Abstract
BACKGROUND
Attempts to achieve rhythm control using direct‐current cardioversion (DCC) are common in those with persistent atrial fibrillation (AF). Although often successful, AF recurs within 1 month in as many as 57% of patients. The aim of this study was to assess whether a baseline left atrial sphericity index (LASI) acquired by 2‐dimensional transthoracic echocardiography (TTE) could be used as a predictor of AF recurrence after successful DCC.
Hypothesis
A baselline LASI assessed by 2D TTE can predict AF recurrence after successful DCC in patients with persistent AF.
Methods
A total of 124 consecutive patients with persistent AF lasting <120 days underwent successful DCC. Other than β‐blockers, no other antiarrhythmic treatment was administered. Prior to DCC, all patients underwent thorough TTE, and LASI was calculated as the fraction of the left atrial width/length of the largest possible left atrial volume in a 4‐chamber view. The primary outcome was a TTE‐estimated baseline LASI as a predictor of AF recurrence after successful DCC for persistent AF.
Results
Anatomically, a more spherical shape of the left atrium (LASI >0.9) proved to be a strong and independent predictor of AF recurrence, with an odds ratio between 4.1 (95% confidence interval: 1.6‐11.9, P = 0.005) and 7.6 (95% confidence interval: 3.3‐19.7; P = 7.2 × 10−6). The receiver operating characteristic curve indicated good power for distinguishing between recurring and nonrecurring AF, and we chose a cutoff of 0.9 because high specificity was a priority for clinical reasons.
Conclusions
In conclusion, baseline LASI >0.9 was associated with significantly greater AF recurrence throughout the 12‐month follow‐up period.
Introduction
Atrial fibrillation (AF) is the most common, sustained cardiac arrhythmia and is increasingly prevalent in an aging population.1, 2 This is a growing public health concern, particularly because AF is associated with a nearly 2‐fold mortality risk and a 5‐fold to 7‐fold increased risk of ischemic stroke.3, 4 Attempts to achieve rhythm control using direct‐current cardioversion (DCC) are common in AF patients, although the condition recurs within a month in as many as 57% of them.5
The pathophysiology of AF is a complex combination of electrical and structural remodeling, which contributes to more heterogeneous conduction and results in the induction and maintenance of the condition.6, 7, 8 The degree of left atrial (LA) structural remodeling is often assessed by measuring LA volume with 2‐dimensional transthoracic echocardiography (TTE). However, as illustrated by earlier studies, this method is subject to great uncertainty, and, when compared with the results produced by magnetic resonance imaging (MRI), the underestimation of LA volumes is in the region of 14% to 37%.9, 10, 11
The inaccuracy of the LA volume method using echocardiography and its relatively poor predictive value with respect to AF recurrence12 have led to sphericity being a new way to describe LA anatomical changes. Previous studies have illustrated an association between the left atrial sphericity index (LASI) and the recurrence of AF, demonstrating that a more spherical LA results in an increased risk of AF recurrence.13, 14 However, earlier studies have used MRI to establish the sphericity of the LA. The increasing prevalence of the condition and the limited capacity of MRI mean that there is a need for a simple and reliable procedure that can be performed routinely when choosing between rhythm‐control or rate‐control treatment. With this in mind, we set out to examine the prognostic value of the baseline LASI acquired by TTE in terms of predicting AF recurrence after successful DCC.
Methods
The aim of this research was to assess whether a baseline LASI could be used as an independent predictor of AF recurrence after successful DCC. The study was approved by the local ethics committee and carried out in accordance with the Declaration of Helsinki. Informed consent was obtained from the patients prior to their inclusion in the research. The study was registered at http://www.ClinicalTrials.gov with the study ID number S‐20110075.
Patients
During a 2‐year period (2011–2013), 141 new‐onset persistent AF patients were enrolled in a randomized controlled trial studying the effects in terms of AF recurrence of early DCC guided by transesophageal echocardiography compared with those of conventional DCC following 3 weeks of new oral anticoagulants. One hundred twenty‐six of these patients underwent successful DCC and were included in this retrospective study.15 All these individuals were either admitted to the Department of Cardiology at Odense University Hospital Svendborg or referred to its outpatient clinic with symptomatic (European Heart Rhythm Association class II/III) persistent AF and an indication for DCC. In accordance with international guidelines, all the patients were given dabigatran etexilate (150 mg twice daily) prior to and for a minimum of 4 weeks after DCC. No antiarrhythmic drugs were administered, but all the subjects received 50 to 100 mg of metoprolol at the time of inclusion in the trial as a rate control and for symptom relief. The treatment was continued after successful DCC.
