ABSTRACT
Background
There are accumulating studies investigating the association between vitamin D status and the risk of atrial fibrillation (AF). However, the results in these studies were inconsistent in regard to the role of vitamin D deficiency in predicting the development of AF.
Hypothesis
Vitamin D deficiency is associated increased risk of AF.
Methods
Using PubMed and Embase databases, we searched for records published before March 2016. Additionally, a manual search was conducted using all review articles on this topic. Of the 587 initially identified records, 8 studies with a total of 27 307 patients were finally analyzed.
Results
In the categorical variable analysis, vitamin D deficiency was associated with the occurrence of AF (odds ratio: 1.31, 95% confidence interval: 1.06‐1.62, P = 0.01). In the continuous variable analysis, higher vitamin D levels appear to protect against the development of AF (odds ratio: 0.92, 95% confidence interval: 0.87‐0.97, P = 0.002). However, the association is weak on the pooled analysis of prospective cohort studies focused on new‐onset AF (P = 0.07 and 0.04), whereas the pooled analysis of case–control studies mainly assessing for chronic AF strongly support such an association (both P < 0.0001).
Conclusions
Vitamin D deficiency modestly increases the risk of AF. Further studies are needed to determine the if there is a direct causal relationship between vitamin D levels and AF and whether vitamin D supplements will prevent new‐onset AF.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in clinical practice and is associated with increased risk of morbidity and mortality in the population. Although the current worldwide prevention and treatment of AF and its associated complications are still inadequate, historical trends showed increasing AF incidence.1, 2 In 2010, the estimated number of subjects with AF globally had reached 33.5 million (20.9 million males and 12.6 million females).2
Although vitamin D is a fundamental micronutrient for individuals, vitamin D deficiency is common in many countries of the Northern Hemisphere.3 Low vitamin D status had been demonstrated to be associated with increased risk of developing diabetes mellitus,4 hypertension,5 congestive heart failure,6 and myocardial infarction,7, 8 which in turn are risk factors for developing AF. In addition, it has been reported that both the occurrence of AF and vitamin D deficiency show a seasonal variation, with the highest incidence in winter and the lowest in summer.9, 10 Collectively, these findings indicate a possible association between vitamin D deficiency and AF. However, there are inconsistent results on the association between vitamin D deficiency and AF.11 Therefore, we aimed to perform a comprehensive meta‐analysis to determine the potential relationship between vitamin D status and risk of AF.
Methods
Meta‐analyses of observational studies present particular challenges because of inherent biases and differences in study designs. Consequently, we performed this analysis according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.12
Inclusion Criteria
We included observational studies with a primary objective to analyze the association between vitamin D status and the risk of AF. Titles and abstracts of all articles were evaluated and rejected after initial screening according to the following inclusion criteria: (1) human subjects; (2) the study design was a prospective cohort study, retrospective cohort study, or case–control study; (3) assessed vitamin D levels at baseline; (4) clearly defined endpoint events of the patients; and (5) the odds ratio (OR) or hazard ratio (HR) and the corresponding 95% confidence interval (CI) for vitamin D levels were reported. Meanwhile, we excluded the studies that only reported unadjusted OR/HR. We included published and unpublished studies without language restriction.
Search Strategy
Two investigators (Z.Z. and Y.Y.) performed a systematic literature search through the online databases of PubMed and Embase to identify relevant studies published before March 2016. We used the following keywords: “vitamin D,” “25(OH)D,” “atrial flutter,” “atrial tachycardia,” and “atrial fibrillation.” Additionally, a manual search was conducted using all review articles on this topic. Titles and abstracts as well as the reference lists of all the retrieved studies were manually checked independently by 2 investigators (Z.Z. and Y.Y.) to include potentially relevant records.
