ABSTRACT
Background
The spatial peaks QRS‐T (SPQRS‐T) angle differentiates hypertrophic cardiomyopathy (HCM) patients from controls. Increased angle confers arrhythmia risk in other populations.
Hypothesis
We predict that the SPQRS‐T angle will identify HCM patients with sustained ventricular arrhythmias (VAs) and those with New York Heart Association class III/IV heart failure.
Methods
Corrected QT interval, QRS duration, and SPQRS‐T angle were assessed in HCM patients with VAs (>30 seconds) and those without VAs.
Results
One hundred HCM patients (mean age, 32.7 ± 17.2 years) were assessed. Twenty patients had VAs. The corrected QT interval identified VA (P = 0.018) and at 460 ms gave positive and negative predictive values of 28.6% and 83.3%, respectively, and an odds ratio of 2.0 (95% confidence interval: 0.7‐5.6). The SPQRS‐T angle differentiated VA from no VA (P < 0.001) and at 124.1 degrees gave positive and negative predictive values and an odds ratio of 36.7%, 96.1%, and 14.2 (95% confidence interval: 3.1‐65.6), respectively.
Conclusions
The SPQRS‐T angle best differentiated patients with VAs.
Introduction
Hypertrophic cardiomyopathy (HCM) has an estimated prevalence of 1 in 500 individuals and its phenotype can include thickened ventricular walls (with or without obstruction of blood flow at the left ventricular outflow tract), increased risk of progression to New York Heart Association (NYHA) III/IV functional class, and increased risk of arrhythmia‐induced sudden cardiac death.1, 2, 3 An increase in the corrected QT interval (QTc) has been associated with increased ventricular arrhythmia (VA) risk in patients with HCM.4
Recent advances in electrocardiographic (ECG) technology, including the ability to automatically transform digital 12‐lead ECG data into orthogonal vectorcardiographic data,5 can add further diagnostic6, 7, 8 and prognostic9, 10, 11, 12, 13, 14, 15, 16, 17 utility to traditional 12‐lead ECGs. Among the derived vectorcardiographic parameters obtainable after such transformations, the spatial peaks QRS‐T (SPQRS‐T) angle has notable diagnostic utility for conditions in which sudden death may be an outcome,10, 11, 12, 15, 17 as well as for identification of HCM in adult18 and pediatric patients.19 The SPQRS‐T angle has also been positively correlated with VA risk in patients with ischemic heart disease,9 whereas an increased angle also correlates with increasing severity of heart failure (HF).7, 8, 14, 16
We hypothesized that the SPQRS‐T angle would differentiate HCM patients with sustained VAs (≥30 seconds) from those without VAs with increased predictive values compared with the QTc interval. A secondary aim was to assess if those with HF (NYHA class III/IV) would also have higher SPQRS‐T angles.
Methods
A retrospective study of HCM patients from 2000 to 2013 at the University of Colorado Hospital and Children's Hospital of Colorado was performed. Internal review board permission was obtained and written permission was waivered. Patients with the diagnosis of HCM by evidence of diastolic dysfunction and 15 mm absolute septal thickness by echocardiogram were identified by first author chart review without knowledge of VA or congestive HF history.
Electrocardiogram, Echocardiogram, and Heart Failure Measures
Electrocardiograms were taken at a 25‐mm/s speed with 10 mm/mV for the limb and precordial leads (Phillips, Best, Netherlands) and ECG recordings (General Electric, Menominee Falls, WI) in sinus rhythm. The diagnosis of HCM was based on echocardiographic evidence of diastolic dysfunction and those with an absolute septal or posterior wall thickness of ≥15 mm by echocardiographic M‐mode measurement during diastole from a parasternal short‐axis view by the cardiologist seeing the particular patient. Conduction abnormalities did not preclude patient participation in this retrospective study.
Congestive HF is defined as those HCM patients who meet criteria as governed by the NYHA for functional classifications for objective or subjective measures (class I–IV).