Direct‐Current Cardioversion
Direct‐current cardioversion was performed using a ZOLL R Series ALS defibrillator (ZOLL Medical Corp., Chelmsford, MA) in accordance with current guidelines, with self‐adhesive electrodes in the anterior‐posterior position.16
Echocardiography
Standard TTE using a Vivid 7 (GE Healthcare, Wauwatosa, WI) and an M4S Matrix array adult cardiac (1.8 MHz) transducer was performed prior to and 1 month post‐DCC. Due to the beat‐to‐beat variations seen during AF, each acquisition consisted of 5 loops that were suitable for averaging during the blinded offline analysis. The analysis was performed using the EchoPac software, version 112 (GE Medical Systems, Horten, Norway). Assessed by TTE, the LASI was calculated as a ratio between the transverse and longitudinal diameter of the LA (LA transverse diameter/LA longitudinal diameter) measured in a 4‐chamber view, with the largest possible LA volume determined just before the opening of the mitral valve (Figure 1A,B). Longitudinal and transverse lines dividing the LA into 4 nearly identical squares were used to measure its length and width. The measurements were repeated 4 times and averaged to reduce intrarater variability and produce a more precise LASI.
Figure 1.
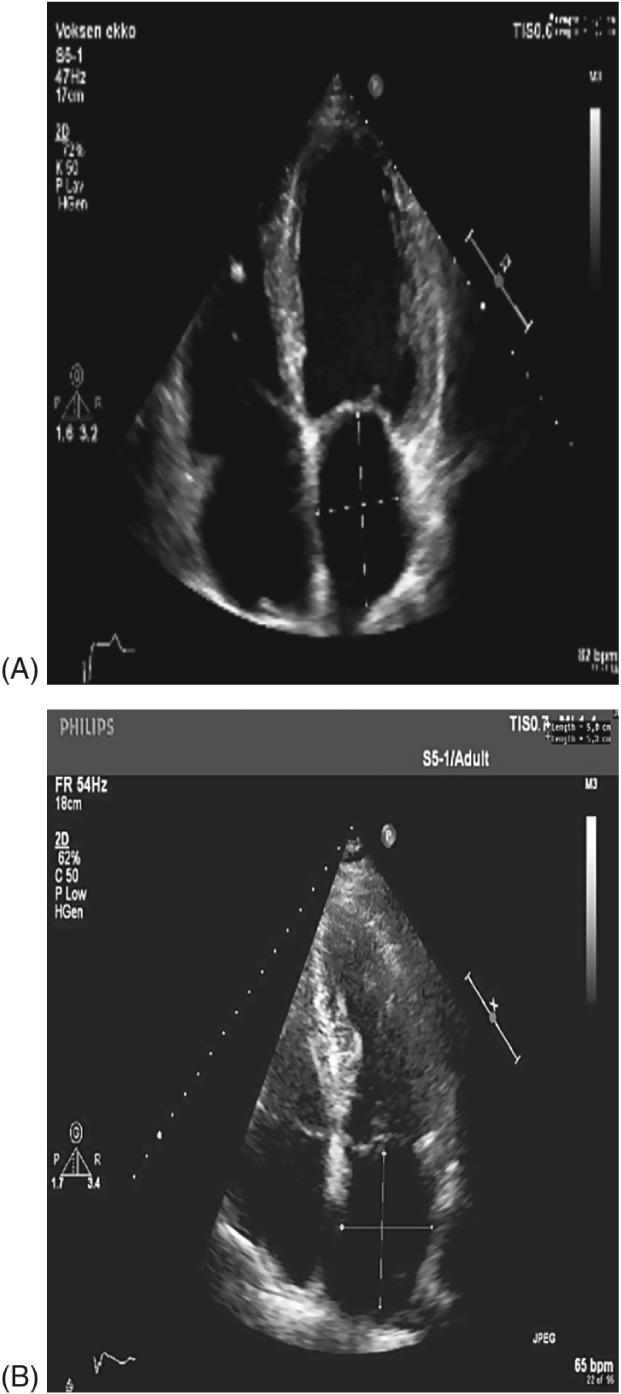
(A) Normal LA. LASI calculated as transverse diameter (3.9 cm)/longitudinal diameter (5.8 cm), for a LASI of 0.67. (B) Spherical remodeling of the LA. LASI calculated as transverse diameter (5.0 cm)/longitudinal diameter (5.3 cm), for a LASI of 0.94. Abbreviations: LA, left atrium; LASI, left atrial sphericity index.