Study Selection
Two investigators (Z.Z. and Y.Y.) independently reviewed the titles and abstracts of the studies from the online databases to identify all potential eligible studies. All potentially relevant records were retrieved as complete manuscripts and assessed for compliance with the inclusion criteria. Any disagreements or uncertainties between the 2 investigators were resolved through consensus after rechecking the source data and consultation with a third investigator (T.L.).
Data Extraction
Two investigators (Z.Z. and Y.Y.) independently performed data extraction using a standard data‐extraction form to determine eligibility for inclusion and to extract data. In the individual primary studies, the results were reported with different variable types (categorical or continuous), so we extracted and analyzed all the multivariate adjusted ORs/HRs and the corresponding 95% CIs in 2 ways (categorical or continuous) to evaluate the association between vitamin D status and AF. In addition, the adjusted ORs/HRs selected for the analysis were those adjusting for the main potential confounders in the multivariate analysis. The extracted data elements of this study included first author's last name, publication year, origin of the studied population, study design, sample size, participants' age and sex, methods of measuring vitamin D levels, season in which the study was conducted, follow‐up duration, medications, mean vitamin D levels, adjusted variables, and endpoint events.
Quality Assessment
To limit the heterogeneity secondary to differences among study designs, the quality of each study was evaluated according to the guidelines developed by the US Preventive Services Task Force13 and the Evidence‐Based Medicine Working Group.14 A scoring system was applied according to the quality of the study. The following key points were assessed: (1) clear description of inclusion and exclusion criteria; (2) study sample representative for the mentioned population; (3) clear description of sample selection; (4) full specification of clinical and demographic variables; (5) sufficient duration of follow‐up; (6) reporting the loss of follow‐up; (7) clear definition of vitamin D deficiency; (8) clear definition of outcomes and outcome assessment; (9) temporality (assessment of vitamin D levels in winter or spring season); and (10) adjustment of possible confounders on the multivariate analysis. If a study did not clearly define one of these key points, we considered that it had not been performed. Consequently, the reported characteristics might have been underestimated.
Table 1.
Characteristics of Studies Included in the Meta‐analysis
| First Author and Year | Location | Study Design | Patients With AF/Total Patients, N | Definition of AF | Methods of AF Detection | Measurement of Vitamin D | Winter or Spring Season | Definition of Vitamin D Deficiency | Follow‐up | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Rienstra 2011 | United States | Prospective cohort | 425/2930 | New‐onset AF | ECGs (clinic visits, external physician visits, Holter monitoring, or hospital records) | A competitive protein‐binding assay (Original cohort) and a radioimmunoassay (Offspring cohort) | 1160 (winter) | 25(OH)D <17 ng/mL for the Original cohort and 13.4 ng/mL for the Offspring cohort | 9.9 ± 4.0 years | 8 |
| Chen 2014 | China | Case control | 162/322 | Nonvalvular persistent AF | ECG | Chemiluminescence assay | 322 (spring) | <20 ng/mL | NA | 8 |
| Demir 2014 | Turkey | Case control | 102/202 | Nonvalvular persistent AF | NA | Electrochemiluminescence method | 202 (winter) | NA | NA | 8 |
| Ozcan 2015 | Turkey | Case control | 137/227 | New‐onset AF | ECG or ambulatory Holter monitoring | Chemiluminescent immunoassay | 227 (winter and spring) | <20 ng/mL | NA | 8 |
| Vitezova 2015 | Netherlands | Prospective cohort | 263/3395 | New‐onset AF | ECG | Electrochemiluminescence immunoassay | NA | <50 nmol/L (20 ng/mL) | 12.0 years | 8 |
| Mathew 2014 (MESA/CHS) | United States | Prospective cohort | 291/6398, 229/1350 | New‐onset AF | Systematic reviews of hospital discharge diagnoses, inpatient and outpatient physician claims data, and study ECGs | Mass spectrometry | NA | NA | 7.7 years, 8.0 years | 8, 8 |
| Alonso 2016 | United States | Prospective cohort | 1866/12 303 | New‐onset AF | ECGs performed during study examinations, hospital discharge codes, and death certificates | Mass spectrometry | NA | <20 ng/mL | 21 years | 9 |
| Belen 2016 | Turkey | Case control | 96/180 | Chronic AF | NA | High‐performance liquid chromatography | All were examined at similar seasonal periods | NA | NA | 8 |
Abbreviations: AF, atrial fibrillation; CHS, Cardiovascular Health Study; ECG, electrocardiogram; MESA, Multi‐Ethnic Study of Atherosclerosis; NA, not applicable.