Sustained Ventricular Arrhythmias
Ventricular arrhythmias were documented by Holter monitoring, exercise stress test, or by implantable cardioverter‐defibrillator (ICD)/pacemaker devices in patients with history of sustained VAs (≥30 seconds) and compared with those HCM patients without sustained VAs. Given the retrospective nature of the study, routine screening was variable dependent on the cardiologist seen by each patient.
Parameters
Electrocardiograms within 3 months prior to VA documentation were assessed. The QTc interval, QRS duration (QRSd), and SPQRS‐T angles were assessed. The vectorcardiographic SPQRS‐T angle was visually derived from conventional 12‐lead ECG recordings based on the method recently described by Cortez et al, utilizing the method described by Kors by the first author, as shown in Figure 1A–C.6, 20 The QRSd and QTc interval were automatically calculated, whereas the Sokolow‐Lion (S‐wave in V1 + R‐wave in V5 ≥ 3.5 mV) and Cornell (R‐wave in aVL + S‐wave in V4 ≥ 2.5 mV) left ventricular hypertrophy voltage criteria and inverted T‐waves in the lateral leads were also assessed. All parameters were assessed under blinded conditions (blinded to outcome).
Figure 1.
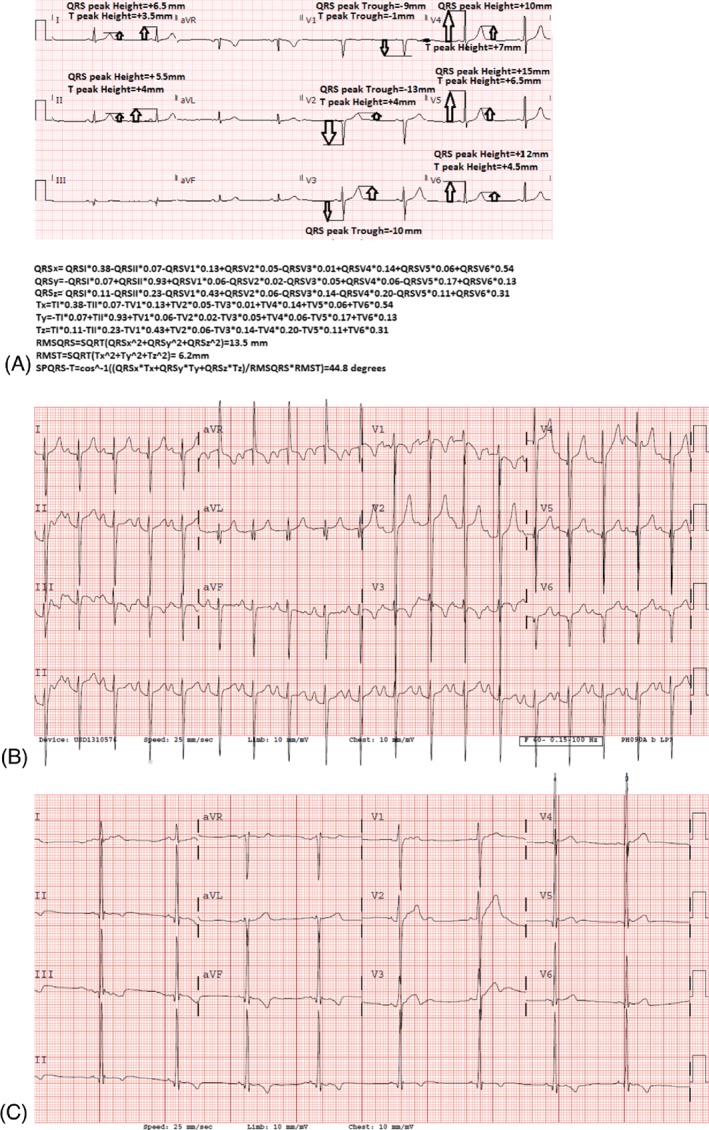
(A) Spatial QRS‐T angle calculation for a patient with mild hypertrophy (maximum thickness, 15 mm). (B) A spatial QRS‐T angle for a patient with HCM with sustained VAs (spatial QRS‐T angle, 141.6 degrees). (C) Sample ECG of an HCM patient without sustained VAs (spatial QRS‐T angle, 83.3 degrees). Abbreviations: ECG, electrocardiogram; HCM, hypertrophic cardiomyopathy; VA, ventricular arrhythmia.