Holter Monitoring
Forty‐eight‐hour Holter monitoring was performed at each visit (28 days, 3 months, 6 months, and 12 months after DCC) using a modular digital Holter recorder (Spacelabs Healthcare, Snoqualmie, WA). Analysis of the Holter recordings was conducted by trained and experienced technicians using the Spacelabs Healthcare Sentinel software. Furthermore, patients with symptoms consistent with AF recurrence were immediately taken for an extra ECG during the 12‐month follow‐up period. A recorded episode of AF ≥30 seconds was classified as AF recurrence.
Statistical Analysis
Continuous variables were expressed as mean ± SD, and categorical variables were presented as counts and percentages. Baseline values were compared using a Wilcoxon test for the numerical measures and a χ2 test for the discrete measures. We produced a receiver operating characteristic (ROC) curve to determine an appropriate cutoff point for sphericity for the prediction of AF recurrence; we chose this point based on clinical requirements with respect to sensitivity and specificity. We then used a survival analysis with a Kaplan‐Meier plot to evaluate recurrence‐free survival (RFS) for patients with a LASI below and above the cutoff point chosen.
We used multivariate logistic regression to model the dependency between the risk of the recurrence of AF and sphericity dichotomized at the chosen cutoff point of 0.9, taking into account other covariates and obtaining odds ratios (ORs) for these effects and corresponding P values for the significance of these ORs. We fitted both a crude model, containing only sphericity, and a model adjusted for treatment (transesophageal echocardiography or conventional), AF duration, and the interaction between them. We only retained additional covariates that were significant at a 0.05 significance level. The goodness of fit was checked with the Hosmer‐Lemeshow test.15 To evaluate the inter‐ and intrarater variability, we produced Bland‐Altman plots to compare the measurements produced by the 2 raters and also compared the first and second measurements of a patient produced by each rater. Furthermore, we fitted a mixed‐effects model to determine the proportion of the total variance that could be explained by intra‐ and interobserver variations. The statistical program R, with the packages MethComp, lme4, plyr, and ResourceSelection, was used for all the analyses (R Foundation for Statistical Computing, Vienna, Austria).17, 18, 19
Results
Patient Population
Screening 199 persistent AF patients led to the exclusion of those with an AF duration >4 months (n = 24), as well as those with reversible causes of AF (thyrotoxicosis: n = 6; infection: n = 14; acute coronary syndrome: n = 3; valvular AF: n = 1320; and spontaneous conversion: n = 11). A total of 126 patients underwent successful DCC. Two were excluded due to missing sphericity measurements, leaving 124 patients with a mean persistent AF duration of 63.2 ± 32.9 days and a mean age of 67.2 ± 8.7 years (Table 1). No significant baseline differences between recurrent and nonrecurrent AF patients were detected.
Table 1.