Table 2.
Patient Characteristics of Included Studies
| First Author and Year | Patients, n | Mean Age, y | Male Sex, % | Vitamin D Supplement, n | CHD, n | Smoking, % | Mean SBP, mm/Hg | Mean BMI, kg/m2 | Medication | Mean LVEF, % | Mean LAD, mm | Mean PASP, mm Hg | Mean 25(OH)D, ng/mL | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholesterol‐Lowering Drug, n (%) | ACEI/ARB, n (%) | β‐Blocker, n (%) | |||||||||||||
| Rienstra 2011 | 2930 | 65 ± 11 | 43.5 | NA | NA | 13.5 | 134 | 27.8 | 366 (12) | 331 (11) | 296 (10) | NA | NA | NA | 23.0 |
| Chen 2014 | 322 | 65.2 | 44.5 | 0 | 0 | 16.5 | 127 | 23.5 | NA | NA | NA | 61.39 | 37.27 | 29.6 | 19.9 |
| Demir 2014 | 202 | 61.94 | 40.6 | 0 | 0 | NA | NA | 23.22 | NA | NA | NA | 63.27 | 40.8 | 31.57 | 8.82 |
| Ozcan 2015 | 227 | 68.0 | 57.7 | 0 | 0 | 30.4 | 134 | 27.0 | 98 (43) | 196 (86) | 9 (4) | 60.8 | 41.6 | 22.6 | 17.9 |
| Vitezova 2015 | 3395 | 71.0 | 40.89 | NA | NA | 16.4 | 143 | 26.9 | 512 (15.1) | NA | NA | NA | NA | NA | 19.7 |
| Mathew 2014 (MESA/CHS) | 6398/1350 | 62.0/77 | 46.5/28.7 | NA | 0/0 | 12.7/7 | 126.2/137 | 28.3/26.5 | 1029 (16.1)/110 (8.1) | NA/NA | NA/NA | NA/NA | NA/38.7 | NA/NA | 25.7/29.2 |
| Alonso 2016 | 12 303 | 56.9 | 43 | NA | 679 | 21.6 | 121.3 | 28.2 | NA | NA | NA | NA | NA | NA | NA |
| Belen 2016 | 180 | 66 ± 8.7 | 53.9 | 0 | 77 | NA | NA | 25.1 | NA | 144 (80) | 144 (80) | 40.4 | NA | NA | 15.2 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CHD, coronary heart disease; CHS, Cardiovascular Health Study; LAD, left atrium diameter; LVEF, left ventricular ejection fraction; MESA, Multi‐Ethnic Study of Atherosclerosis; NA, not applicable; PASP, pulmonary artery systolic pressure; SBP, systolic blood pressure.