Statistical Analysis
Normality was tested and t testing was used to evaluate differences between parameters tested between the HCM patients with VAs and the HCM patients without VAs. A P value ≤0.05 was deemed significant. Odds ratios (ORs) were also calculated for each of the criteria, as well as positive and negative predictive values (PPV, NPV) based on receiver operating characteristic curve analysis optimum cutoff values. All statistics were performed using GNU PSPP software (http://www.gnu.org/software/pspp/). Logistic regression was used to assess for independent predictors of VA with a P value cutoff of 0.05. Intra‐ and interobserver variability for the SPQRS‐T angle has been previously presented in a cohort that included a subset of the patients presented here in this manuscript.20
Results
Patient Population
Electrocardiographic results from 100 HCM patients (mean age, 32.7 ± 17.2 years) were assessed. Twenty patients with VAs were identified. Five patients with NYHA functional class III or IV HF were identified. Syncope differentiated those with vs those without VAs (Table 1). Four patients with VA had ICDs, and 1 patient without VA (but with atrial fibrillation) had an ICD. Holter monitoring identified 8 patients with VA; loop recording, exercise stress testing, and inpatient telemetry identified 3, 2, and 4 patients, respectively.
Table 1.
Patient Demographics and Results for HCM Patients With Sustained VAs and Those Without Sustained VAs
| VAs, n = 20 | No VAs, n = 80 | P Value | |
|---|---|---|---|
| Age, years | 32.7 ± 17.2 | 33.8 ± 18.4 | 0.418 |
| Male sex | 13 (65) | 53 (66.3) | 0.833 |
| History of syncope | 7 (35.0) | 2 (2.50) | <0.001 |
| Atrial arrhythmias | 3 (15.0) | 8 (10) | 0.689 |
| Nonsustained VAs | 2 (10) | 3 (3.8) | 0.261 |
| NYHA class III/IV | 4 (5.0) | 4 (5.0) | 1.000 |
| Maximum septal thickness, cm | 2.4 ± 0.5 | 2.17 ± 0.4 | 0.107 |
| LVOT obstructions presence | 2 (10.0) | 8 (10.0) | 1.000 |
| Sokolow‐Lion voltage criteria | 4 (20.0) | 27 (33.8) | 0.358 |
| Cornell voltage criteria | 8 (40.0) | 18 (22.5) | 0.190 |
| Inverted T‐waves V4 through V6 | 10 (50.0) | 33 (41.3) | 0.650 |
| QRSd, ms | 116.8 ± 36.1 | 98.8 ± 22.0 | 0.137 |
| QTc, ms | 472.0 ± 43.4 | 440.7 ± 48.1 | 0.013 |
| SPQRS‐T, degrees | 144.0 ± 26.7 | 108.2 ± 45.9 | <0.001 |
Abbreviations: HCM, hypertrophic cardiomyopathy; HF, heart failure; LVOT, left ventricular outflow tract; NYHA, New York Heart Association; QRSd, QRS duration; QTc, corrected QT interval; SD, standard deviation; SPQRS‐T, spatial peaks QRS‐T angle; VA, ventricular arrhythmia.
Data are presented as n (%) or mean ± SD.
Corrected QT Interval and QRS Duration
The QTc interval differentiated those with VAs from those without VAs with values of 440.7 ± 48.1 ms and 472.0 ± 43.4 ms, respectively (P = 0.013; Table 1, Figure 2). A QTc interval at a cutoff value of 460 ms gave PPV, NPV, and OR of 28.6%, 83.3%, and 2.0, respectively (95% confidence interval [CI]: 0.7‐5.6). The QRSd did not differentiate HCM patients with VAs from those without VAs.
Figure 2.