Clinical and Echocardiographic Baseline Characteristics of the Randomized Treatment Groups
| All, N = 124 | No Recurrence Within 12 Months, n = 29 | Recurrence Within 12 Months, n = 95 | P Value | |
|---|---|---|---|---|
| Female sex | 19 (15) | 6 (21) | 13 (14) | 0.53 |
| Mean age, y | 67.1 ± 8.7 | 65.0 ± 8.3 | 67.7 ± 8.8 | 0.09 |
| AF duration, d | 62.8 ± 32.8 | 58.6 ± 35.1 | 64.1 ± 32.2 | 0.43 |
| DM | 14 (11) | 2 (7) | 12 (13) | 0.59 |
| HTN | 61 (50) | 17 (59) | 44 (47) | 0.37 |
| Dyslipidemia | 32 (26) | 5 (17) | 27 (29) | 0.32 |
| BMI, kg/m2 | 29.3 ± 4.64 | 29.6 ± 5.7 | 29.2 ± 4.3 | 0.95 |
| EHRA class (I/II/III/IV) | 1/64/59/0 | 0/14/15/0 | 1/50/44/0 | |
| CHA2DS2‐VASc score total | 1.67 ± 1.22 | 1.55 ± 1.09 | 1.70 ± 1.26 | 0.61 |
| HAS‐BLED score total | 1.11 ± 0.77 | 1.10 ± 0.82 | 1.12 ± 0.76 | 0.94 |
| Medications | ||||
| ACEI | 15 (12) | 4 (14) | 11 (12) | 1 |
| ARB | 16 (13) | 2 (7) | 14 (15) | 0.43 |
| Statin | 29 (23) | 5 (17) | 24 (25) | 0.52 |
| Dabigatran etexilate | 124 (100) | 29 (100) | 95 (100) | 1 |
| β‐Blocker | 103 (83) | 24 (83) | 79 (83) | 1 |
| CCB | 20 (16) | 6 (21) | 14 (15) | 1 |
| Digitalis | 15 (12) | 3 (10) | 12 (13) | 0.63 |
| Echocardiographic parameters | ||||
| LA volume, mL | 72.2 ± 9.66 | 72.0 ± 9.45 | 72.3 ± 9.77 | 1 |
| LVEF, % | 51.6 ± 8.32 | 52.2 ± 9.92 | 51.5 ± 7.81 | 0.46 |
| LV volume, mL | 105.0 ± 15.2 | 104.4 ± 16.2 | 105.2 ± 14.9 | 0.66 |
| LVESD, cm | 3.79 ± 0.487 | 3.82 ± 0.529 | 3.78 ± 0.476 | 0.80 |
| LVEDD, cm | 5.10 ± 0.372 | 5.13 ± 0.368 | 5.09 ± 0.375 | 0.75 |
| E/a (after DCC) | 1.12 ± 0.444 | 0.99 ± 0.215 | 1.23 ± 0.554 | 0.25 |
| E/E′ | 7.97 ± 2.34 | 8.55 ± 2.31 | 7.79 ± 2.33 | 0.06 |
| E′, lateral/medial average | 10.1 ± 1.47 | 10.1 ± 1.21 | 10.1 ± 1.55 | 0.94 |
| Deceleration time, ms | 199.2 ± 64.0 | 198.4 ± 35.9 | 199.4 ± 70.3 | 0.36 |
| LV septum, cm | 1.07 ± 0.127 | 1.09 ± 0.122 | 1.07 ± 0.130 | 0.40 |
| LV global strain baseline | 13.2 ± 2.65 | 13.4 ± 3.43 | 13.1 ± 2.39 | 0.58 |
| LA transverse diameter | 4.69 ± 0.593 | 4.47 ± 0.524 | 4.76 ± 0.598 | 0.03 |
| LA longitudinal diameter | 5.39 ± 0.520 | 5.48 ± 0.567 | 5.37 ± 0.505 | 0.255 |
| LASI | 0.869 ± 0.102 | 0.818 ± 0.078 | 0.885 ± 0.104 | 0.0006 |
Abbreviations: a, late diastolic filling velocity; ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; CCB, calcium channel blocker; CHA2DS2‐VASc, congestive heart failure, HTN, age ≥75 y, DM, stroke/TIA, vascular disease, age 65–74 y, sex category (women); DCC, direct‐current cardioversion; DM, diabetes mellitus; e′, early diastolic mitral annular velocity; E, early diastolic filling velocity; EHRA, European Heart Rhythm Association; HAS‐BLED, hypertension, abnormal renal/liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol; HTN, hypertension; INR, international normalized ratio; LA, left atrial; LASI, left atrial sphericity index; LV, left ventricular; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; SD, standard deviation; TIA, transient ischemic attack.
Data are presented as n (%) or mean ± SD.