Statistical Analysis
Pooled‐effect sizes were presented as ORs with 95% CIs. The HR value using multivariate Cox proportional hazards model in each primary study was directly considered as OR. As the studies included in the meta‐analysis used vitamin D levels as either a categorical or continuous variable, we performed a separate meta‐analysis for both types of variable to evaluate the association between vitamin D status and the occurrence of AF. To evaluate the heterogeneity across studies, we used I 2 derived from the χ2 test, which describes the percentage of the variability in effect estimates resulting from heterogeneity, rather than sampling error. An I 2 > 50% indicates at least moderate statistical heterogeneity.15 When pooled analysis resulted in significant heterogeneity, the random‐effects model was used. We conducted fixed‐effects meta‐analysis using the inverse variance method for pooling effect sizes, and random‐effects meta‐analysis using the inverse variance heterogeneity method. The sensitivity analysis was also done in a random predefined manner. We also performed separate subgroup analyses based on the different study designs. Publication bias was evaluated using the funnel plot. Statistical significance was defined as a 2‐tailed P value of 0.05. All statistical analyses were performed using Review Manager (RevMan) version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Results
A flow diagram of the data search and study selection is presented in Figure 1. A total of 587 records were initially identified by our literature search strategy. After careful assessment of the retrieved studies, 8 studies were finally included in the present meta‐analysis. These remaining 8 studies16, 17, 18, 19, 20, 21, 22, 23 included a total of 27 307 subjects with 3571 patients who had incident AF, all using multivariate analysis with adjustment of possible confounders. The characteristics and quality scores of each study are listed in Table 1, and the patient characteristics of each study are listed in Table 2. For the reference of vitamin D deficiency and the magnitude of vitamin D change that the OR refers to, see Supporting Information, Table 1, in the online version of this article. Furthermore, confounding factors used in multivariate analyses in the included studies are provided in Supporting Information, Table 2, in the online version of this article.
Figure 1.
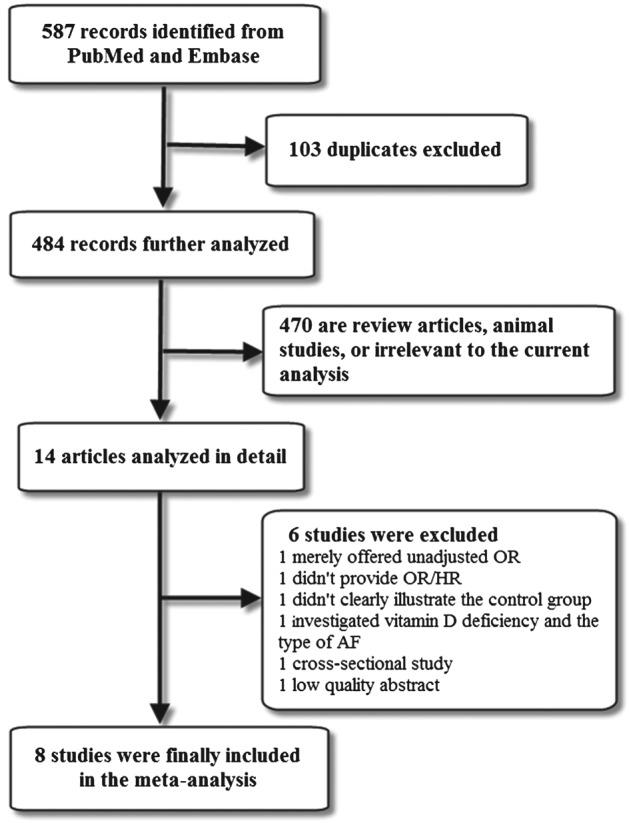
Flow diagram of the study selection process. Abbreviations: HR, hazard ratio; OR, odds ratio.