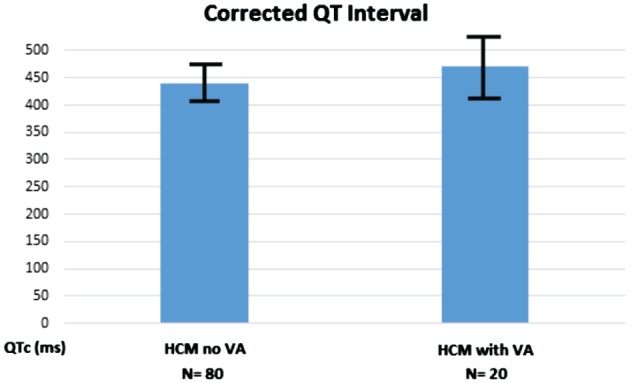
Corrected QT interval for HCM patients with sustained VAs and those without VAs (P = 0.018, which gives a significant difference between the patients). Abbreviations: HCM, hypertrophic cardiomyopathy; VA, ventricular arrhythmia.
Spatial QRS‐T Angle
The SPQRS‐T angle differentiated those with sustained VAs from those without VAs (108.2 ± 45.9 degrees vs 144.0 ± 26.7 degrees; P < 0.001; Table 1, Figure 2). At an optimum cutoff value of 124.1 degrees, PPV and NPV were 36.7% and 96.1%, respectively, with an OR of 14.2 (95% CI: 3.1‐65.6; Figure 3). Even in those without syncope, the SPQRS‐T angle significantly differentiated those with vs those without VAs (109.3 ± 45.0 degrees vs 142.1 ± 28.6 degrees; P < 0.001).
Figure 3.
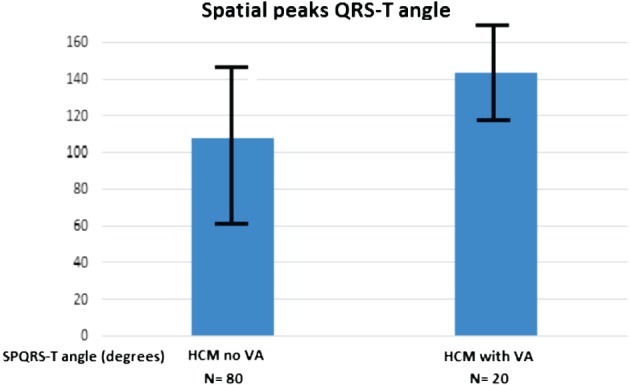
Spatial peaks QRS‐T angle (angles) for HCM patients with sustained VAs and those without Vas (P < 0.001, which gives a significant difference between the patients). Abbreviations: HCM, hypertrophic cardiomyopathy; VA, ventricular arrhythmia.
Heart Failure
Only the SPQRS‐T angle differentiated those with NYHA class III/IV HF from those without NYHA III/IV HF. There were 5 (5%) HCM patients with NYHA III/IV classification, all with systolic HF (1 with preserved ejection fraction), with a mean SPQRS‐T angle of 150.3 ± 9.7 degrees vs 113.9 ± 45.3 degrees (P < 0.001). At an optimum cutoff value of 153.4 degrees, the PPV, NPV, and OR were 15.0%, 98.8%, and 13.9, respectively (95% CI: 1.4‐142.3). When those with congestive HF or VAs were compared with HCM patients without congestive HF or VA, the PPV, NPV, and OR were 44.9%, 96.1%, and 20.0, respectively (95% CI: 4.4 to 91.5), at a cutoff value of 124.1 degrees.
Multivariate Analysis
Logistic regression was used for multivariate analysis utilizing septal thickness, syncope, the QTc, and the SPQRS‐T angle in the regression. Only syncope and the SPQRS‐T angle both remained independent predictors of sustained VAs (both P < 0.001). The OR for the SPQRS‐T angle was 1.41 (95% CI: 1.20‐1.65).
The R2 value for spatial QRS‐T angle vs septal thickness was 0.27. The R2 for the QRSd and QTc compared with septal thickness were 0.63 and 0.26, respectively.
Discussion
Corrected QT Interval and QRS Duration
Corrected QT interval prolongation has been previously described in HCM patients, with 13% having values >480 ms.21 However, the association between a prolonged QTc interval, specifically >460 ms, and VAs, as well as sudden cardiac death in HCM, has been described.4 In this case, the QTc interval had too low of a predictive value (positive or negative) for any clinical usefulness.