Baseline Characteristics
The baseline LA volume and the LA indexed volume, calculated by the Simpson biplane method using the apical 4‐ and 2‐chamber views, had no predictive value in terms of AF recurrence during the 12 months of follow‐up. In 93.7% of patients, β‐blockers were tolerated; in the remaining 6.3%, the treatment was stopped due to side effects. The recurrence rates in those receiving a maintenance dose of β‐blockers and those who did not tolerate the treatment did not differ significantly. Furthermore, the logistic regression showed no predictive value in terms of AF recurrence with respect to age, sex, body mass index, LA diameter, LA volume, LA index volume, smoking, β‐blockers, dyslipidemia, and diabetes mellitus. As a result, these factors are not included (Table 2).
Table 2.
AF Recurrence Counts and Results of Logistic Regression With Respect to LASI ≥/< 0.9 at Different Follow‐up Times
| LASI <0.9, n = 69 | LASI ≥0.9, n = 55 | Proportion P Value | Crude OR | Crude 95% CI | Crude P Value | Adjusted OR | Adjusted 95% CI | Adjusted P Value | |
|---|---|---|---|---|---|---|---|---|---|
| 28 days | 26 (38%) | 45 (82%) | 2 × 10−6 | 7.4 | (3.3‐18.0) | 2.9 × 10−6 | 7.7 | (3.3‐20.0) | 7.5 × 10−6 |
| 3 months | 30 (43%) | 47 (85%) | 4 × 10−6 | 7.6 | (3.3‐19.7) | 7.2 × 10−6 | 7.5 | (3.2‐19.7) | 1.4 × 10−6 |
| 6 months | 36 (52%) | 47 (85%) | 0.0002 | 5.4 | (2.3‐13.8) | 0.0002 | 5.2 | (2.2‐13.5) | 0.0003 |
| 12 months | 46 (67%) | 49 (89%) | 0.007 | 4.1 | (1.6‐11.9) | 0.005 | 3.9 | (1.5‐11.5) | 0.008 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; HTN, hypertension; LA, left atrial; LASI, left atrial sphericity index; LV, left ventricular; LVEF, left ventricular ejection fraction; OR, odds ratio; TEE, transesophageal echocardiography.
In the adjusted model, we adjusted for treatment (TEE or conventional), AF duration, and interactions between treatment and AF duration. The LA volume, LVEF, LV volume, age, sex, smoking, HTN, dyslipidemia, β‐blockers, IHD, height, weight, and BMI were not included, as they did not significantly improve the fit.
Clinically Relevant Cutoff
The ROC curve indicated good power when it came to distinguishing between recurring and nonrecurring AF, with an area under the curve of 0.71. We chose a cutoff of 0.9, resulting in a specificity of 0.793 and a sensitivity of 0.518, as high specificity was a priority for clinical reasons (Figure 2). The Kaplan‐Meier plot using this cutoff illustrates a clear difference in RFS at all 4 follow‐up times, with nonoverlapping confidence bands. This points to there being significant differences in RFS between patients with a LA sphericity above and below 0.9 (Figure 3).
Figure 2.
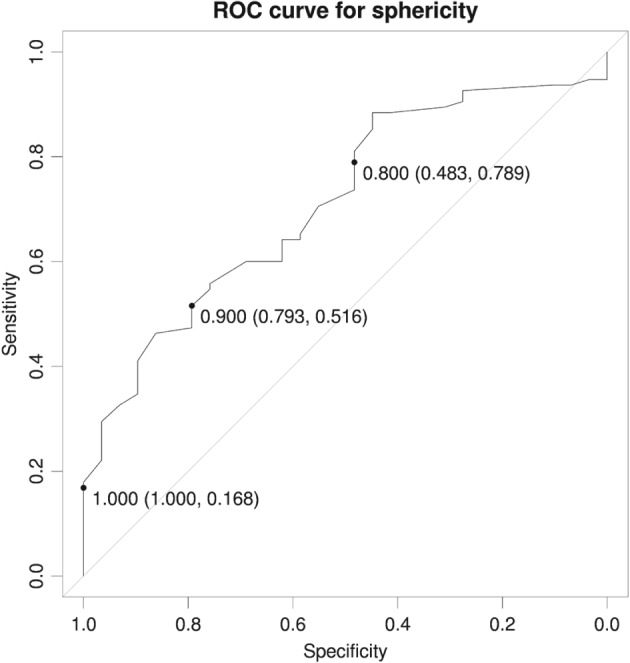
ROC curve for specificity. Abbreviations: ROC, receiver operating characteristic.