Of the 8 included studies, 4 case–control studies17, 18, 19, 23 that mainly assessed for chronic AF and were performed during winter or spring suggested that vitamin D deficiency is associated with the development of AF, whereas the remaining 4 cohort studies16, 20, 21, 22 focused on new‐onset AF, were conducted without seasonal restriction, and showed no significant association between vitamin D status and risk of AF after multivariate analysis. Five studies16, 17, 19, 20, 22 with OR/HR values were obtained in which vitamin D levels were analyzed as a categorical variable; 8 studies16, 17, 18, 19, 20, 21, 22, 23 in which vitamin D levels were analyzed as a continuous variable had been included in a separate analysis. Of the 8 included studies, 5 of them16, 17, 19, 20, 22 analyzed vitamin D levels as both a categorical variable and continuous variable. Intriguingly, 1 study showed inconsistent results between categorical variable and continuous variable analyses,19 and 1 study reported 2 separate results.21
Overall, the pooled analysis of the categorical variable suggested that vitamin D deficiency is modestly associated with the occurrence of AF. There is a statistically significant 31% increase in the risk of AF (OR: 1.31, 95% CI: 1.06‐1.62, P = 0.01; Figure 2) with significant heterogeneity among the studies (P = 0.04, I 2 = 60%). This association remains on a pooled analysis of case–control studies (OR: 1.83, 95% CI: 1.35‐2.48, P < 0.0001; heterogeneity: P = 0.61, I 2 = 0%). However, there appears to be no association between vitamin D deficiency and AF on pooled analysis of cohort studies (OR: 1.11, 95% CI: 0.99‐1.25, P = 0.07; heterogeneity: P = 0.87, I 2 = 0%).
Figure 2.
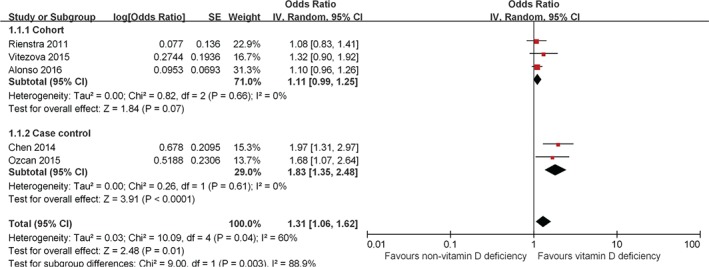
Forest plot showing the association between vitamin D deficiency and the risk of AF in the meta‐analysis with vitamin D levels as a categorical variable. Abbreviations: AF, atrial fibrillation; CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
In addition, the pooled analysis of the continuous variable also suggested that higher vitamin D levels were associated with reduced risk of AF (OR: 0.92, 95% CI: 0.87‐0.97, P = 0.002; Figure 3) with significant heterogeneity among the studies (P = 0.006, I 2 = 63%). However, the association is weak in the pooled analysis of cohort studies (OR: 0.96, P = 0.04), whereas the pooled analysis of case–control studies strongly support this association (OR: 0.85, P < 0.0001).
Figure 3.
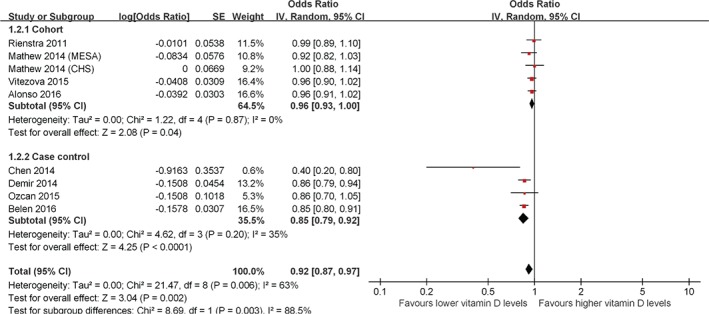
Forest plot showing the association between vitamin D deficiency and the risk of AF in the meta‐analysis with vitamin D levels as a continuous variable. Abbreviations: AF, atrial fibrillation; CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
There was significant heterogeneity when the different study designs were pooled together (P = 0.003, I 2 = 88.9%, Figure 2; and P = 0.003, I 2 = 88.5%, Figure 3). Finally, the results of the funnel plot showed that publication bias may be present, although the small number of studies made this somewhat difficult to interpret (see Supporting Information, Figure 1, in the online version of this article).
Discussion
The present meta‐analysis suggests that vitamin D deficiency is a moderate predictor of AF in both categorical‐ and continuous‐variable analysis. The different study designs appear to have been the potential reason behind the significant heterogeneity in our meta‐analysis, as the cohort studies focused on new‐onset AF and the case–control studies mainly assessed for chronic AF. Also, the measurements of vitamin D levels were performed after the diagnosis of AF in the case–control studies. Therefore, it is possible that behavioral changes such as reduction in outdoor activities may have had an impact on the vitamin D levels in these patients.
