ABSTRACT
Background
The use of oral anticoagulation or dual antiplatelet therapy (DAPT) is recommended within the first 45 days after left atrial appendage (LAA) closure using the Watchman device because of incomplete device endothelialization. This study reports for the first time the feasibility of novel oral anticoagulants (NOAC) in these patients.
Hypothesis
NOAC therapy is safe and effective after LAA closure.
Methods
Interventional LAA closure was performed successfully in 45 patients. Of these, 18 patients received NOAC during the first 45 days after implantation and 27 patients received DAPT. Transesophageal echocardiography was conducted 45 days after implantation. The primary study endpoint was abnormal thrombus apposition 45 days after implantation. Secondary study endpoints were death from any cause, major adverse cardiac and cerebrovascular events (MACCE), and major bleedings.
Results
After 45 days, transesophageal echocardiography revealed no abnormal thrombus apposition. During a follow‐up of 417 ± 323 days, 7 patients died. No stroke or transient ischemic attack occurred. Nonfatal myocardial infarction occurred in 1 patient. There was a nonsignificant trend for lower all‐cause mortality (P = 0.159) and occurrence of MACCE (P = 0.096) in the NOAC group compared with the DAPT group. Overall, 6 patients suffered from a major bleeding (NOAC, n = 3; DAPT, n = 3). In NOAC group, major bleedings (at day 205, 688, and 736) occurred long after termination of NOAC therapy. There was no significant difference in the frequency of major bleedings in different groups.
Conclusions
Our pilot study suggests that NOAC therapy within the first 45 days after interventional LAA closure is safe and effective.
Introduction
Atrial fibrillation (AF) is a common arrhythmia associated with thromboembolic stroke and transient ischemic attack (TIA). In nonvalvular AF patients, >90% of atrial thrombi originate from the left atrial appendage (LAA).1 For several years, interventional LAA closure systems have been available that enable nonpharmacological stroke prevention.
In the 3.8‐year follow‐up of Watchman Left Atrial Appendage Closure Technology for Embolic Protection in Patients With Atrial Fibrillation (PROTECT‐AF), percutaneous LAA closure met criteria for superiority, compared with warfarin, for preventing stroke.2 Despite these favorable results, some serious side effects, such as device‐associated strokes, are described. In particular, the first 45 days after implantation are a critical transition period. After this time, a complete endothelialization of the device is expected.3, 4 An incomplete endothelialization, however, is associated with the risk of thrombus formation and stroke. According to the manufacturer's suggestion, oral anticoagulation (OAC) with warfarin is therefore recommended during the first 45 days after implantation.2, 3, 4 A recent study suggests that dual antiplatelet therapy (DAPT) is also reasonable in patients with absolute contraindications to OAC.5 Despite this therapy, device‐associated abnormal thrombus apposition occurred in about 4% of all patients.4, 5, 6
Methods
This single‐center study aims to investigate the use of novel oral anticoagulants (NOAC) during the first 45 days after implantation of an LAA occlusion device (Watchman; Boston Scientific, Marlborough, MA). It is a retrospective analysis of prospectively gained data. The local ethics committee approved the protocol.
Inclusion Criteria
The inclusion criteria were a history nonvalvular AF and a CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 y, diabetes mellitus, stroke/TIA, vascular disease, age 65–74 y, sex category [female]) score >1. Exclusion criteria were mechanical prosthetic heart valve, left ventricular ejection fraction <30%, and intracardiac thrombus.7 All patients with a successfully implanted device were included in the main analysis.
Device Implantation and Anticoagulation
The Watchman device and the implantation procedure have been described in detail elsewhere.3 Briefly, the Watchman implant is a nitinol frame structure with fixation barbs and a permeable polyester fabric that covers the atrial‐facing surface of the device.3 The device was implanted under echocardiographic and fluoroscopic guidance via femoral venous access via the transseptal route into the LAA. Accurate device position was confirmed by angiography and echocardiography.3, 7
After implantation, an individualized drug therapy was performed. Patients with contraindications to OAC received a DAPT with aspirin 100 mg/d and clopidogrel 75 mg/d for 6 months, followed by a lifelong therapy with aspirin 100 mg.