Figure 3.
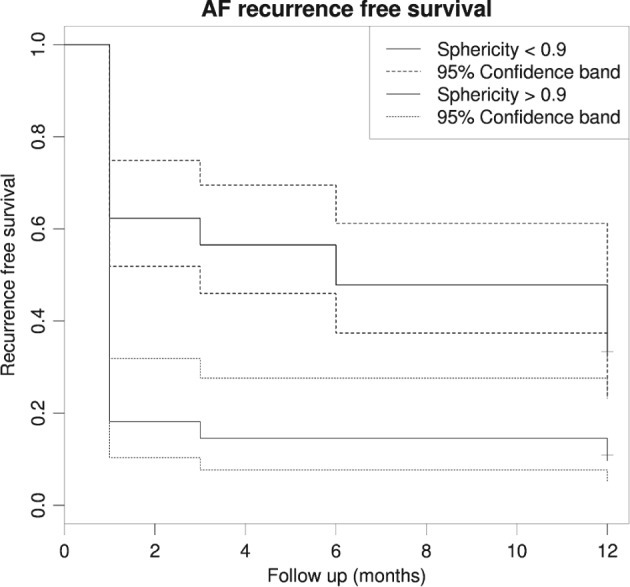
Kaplan‐Meier plot showing recurrence free survival after sphericity stratification < and > 0.9. Abbreviations: AF, atrial fibrillation.
Left Atrial Sphericity Index to Predict Atrial Fibrillation Recurrence
A higher LASI, as seen in the patients with a more spherical LA, significantly increased the AF recurrence risk after successful DCC during the 12‐month follow‐up period. The recurrence risk (expressed as an OR) during the 12 month follow‐up was between 4.1 (95% confidence interval [CI]: 1.6‐11.9, P = 0.005) and 7.6 (95% CI: 3.3‐19.7, P = 7.2 × 10−6) for the crude model, as portrayed in Table 2. The results for the adjusted model were similar (Table 2). We investigated whether the LASI could be replaced with the LA longitudinal diameter or LA transverse diameter in terms of predicting recurrence. The former had no significant predictive value, whereas the latter had a slightly significant, but much lower, predictive value than the LASI.
Observer Variability
The Bland‐Altman plot revealed good inter‐ and intrarater agreement, with only a few differences close to the limits of agreement identified. However, we also observed a slight bias between the 2 observers. The inter‐rater variation explained 5%, and the intrarater variation 12%, of the total variation.
Discussion
Our findings suggest that LA remodeling toward a more spherical shape significantly increases the risk of AF recurrence. The baseline LASI acquired by TTE in our study proved to be an independent predictor of AF recurrence after successful DCC in patients with persistent AF.
Left Atrial Volume
Traditionally, LA structural remodeling has been quantified by TTE LA volume measurements.21 However, this modality is known to underestimate LA volumes by 14% to 37% compared with those acquired by MRI.9, 10, 11 In our study, we included patients with a mean persistent AF of 63.2 days prior to DCC and a moderately dilated LA. However, the statistical analysis showed no significant prognostic value of LA volume or the LA index volume on AF recurrence. The discrepancy between the TTE‐estimated volume and the actual LA volume, and the rather poor predictive value of LA volume on AF recurrence, as demonstrated in several studies,16 including our own, highlight the need for a new, easy, and reliable method to predict whether AF will recur.