Several pathophysiological mechanisms have been proposed for the association between vitamin D deficiency and AF. One of the most important mechanisms is the activation of the renin‐angiotensin‐aldosterone system (RAAS), as it is responsible for both structural and electrical remodeling of the atrium. The antiarrhythmic properties of RAAS inhibition have been well reported, as it decreases the risk of AF.24 In particular, inhibition of the RAAS by vitamin D and an inverse correlation of serum 25(OH)D with angiotensin II levels have been observed previously.25 Therefore, vitamin D deficiency can be harmful secondary to its negative regulatory property of the RAAS.26
Our previous studies have demonstrated that the inflammatory state manifested by elevated C‐reactive protein levels was associated with an increased risk of AF.27, 28 Low vitamin D status leads to an increase in the synthesis of C‐reactive protein through direct and indirect mechanisms.29, 30 The increased oxidative stress in AF plays a major role in the pathogenesis and perpetuation of AF. Vitamin D has antioxidant properties that protect against oxidative stress in the atrium.31 More recently, Canpolat et al32 demonstrated that P‐wave dispersion and atrial electromechanical delay, which are associated with increased risk for the development of AF,33, 34 were significantly prolonged in patients with vitamin D deficiency compared with controls (P < 0.001). Meanwhile, we have previously hypothesized that prolonged P‐wave dispersion and atrial electromechanical delay may be due to electrical and structural remodeling of the atrium secondary to inflammation and oxidative stress.35 Therefore, the inflammatory process and oxidative stress appear to be the link between vitamin D deficiency and the development of AF. By modulating the immune response, and thus, inflammatory pathways, vitamin D may exert additional effects on AF pathogenesis. Finally, Hanafy et al36 found that 1,25[OH]2D increased the action potential duration and contractility in the left atrium of heart failure rabbits, suggested the direct electromechanical effects of vitamin D for enabling prevention or termination of AF.
Atrial fibrillation has been known to have multiple risk factors that contribute to its development. The most important risk factors include age, sex, hypertension, obesity, alcohol, and smoking. In the present meta‐analysis, the HRs or ORs combined in our study were adjusted for confounding risk factors such as age, sex, comorbidities, medications, and ejection fraction. However, further studies are needed to determine whether there is a direct association between vitamin D levels and AF.
Study Limitations
The present meta‐analysis has limitations that should be considered. First, our analysis is based only on observational studies, which may reduce the reliability of the results due to the limitations of such studies. Second, as vitamin D levels were analyzed as either a categorical or continuous variable in the individual studies, we could not pool all of the studies together. However, the final results corroborate each other. Third, the cohort studies did not clearly report the number of participants using vitamin D supplements or the number of patients with coronary heart disease; these may indicate latent bias in this meta‐analysis. Fourth, substantial heterogeneity was found among the included studies in both categorical‐and continuous‐variable analyses. Fifth, the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) defined 25(OH)D levels <10 ng/mL as vitamin D deficiency37; however, most of the studies included in this meta‐analysis defined vitamin D deficiency as a 25(OH)D level of <20 ng/mL. The effects of “real” vitamin D deficiency on the risk of AF need to be further investigated.
Conclusion
This meta‐analysis suggested a positive association between vitamin D deficiency and the risk of AF, although there was a significant heterogeneity secondary to the different study designs. Further studies are needed to determine whether there is a direct causal relationship between vitamin D deficiency and AF, as well as whether vitamin D supplementation will prevent new‐onset AF.
Supporting information
Supplement Figure. Funnel plot of meta‐analysis. Left panel for the studies that used vitamin D levels as a categorical variable; right panel for the studies that used vitamin D levels as a continuous variable. SE = standard error.
