinical efficacy of ivabradine in patients with inappropriate sinus tachycardia: a prospective, randomized, placebo‐controlled, double‐blind, crossover evaluation. J Am Coll Cardiol. 2012;60:1323–1329. [DOI] [PubMed] [Google Scholar]
- 19. Villano A, Di Franco A, Nerla R, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013;112:8–13. [DOI] [PubMed] [Google Scholar]
- 20. Dominguez‐Rodriguez A, Consuegra‐Sanchez L, Blanco‐Palacios G, et al. Anti‐inflammatory effects of ivabradine in patients with acute coronary syndrome: a pilot study. Int J Cardiol. 2012;158:160–162. [DOI] [PubMed] [Google Scholar]
- 21. Fasullo S, Cannizzaro S, Maringhini G, et al. Comparison of ivabradine versus metoprolol in early phases of reperfused anterior myocardial infarction with impaired left ventricular function: preliminary findings. J Card Fail. 2009;15:856–863. [DOI] [PubMed] [Google Scholar]
- 22. Tardif JC, Ponikowski P, Kahan T; ASSOCIATE Study Investigators . Efficacy of the I(f) current inhibitor ivabradine in patients with chronic stable angina receiving β‐blocker therapy: a 4‐month, randomized, placebo‐controlled trial. Eur Heart J . 2009;30:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. European Medicines Agency . Procoralan, European public assessment report (EPAR). Procedural steps taken before authorization. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Procedural_steps_taken_before_authorisation/human/000597/WC500043588.pdf. Accessed January 2016.
- 24. Ruzyllo W, Tendera M, Ford I, et al. Antianginal efficacy and safety of ivabradine compared with amlodipine in patients with stable effort angina pectoris: a 3‐month randomised, double‐blind, multicentre, noninferiority trial. Drugs. 2007;67:393–405. [DOI] [PubMed] [Google Scholar]
- 25. European Medicines Agency. Ivabradine, scientific discussion . http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Scientific_Discussion_‐_Variation/human/000598/WC500063748.pdf. Accessed January 2016.
- 26. Fox K, Komajda M, Ford I, et al. Effect of ivabradine in patients with left‐ventricular systolic dysfunction: a pooled analysis of individual patient data from the BEAUTIFUL and SHIFT trials. Eur Heart J. 2013;34:2263–2270. [DOI] [PubMed] [Google Scholar]
- 27. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. [DOI] [PubMed] [Google Scholar]
- 28. Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 29. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998;82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 30. Baruscotti M, Bucchi A, Difrancesco D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol Ther. 2005;107:59–79. [DOI] [PubMed] [Google Scholar]
- 31. Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 32. Chen YC, Pan NH, Cheng CC, et al. Heterogeneous expression of potassium currents and pacemaker currents potentially regulates arrhythmogenesis of pulmonary vein cardiomyocytes. J Cardiovasc Electrophysiol. 2009;20:1039–1045. [DOI] [PubMed] [Google Scholar]
- 33. Ellinor PT, Lunetta KL, Albert CM, et al. Meta‐analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. den Hoed M, Eijgelsheim M, Esko T, et al. Identification of heart rate–associated loci and their effects on cardiac conduction and rhythm disorders. Nat Genet. 2013;45:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]