Contraindications were considered as hemorrhagic/bleeding tendencies defined as active peptic ulcer disease or history of overt bleeding of the gastrointestinal, genitourinary, or respiratory tract; central nervous system hemorrhage; cerebral aneurysms; dissection of the aorta; pericarditis/pericardial effusions, or bacterial endocarditis; blood dyscrasias; unsupervised patients with senility and/or high fall risk; and other documented reason (including hypersensitivity to phenprocoumon).5
In addition, in patients already receiving DAPT for other reasons (recent or recurrent implantation of drug‐eluting coronary stent (DES), severe peripheral vascular disease, or transjugular intrahepatic portosystemic stent shunting stenosis), therapy was continued after LAA closure. These patients did not receive OAC.
Patients without preexisting DAPT or contraindication for OAC received NOAC (dabigatran or rivaroxaban) for ≥45 days according to operator's decision. After 45 days, OAC was usually stopped and clopidogrel 75 mg/d and aspirin 100 mg/d were substituted until 6 months after device implantation. After that, clopidogrel was stopped and aspirin monotherapy was continued.
Transesophageal Echocardiography and Follow‐up
Transesophageal echocardiography (TEE) was performed 45 days after implantation. In particular, device stability and positioning, abnormal thrombus apposition, and residual peri‐device flow device were evaluated. We defined an abnormal thrombus apposition as a visible thrombus on echocardiography, adhering to the device externally. Novel OAC therapy was stopped if the 45‐day TEE showed complete sealing of the LAA or minimal residual peri‐device flow of ≤5 mm.8 Follow‐up information was obtained during routine ambulatory visit 45 days after implantation and annually after implantation. In deceased patients, medical records were examined to determine the cause of death. A death of unknown cause was assumed if the cause of death was not clear to determine.
Study Endpoints
The primary endpoint was defined as abnormal thrombus apposition at the device within the first 45 days after implantation. Secondary study endpoints were the following: (1) all‐cause mortality; (2) major adverse cardiac and cerebrovascular events (MACCE), defined as death, myocardial infarction (MI), and stroke/TIA; and (3) major bleeding, defined as intracranial bleeding, hospitalization due to bleeding, hemoglobin decrease >2 mg/dL and/or transfusion of red blood cells.
Statistical Analysis
Numeric values are expressed as mean ± SD. Continuous variables were compared between groups using an unpaired t test (for normally distributed variables) or Mann–Whitney U test (for none normally distributed variables). The χ2 analysis was used to compare categorical variables. Categorical data are expressed as numbers of patients and percentages.
Freedom from all‐cause mortality was analysed by the Kaplan‐Meier method, and survival curves were compared by the log‐rank test. A P value <0.05 was considered significant. All probability values reported are 2‐sided. Analyses were performed with the SPSS statistical software package, version 20.0 (IBM Corp., Armonk, NY).
Results
Procedure and Patient Characteristics
From 2012 to 2014, 47 patients underwent LAA occlusion. Mean procedure time was 77 ± 25 minutes. The device size was chosen 8% to 20% greater than the LAA diameter, as suggested.3, 4, 9 Selected device sizes were 21 mm (n = 16), 24 mm (n = 25), 27 mm (n = 5), and 33 mm (n = 1). Procedural complications in study patients are given in Table 1.
Table 1.
Procedure‐Related Adverse Events in All Patients Within the First 7 Days (n = 47)
| Adverse Event | No. (%) |
|---|---|
| Death | 1 (2) |
| Stroke/TIA | 0 (0) |
| Device embolization | 1 (2) |
| Pericardial effusion requiring surgery | 0 (0) |
| Pericardial effusion with pericardiocentesis | 1 (2) |
| Major bleeding | 0 (0) |
Abbreviations: TIA, transient ischemic attack.
Overall, 2 patients were excluded from main analysis as they did not receive postprocedural anticoagulation: 1 patient suffered from a device embolization, which could be successfully retrieved percutaneously. The other patient died during procedure due to fatal pericardial tamponade (probably as a result of an incorrect transseptal puncture).
Thus, a total of 45 patients formed the final study cohort. During implantation, device repositioning was necessary in 8 patients (17%). No stroke/TIA occurred. In 1 patient, residual peri‐device flow device >5 mm arose.