Spatial Peaks QRS‐T Angle
The SPQRS‐T angle has been previously been shown to identify patients with HCM18, 19 and to predict VAs in adults with myocardial ischemia9; thus, it seems logical that it would predict VAs in patients with HCM. Hypertrophic cardiomyopathy, however, can involve subendocardial ischemia; thus, the SPQRS‐T angle is not as robust a predictor as one might have thought. Given its positive correlation with worsening HF, it is likely that the SPQRS‐T angle, given a larger population size, would identify HF in patients with HCM.7, 8, 14, 16 However, identification of significant morbidity from either HF or VAs is obtained by utilizing the SPQRS‐T angle with improved ORs at the same VA optimum cutoff value for the SPQRS‐T angle. Thus, arrhythmia and HF risk are both conferred by an increase in the SPQRS‐T angle. The spatial QRS‐T angle correlates with reduced left ventricular ejection fraction, left ventricular dilation, and increased left ventricular mass and is associated with ischemic scar, which are known risk factors for VAs.22 Furthermore, the derived spatial QRS‐T angle from the 12‐lead ECG, which was the method used in this study, shows similar diagnostic ability to the vectorcardiographically measured spatial QRS‐T angle with tight Bland‐Altman 95% limits of agreement; thus, is a reasonable and clinically available tool for risk assessment in HCM patients.19, 20
Study Limitations
This study had limitations, including the size of the HCM group. This was also a retrospective study, and thus not prospective in nature, as would be needed to validate this method. New York Heart Association class III/IV HF patients were in limited number, so comparisons of these patients with others may not represent true population comparisons. Also, although by definition all HCM patients had some component of diastolic dysfunction, specific evaluation for diastolic HF was not performed due to limited numbers of patients. Another limitation in any study assessing VAs includes identification of VAs in patients without ICDs, as all VAs might not be documented. The last ECG prior to arrhythmia diagnosis was used to calculate the parameters assessed; all patients in the study happened to have an ECG within 3 months of diagnosis, so these were the ones used; and, unfortunately, given the retrospective nature of the study, further time assessment (closer to VA timing) was not available in all patients, thus this is viewed as a limitation of the study, regarding optimal timing for spatial QRS‐T angle assessment.
Conclusion
In our HCM cohort, the SPQRS‐T angle and the QTc interval differentiated HCM patients with VAs from those without VAs. The SPQRS‐T angle best differentiated patients with VAs with highest predictive values and ORs. The spatial QRS‐T angle had the best predictive values for either VA or NYHA class III or IV HF identification in HCM patients. Prospective studies are needed to validate this method.
Acknowledgments
No disclosures.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Olivotto I, Girolami F, Ackerman MJ, et al. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin Proc. 2008;83:630–638. [DOI] [PubMed] [Google Scholar]
- 2. Spirito P, Autore C, Rapezzi C, et al. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2009;119:1703–1710. [DOI] [PubMed] [Google Scholar]
- 3. Elliott PM, Poloniecki J, Dickie S, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. [DOI] [PubMed] [Google Scholar]
- 4. Debonnaire P, Katsanos S, Joyce E, et al. QRS fragmentation and QTc duration relate to malignant ventricular tachycarrhythmias and sudden cardiac death in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2015;26:547–555. [DOI] [PubMed] [Google Scholar]
- 5. Kors JA, van Herpen G, Sittig AC, et al. Reconstruction of the Frank vectorcardiogram from standard electrocardiographic leads: diagnostic comparison of different methods. Eur Heart J. 1990;11:1083–1092. [DOI] [PubMed] [Google Scholar]