Left Atrial Sphericity Index
The relatively new methods using MRI or cardiac computed tomography (CCT)13, 22 to assess asymmetric changes to the LA produce very promising results. Nedios et al emphasized the importance of the asymmetrical remodeling of the LA by illustrating the limitations to the currently used LA volume method, which does not sufficiently distinguish between patients with persistent AF and those with long‐term persistent AF. In contrast, the asymmetry index not only proved to be a strong predictor of AF recurrence following pulmonary vein isolation, but also differed significantly in identifying patients with persistent and long‐term persistent AF. In another study, Nedios et al illustrated the superiority of CCT‐assessed asymmetrical remodeling over conventionally acquired LA diameter measurements obtained by TTE in terms of predicting the recurrence of AF.17 Previous research13, 17, 18, 22 shows great advances in predicting AF recurrence by assessing LA geometry, but it also comes with limitations, as the radiation produced by CCT and the limited access to MRI make it nearly impossible to use these methods for all AF patients. In our study, we applied a routinely available method (TTE) to estimate the LASI, demonstrating a significant predictive value of this approach with respect to AF recurrence.
We observed that a higher LASI, which means a more spherical LA, reduced the chances of sinus‐rhythm maintenance during the 12‐month follow‐up period. Our observations may not come as a surprise, as one would expect reduced remodeling to increase the chances of sinus‐rhythm maintenance. However, the introduction of a clinically applicable cutoff value of a LASI < or > 0.9 may help to identify the patients with a significantly increased risk of AF recurrence. It may also be used to prevent attempts to achieve rhythm control in patients with a spherical LA (LASI >0.9), where AF recurrence is very likely. We also investigated whether the LASI could be replaced with LA longitudinal or transverse diameter measurements to predict AF recurrence, as suggested in earlier studies17; but the former had no significant predictive value, whereas the latter had a slightly significant, but much lower, predictive value than the LASI. Finally, the results of our research are consistent with the findings of the studies mentioned above,13, 17, 18, 22 although further validation of this new method is needed before it can be routinely applied.
Left Atrial Sphericity Index Cutoff
The very low level of complications associated with the DCC procedure19 allowed us to give priority to high specificity when choosing a cutoff value for the LASI. The ROC curve illustrated specificity in the region of 80%, whereas the sensitivity was about 52% for a LASI of 0.9, which was selected as the most relevant cutoff point from a clinical perspective. Although this new method does seem to be promising, its full potential may only be achieved when combined with the novel 3‐dimensional TTE method, which enables us to estimate the LASI even more precisely than is the case with 2‐dimensional TTE. Furthermore, there is a need for studies that verify this method, preferably by comparing the results with those obtained by MRI.
Study Limitations
The study has several limitations. The recurrence of AF was documented by 48‐hour Holter monitoring or an ECG performed at each hospital visit. Furthermore, patients with symptoms consistent with AF recurrence were immediately taken in for an extra ECG. Nevertheless, the monitoring method leaves room for episodes of silent AF that could pass undetected, although in daily clinical practice it is impossible to continuously monitor all persistent‐AF patients treated with DCC. Undetected and asymptomatic short paroxysmal AF has no clinical relevance, as all patients already receive appropriate treatment. As with all TTE‐acquired estimates, LA sphericity is also subject to the uncertainty of the measurements. However, even if an MRI demonstrates discrepancies when compared with the TTE estimate, the precise individual measurements of length or width may not be as clinically important as the ratio between them, which is the sphericity index we applied. The reproducibility of TTE‐acquired LASI may be questioned. However, the statistical analysis of inter‐ and intrarater variability revealed good agreement in both. Indeed, inter‐rater variability in the region of 5% may be regarded as very reasonable from a clinical perspective, and the slightly higher intrarater variability (12%) was substantially reduced by averaging all the applied measurements.