Mean age of study patients was 75 ± 7 years, mean CHA2DS2‐VASc score was 4 ± 1.4, and mean HAS‐BLED (hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratios, elderly [age ≥65], drug therapy) score was 3.5 ± 0.8. The patients' baseline characteristics are given in Table 2. In study patients, the indications for interventional LAA occlusion were as follows: recurrent gastrointestinal bleeding (n = 20); recurrent genitourinary bleeding (n = 1); recurrent severe epistaxis (n = 1); need for therapy with aspirin and clopidogrel, due to (1) recently implanted DES (n = 5), (2) severe peripheral artery disease (n = 1), and (3) thrombosis of transjugular intrahepatic portosystemic stent shunting (n = 1); intracerebral bleeding (n = 3); toxic hepatitis under treatment with phenprocoumon (n = 1); unwilling to take any anticoagulation (n = 4); intolerances against anticoagulation (n = 4); recurrent falls with hematoma (n = 1); vasculitis and Osler‐Weber‐Rendu disease with contraindication of anticoagulation (n = 2); and macular degeneration with contraindication of anticoagulation (n = 1).
Table 2.
Baseline Characteristics of Study Patients
| All, N = 45 | NOAC Group, n = 18 | DAPT Group, n = 27 | P Value | |
|---|---|---|---|---|
| Age, y | 75 ± 7 | 74 ± 8 | 76 ± 7 | 0.430 |
| Female sex | 19 (42) | 8 (44) | 11 (41) | 1.000 |
| CHA2DS2‐VASc score | 4 ± 1.4 | 4.1 ± 1.2 | 4 ± 1.6 | 0.960 |
| HAS‐BLED score | 3.5 ± 0.8 | 3.1 ± 0.9 | 3.7 ± 0.6 | 0.011 |
| BMI, kg/m2 | 27 ± 6 | 27 ± 4 | 27 ± 6 | 0.966 |
| HTN | 41 (91) | 17 (94) | 24 (89) | 0.640 |
| DM | 15 (34) | 4 (24) | 11 (41) | 0.333 |
| PAD | 8 (18) | 2 (11) | 6 (22) | 0.445 |
| Previous stroke | 14 (31) | 8 (44) | 6 (22) | 0.188 |
| CAD | 24 (53) | 7 (39) | 17 (63) | 0.138 |
| Previous MI | 19 (42) | 5 (28) | 14 (52) | 0.134 |
| LVEF | 54 ± 12 | 58 ± 6 | 51 ± 14 | 0.034 |
| Mitral regurgitation (moderate/severe) | 14 (31) | 7 (39) | 7 (26) | 0.521 |
| Cr, mg/dL | 1.4 ± 0.7 | 1.3 ± 0.9 | 1.5 ± 0.6 | 0.338 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CHA2DS2‐VASc, congestive heart failure, HTN, age ≥75 y, DM, stroke/TIA, vascular disease, age 65–74 y, sex category (women); Cr, creatinine; DAPT, dual antiplatelet therapy; DM, diabetes mellitus; HAS‐BLED, hypertension, abnormal renal or liver function, stroke, bleeding, labile INRs, elderly (age ≥65), drug therapy; HTN, hypertension; INR, international normalized ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NOAC, novel oral anticoagulants; PAD, peripheral arterial disease; SD, standard deviation; TIA, transient ischemic attack.
Data are presented as n (%) or mean ± SD.
Follow‐up
After implantation, 27 patients (60%) received DAPT (aspirin 100 mg/d and clopidogrel 75 mg/d) for ≥6 months. Eighteen patients (40%) received NOAC (dabigatran 2× 110 mg/d [n = 16] or rivaroxaban 1× 20 mg/d [n = 2]) for ≥45 days. All patients were followed up for ≥45 days.
Transesophageal echocardiography was performed 45 days after implantation in all patients receiving NOAC and in 24 of 27 patients receiving DAPT. Transesophageal echocardiography revealed neither thrombus formation nor late device embolization. Immediately after implantation, residual peri‐device flow >5 mm arose in 1 patient being no longer observable in TEE control.
During a follow‐up of 417 ± 323 days (range, 45–1111 days), 7 patients (16%) died. Causes of death were MI (n = 1), heart pump failure (n = 1), sudden cardiac death (n = 1), hemorrhagic shock (n = 1), malignant melanoma (n = 1), amyotrophic lateral sclerosis (n = 1), and unknown death (n = 1).
During the study period, no stroke or TIA occurred. Nonfatal MI occurred in 1 patient. There was a nonsignificant trend for lower all‐cause mortality and occurrence of MACCE in the NOAC group compared with the DAPT group (Figure 1A,B). However, this could be due to the different characteristics of our patients in the NOAC group in comparison with the DAPT group (Table 2).