- 6. Dilaveris P, Gialafos E, Pantazis A, et al. The spatial QRS‐T angle as a marker of ventricular repolarisation in hypertension. J Hum Hypertens. 2001;15:63–70. [DOI] [PubMed] [Google Scholar]
- 7. Triola B, Olson MB, Reis SE, et al. Electrocardiographic predictors of cardiovascular outcome in women: the National Heart, Lung, and Blood Institute–sponsored Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 2005;46:51–56. [DOI] [PubMed] [Google Scholar]
- 8. Voulgari C, Tentolouris N, Moyssakis I, et al. Spatial QRS‐T angle: association with diabetes and left ventricular performance. Eur J Clin Invest. 2006;36:608–613. [DOI] [PubMed] [Google Scholar]
- 9. Borleffs CJ, Scherptong RW, Man SC, et al. Predicting ventricular arrhythmias in patients with ischemic heart disease: clinical application of the ECG‐derived QRS‐T angle. Circ Arrhythm Electrophysiol. 2009;2:548–554. [DOI] [PubMed] [Google Scholar]
- 10. de Torbal A, Kors JA, van Herpen G, et al. The electrical T‐axis and the spatial QRS‐T angle are independent predictors of long‐term mortality in patients admitted with acute ischemic chest pain. Cardiology. 2004;101:199–207. [DOI] [PubMed] [Google Scholar]
- 11. Kardys I, Kors JA, van der Meer IM, et al. Spatial QRS‐T angle predicts cardiac death in a general population. Eur Heart J. 2003;24:1357–1364. [DOI] [PubMed] [Google Scholar]
- 12. Kors JA, Kardys I, van der Meer IM, et al. Spatial QRS‐T angle as a risk indicator of cardiac death in an elderly population. J Electrocardiol. 2003;(36 suppl):113–114. [DOI] [PubMed]
- 13. Rautaharju PM, Ge S, Nelson JC, et al. Comparison of mortality risk for electrocardiographic abnormalities in men and women with and without coronary heart disease (from the Cardiovascular Health Study). Am J Cardiol. 2006;97:309–315. [DOI] [PubMed] [Google Scholar]
- 14. Rautaharju PM, Kooperberg C, Larson JC, et al. Electrocardiographic predictors of incident congestive heart failure and all‐cause mortality in postmenopausal women: the Women's Health Initiative. Circulation. 2006;113:481–489. [DOI] [PubMed] [Google Scholar]
- 15. Rautaharju PM, Kooperberg C, Larson JC, et al. Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: the Women's Health Initiative. Circulation. 2006;113:473–480. [DOI] [PubMed] [Google Scholar]
- 16. Rautaharju PM, Prineas RJ, Wood J, et al. Electrocardiographic predictors of new‐onset heart failure in men and in women free of coronary heart disease (from the Atherosclerosis in Communities [ARIC] Study). Am J Cardiol. 2007;100:1437–1441. [DOI] [PubMed] [Google Scholar]
- 17. Yamazaki T, Froelicher VF, Myers J, et al. Spatial QRS‐T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–78. [DOI] [PubMed] [Google Scholar]
- 18. Potter SL, Holmqvist F, Platonov PG, et al. Detection of hypertrophic cardiomyopathy is improved when using advanced rather than strictly conventional 12‐lead electrocardiogram. J Electrocardiol. 2010;43:713–718. [DOI] [PubMed] [Google Scholar]
- 19. Cortez D, Sharma N, Cavanaugh J, et al. The spatial QRS‐T angle outperforms the Italian and Seattle ECG‐based criteria for detection of hypertrophic cardiomyopathy in pediatric patients. J Electrocardiol. 2015;48:826–833. [DOI] [PubMed] [Google Scholar]
- 20. Cortez D, Sharma N, Devers C, et al. Visual transform applications for estimating the spatial QRS‐T angle from the conventional 12‐lead ECG: Kors is still most Frank. J Electrocardiol. 2014;47:12–19. [DOI] [PubMed] [Google Scholar]
- 21. Johnson JN, Grifoni C, Bos JM, et al. Prevalence and clinical correlates of QT prolongation in patients with hypertrophic cardiomyopathy. Eur Heart J. 2011;32:1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi B, Ferrier KA, Sasse A, et al. Correlation between vectorcardiographic measures and cardiac magnetic resonance imaging of the left ventricle in an implantable cardioverter defibrillator population. J Electrocardiol. 2014;47:52–58. [DOI] [PubMed] [Google Scholar]