Conclusion
A baseline LASI >0.9 was associated with a significantly higher risk of AF recurrence at all follow‐up times during the 12‐month follow‐up period. This relatively new method of predicting AF recurrence is fast and easy and has reasonable inter‐ and intrarater variability, potentially making the LASI a valuable method for future decision‐making when choosing between a rhythm‐control or rate‐control strategy in patients with AF.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648–661. [DOI] [PubMed] [Google Scholar]
- 2. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence [published correction appears in Circulation. 2006;114:e498]. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 4. Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–e367. [DOI] [PubMed] [Google Scholar]
- 5. Tieleman RG, Van Gelder IC, Crijns HJ, et al. Early recurrences of atrial fibrillation after electrical cardioversion: a result of fibrillation‐induced electrical remodeling of the atria? J Am Coll Cardiol. 1998;31:167–173. [DOI] [PubMed] [Google Scholar]
- 6. Park JH, Park SW, Kim JY, et al. Characteristics of complex fractionated atrial electrogram in the electroanatomically remodeled left atrium of patients with atrial fibrillation. Circ J. 2010;74:1557–1563. [DOI] [PubMed] [Google Scholar]
- 7. Ausma J, Wijffels M, Thoné F, et al. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–3163. [DOI] [PubMed] [Google Scholar]
- 8. Wijffels MC, Kirchhof CJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. [DOI] [PubMed] [Google Scholar]
- 9. Rodevan O, Bjornerheim R, Ljosland M, et al. Left atrial volumes assessed by three‐ and two‐dimensional echocardiography compared to MRI estimates. Int J Cardiac Imaging. 1999;15:397–410. [DOI] [PubMed] [Google Scholar]
- 10. Agner BF, Kühl JT, Linde JJ, et al. Assessment of left atrial volume and function in patients with permanent atrial fibrillation: comparison of cardiac magnetic resonance imaging, 320‐slice multidetector computed tomography, and transthoracic echocardiography. Eur Heart J Cardiovasc Imaging. 2014;15:532–540. [DOI] [PubMed] [Google Scholar]
- 11. Kühl JT, Lønborg J, Fuchs A, et al. Assessment of left atrial volume and function: a comparative study between echocardiography, magnetic resonance imaging and multislice computed tomography. Int J Cardiovasc Imaging. 2012;28:1061–1071. [DOI] [PubMed] [Google Scholar]
- 12. Hirai T, Cotseones G, Makki N, et al. Usefulness of left ventricular diastolic function to predict recurrence of atrial fibrillation in patients with preserved left ventricular systolic function. Am J Cardiol. 2014;114:65–69. [DOI] [PubMed] [Google Scholar]
- 13. Bisbal F, Guiu E, Calvo N, et al. Left atrial sphericity: a new method to assess atrial remodeling. Impact on the outcome of atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2013;24:752–759. [DOI] [PubMed] [Google Scholar]
- 14. Bisbal F, Guiu E, Cabanas P, et al. Reversal of spherical remodelling of the left atrium after pulmonary vein isolation: incidence and predictors. Europace. 2014;16:840–847. [DOI] [PubMed] [Google Scholar]
- 15. Hosmer DW, Lemeshow S. Applied Logistic Regression New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 16. Fornengo C, Antolini M, Frea S, et al. Prediction of atrial fibrillation recurrence after cardioversion in patients with left‐atrial dilation. Eur Heart J Cardiovasc Imaging. 2015;16:335–341. [DOI] [PubMed] [Google Scholar]
- 17. Nedios S, Kosiuk J, Koutalas E, et al. Comparison of left atrial dimensions in CT and echocardiography as predictors of long‐term success after catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2015;43:237–244. [DOI] [PubMed] [Google Scholar]
- 18. Hoffmeister PS, Chaudhry GM, Mendel J, et al. Evaluation of left atrial and posterior mediastinal anatomy by multidetector helical computed tomography imaging: relevance to ablation. J Interv Card Electrophysiol. 2007;18:217–223. [DOI] [PubMed] [Google Scholar]
- 19. Seidl K, Rameken M, Drögemüller A, et al. Embolic events in patients with atrial fibrillation and effective anticoagulation: value of transesophageal echocardiography to guide direct‐current cardioversion. Final results of the Ludwigshafen Observational Cardioversion Study. J Am Coll Cardiol. 2002;39:1436–1442. [DOI] [PubMed] [Google Scholar]
- 20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society [published correction appears in J Am Coll Cardiol. 2014;64:2305–2307]. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 21. Tsang TS, Barnes ME, Gersh BJ, et al. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289. [DOI] [PubMed] [Google Scholar]
- 22. Nedios S, Tang M, Roser M, et al. Characteristic changes of volume and three‐dimensional structure of the left atrium in different forms of atrial fibrillation: predictive value after ablative treatment. J Interv Card Electrophysiol. 2011;32:87–94. [DOI] [PubMed] [Google Scholar]