Figure 1.
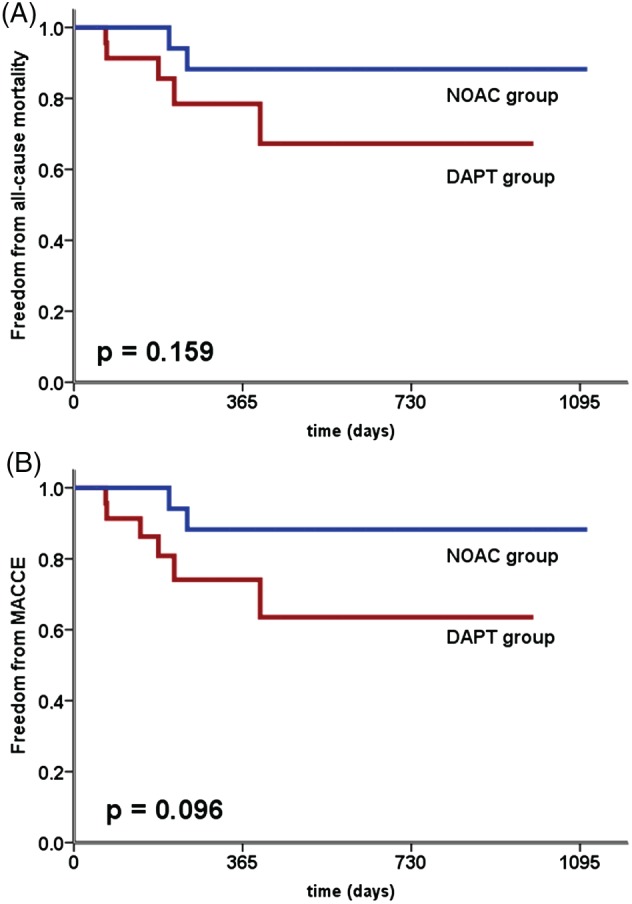
(A) Kaplan‐Meier estimates of freedom from all‐cause mortality based on the use of DAPT or NOAC. (B) Kaplan‐Meier estimates of freedom from MACCE based on the use of DAPT or NOAC. Abbreviations: DAPT, dual antiplatelet therapy; MACCE, major adverse cardiac and cerebrovascular events; NOAC, novel oral anticoagulants.
A total of 6 patients suffered from a major bleeding (NOAC group, n = 3; DAPT group, n = 3). In patients in the NOAC group, major bleeding occurred at day 205, 688, and 736. In contrast, a major bleeding in the DAPT group appeared at day 28, 382, and 819. However, there was no significant difference in the frequency of a major bleeding in different groups (Figure 2).
Figure 2.
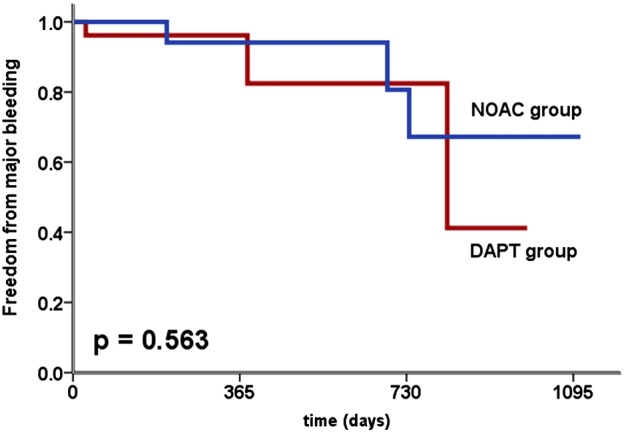
Kaplan‐Meier estimates of freedom from major bleeding based on the use of DAPT or NOAC. Abbreviations: DAPT, dual antiplatelet therapy; NOAC, novel oral anticoagulants.
Discussion
The present study investigates the use of NOAC during the first 45 days after LAA closure with the Watchman device. This transitional period is particularly important, as complete device endothelialization is assumed within this period. After the first 45 days, a thrombus formation is unlikely.3, 10 Our study suggests that NOAC therapy within the first 45 days after Watchman implantation is at least as safe and effective as therapy with aspirin 100 mg/d and clopidogrel 75 mg/d (figures 1 and 2).
However, there is no valid direct comparison between different ways of OAC after LAA closure. Therefore, it is not clear whether the use of NOAC provides a superior outcome compared with use of warfarin.
Adverse Events
Until now, 2 randomized clinical trials of percutaneous LAA closure have been completed and published: the PROTECT‐AF study and the recently published Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long‐Term Warfarin Therapy (PREVAIL) study.
Both studies prove the noninferiority of percutaneous LAA closure with the Watchman device compared with standard therapy with warfarin. In addition, in the 3.8‐year follow‐up of PROTECT‐AF, percutaneous LAA closure met criteria for superiority, compared with warfarin, for preventing stroke, systemic embolism, and cardiovascular death and all‐cause mortality.2
Despite these favorable results, procedural complications have to be considered. PROTECT‐AF and PREVAIL reported a rate of serious procedural complications of 8.7% and 4.5%, respectively. Serious procedural complications were defined as pericardial effusion requiring surgery, pericardial effusion with pericardiocentesis, procedure‐related strokes, device embolization, and major bleeding.4, 11
In our study, serious procedural complications occurred in 6.4% of patients, which is within the range of described rates of complications in PROTECT‐AF.4 What is noteworthy in our study is that no procedural stroke/TIA occurred, in contrast to a procedural stroke rate of 1.1% and 0.7% in PROTECT‐AF and PREVAIL, respectively.4, 11 In our study cohort, however, 1 patient died due to procedural complication (pericardial tamponade; Table 1).
Compared with the recently published PREVAIL study, the rate of complications is relatively high in our study.11 However, it should be noted that these are the first patients treated with percutaneous LAA closure in our hospital. By contrast, the PREVAIL study was conducted at centers that already had experience with the method.4, 11
Anticoagulation Therapy
Both PROTECT‐AF and PREVAIL only included patients without absolute contraindications to OAC. To ensure complete endothelialization of the device, patients were given an OAC with warfarin (with a target international normalized ratio between 2.0 and 3.0) over a period of ≥45 days.4, 11 However, there is a large group of patients with AF with absolute contraindication to OAC.1, 10
The recently published ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology (ASAP) suggests that DAPT is similarly effective and as safe as warfarin therapy.5 The smaller study by Meinke et al demonstrated similar results.6
Our study is in line with these results. In our investigation, 60% of patients received DAPT within the first 45 days after LAA closure. Compared with the ASAP study, in our study patients had similar high CHA2DS2‐VASc scores (4.4 ± 1.7 vs 4 ± 1.6 points, respectively).5
In both studies, the main reasons for DAPT were absolute contraindications to OAC. However, in our study, preexisting DAPT due to other indications (eg, recent coronary stenting with a DES) was also a reason for DAPT.
In the ASAP study, there were 6 cases of thrombus formation.5 By contrast, in our study no abnormal thrombus apposition occurred (Table 3). However, our study had the smaller number of patients.
Table 3.
Postprocedural Adverse Events in Study Patients (N = 45)
| Adverse Event | NOAC Group, n = 18, No. (%) | DAPT Group, n = 27, No. (%) |
|---|---|---|
| All‐cause death | 2 (11) | 5 (19) |
| CV death | 0 (0) | 3 (11) |
| Stroke/TIA | 0 (0) | 0 (0) |
| Nonfatal MI | 0 (0) | 1 (4) |
| Major bleeding | 3 (17) | 3 (11) |
| Device embolization | 0 (0) | 0 (0) |
| Device‐related thrombus after 45 d | 0 (0) | 0 (0) |
| Peri‐device flow >5 mm after 45 d | 0 (0) | 0 (0) |
| Intracardiac thrombi after 45 d | 0 (0) | 0 (0) |
Abbreviations: CV, cardiovascular; DAPT, dual antiplatelet therapy; MI, myocardial infarction; NOAC, novel oral anticoagulant; TIA, transient ischemic attack.
For some years, safe and effective new anticoagulants have been available. These NOAC (factor Xa inhibitors and 1 direct thrombin inhibitor) have some advantages over warfarin in AF: They act more quickly, do not require laboratory control, and have a more favorable side‐effect profile.8, 9, 12, 13, 14
However, compared with warfarin therapy, NOAC do not have advantages in all disorders. For example, in patients with mechanical heart valves, the use of dabigatran was reported to be associated with increased rates of thromboembolic and bleeding complications.15 Therefore, it was uncertain whether NOAC could be used in patients after LAA closure implantation.
Our study examined the use of NOAC in patients with interventional LAA closure for the first time. Our results suggest that NOAC therapy is safe and effective in patients without absolute contraindications to OAC. Within the first 45 days after LAA closure, neither MACCE nor thrombus formation occurred (Table 3). No major bleeding appeared within the first 45 days, but in our long‐term follow‐up, major bleedings occurred on day 205, 688, and 736. At that time, NOAC therapy had already been finished for long time (Figure 2).
Notably, there was a nonsignificant trend for lower all‐cause mortality and occurrence of MACCE in the NOAC group compared with the DAPT group (Figure 1A,B). However, this could be due to the different characteristics of our patients in the NOAC group in comparison with the DAPT group (Table 2).
Study Limitations
The main limitation of the present study is the small sample size and its single‐center character. However, our present study is the first study investigating the use of NOAC during the first 45 days after interventional LAA closure. Moreover, a direct comparison between NOAC and warfarin might provide additional important information. However, in our study population, recurrent bleedings were one of the main indications for LAA closure (contraindication to OAC). Moreover, our study exclusively examined the Watchman device. Our results should therefore not be transferred to other percutaneous LAA closure systems.
Conclusion
Our study suggests that NOAC therapy within the first 45 days of interventional LAA closure with the Watchman device is effective. Novel OAC therapy allows a simple and fast‐acting OAC. It could therefore improve patient compliance in this critical period of complete device endothelialization and lead to a shorter duration of hospitalization. This pilot study may encourage larger‐scale studies. What is more, novel antiplatelet agents have been available for some years. It should also be examined whether prasugrel and ticagrelor, preferably without acetylsalicylic acid, provide an alternative treatment after LAA closure.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. John Camm A, Colombo A, Corbucci G, et al. Left atrial appendage closure: a new technique for clinical practice. Heart Rhythm. 2014;11:514–521. [DOI] [PubMed] [Google Scholar]
- 2. Reddy VY, Sievert H, Halperin J, et al; PROTECT‐AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial [published correction appears in JAMA. 2015;313:1061]. JAMA. 2014;312:1988–1998. [DOI] [PubMed] [Google Scholar]
- 3. Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007;49:1490–1495. [DOI] [PubMed] [Google Scholar]
- 4. Holmes DR, Reddy VY, Turi ZG, et al; PROTECT‐AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non‐inferiority trial [published correction appears in Lancet. 2009;374:1596]. Lancet. 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 5. Reddy VY, Möbius‐Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013;61:2551–2556. [DOI] [PubMed] [Google Scholar]
- 6. Meincke F, Schmidt‐Salzmann M, Kreidel F, et al. New technical and anticoagulation aspects for left atrial appendage closure using the WATCHMAN device in patients not taking warfarin. EuroIntervention. 2013;9:463–468. [DOI] [PubMed] [Google Scholar]
- 7. Reddy VY, Doshi SK, Sievert H, et al; PROTECT‐AF Investigators. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3‐year follow‐up of the PROTECT‐AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013;127:720–729. [DOI] [PubMed] [Google Scholar]
- 8. Savelieva I, Camm AJ. Practical considerations for using novel oral anticoagulants in patients with atrial fibrillation. Clin Cardiol. 2014;37:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dogliotti A, Paolasso E, Giugliano RP. Novel oral anticoagulants in atrial fibrillation: a meta‐analysis of large, randomized, controlled trials vs warfarin. Clin Cardiol. 2013;36:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergmann MW, Landmesser U. Left atrial appendage closure for stroke prevention in nonvalvular atrial fibrillation: rationale, devices in clinical development and insights into implantation techniques. EuroIntervention. 2014;10:497–504. [DOI] [PubMed] [Google Scholar]
- 11. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long‐term warfarin therapy: the PREVAIL trial [published correction appears in J Am Coll Cardiol. 2014;64:1186]. J Am Coll Cardiol. 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 12. Patel MR, Mahaffey KW, Garg J, et al; ROCKET‐AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 13. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE‐LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation [published correction appears in N Engl J Med. 2010;363:1877]. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 14. Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 15. Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE‐ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. [DOI] [PubMed] [Google Scholar]
- 16. Viles‐Gonzalez JF, Kar S, Douglas P, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman device in patients with atrial fibrillation: a PROTECT‐AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol. 2012;59:923–929. [DOI] [PubMed] [Google Scholar]