Supplement table 1. Reference of vitamin D deficiency and the magnitude of vitamin D change that the OR refers to.
Supplement table 2. Confounding factors used in multivariate analysis in the 6 included studies.
This work was supported by grants (81270245 and 81570298 to T.L.) from the National Natural Science Foundation of China.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Ball J, Carrington MJ, McMurray JJ, et al. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013;167:1807–1824. [DOI] [PubMed] [Google Scholar]
- 2. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spiro A, Buttriss JL. Vitamin D: an overview of vitamin D status and intake in Europe. Nutr Bull. 2014;39:322–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pittas AG, Dawson‐Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–656. [DOI] [PubMed] [Google Scholar]
- 5. Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25‐hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. [DOI] [PubMed] [Google Scholar]
- 6. Zittermann A, Schleithoff SS, Tenderich G, et al. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–112. [DOI] [PubMed] [Google Scholar]
- 7. Giovannucci E, Liu Y, Hollis BW, et al. 25‐Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brøndum‐Jacobsen P, Benn M, Jensen GB, et al. 25‐Hydroxyvitamin D levels and risk of ischemic heart disease, myocardial infarction, and early death: population‐based study and meta‐analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol. 2012;32:2794–2802. [DOI] [PubMed] [Google Scholar]
- 9. Murphy NF, Stewart S, MacIntyre K, et al. Seasonal variation in morbidity and mortality related to atrial fibrillation. Int J Cardiol. 2004;97:283–288. [DOI] [PubMed] [Google Scholar]
- 10. Shoben AB, Kestenbaum B, Levin G, et al. Seasonal variation in 25‐hydroxyvitamin D concentrations in the cardiovascular health study. Am J Epidemiol. 2011;174:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thompson J, Nitiahpapand R, Bhatti P, et al. Vitamin D deficiency and atrial fibrillation. Int J Cardiol. 2015;184:159–162. [DOI] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 13. Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 suppl):21–35. [DOI] [PubMed] [Google Scholar]
- 14. Levine M, Walter S, Lee H, et al; Evidence‐Based Medicine Working Group. Users' guides to the medical literature. IV. How to use an article about harm. JAMA. 1994;271:1615–1619. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rienstra M, Cheng S, Larson MG, et al. Vitamin D status is not related to development of atrial fibrillation in the community. Am Heart J. 2011;162:538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen WR, Liu ZY, Shi Y, et al. Relation of low vitamin D to nonvalvular persistent atrial fibrillation in Chinese patients. Ann Noninvasive Electrocardiol. 2014;19:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Demir M, Uyan U, Melek M. The effects of vitamin D deficiency on atrial fibrillation. Clin Appl Thromb Hemost. 2014;20:98–103. [DOI] [PubMed] [Google Scholar]
- 19. Ozcan OU, Gurlek A, Gursoy E, et al. Relation of vitamin D deficiency and new‐onset atrial fibrillation among hypertensive patients. J Am Soc Hypertens. 2015;9:307–312. [DOI] [PubMed] [Google Scholar]
- 20. Vitezova A, Cartolano NS, Heeringa J, et al. Vitamin D and the risk of atrial fibrillation—the Rotterdam Study. PLoS One. 2015;10:e0125161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathew JS, Sachs MC, Katz R, et al. Fibroblast growth factor‐23 and incident atrial fibrillation: the Multi‐Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS). Circulation. 2014;130:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alonso A, Misialek JR, Michos ED, et al. Serum 25‐hydroxyvitamin D and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Europace. 2016. doi: 10.1093/europace/euv395. [DOI] [PMC free article] [PubMed]
- 23. Belen E, Aykan AC, Kalaycioglu E, et al. Low‐level vitamin D is associated with atrial fibrillation in patients with chronic heart failure. Adv Clin Exp Med. 2016;25:51–57. [DOI] [PubMed] [Google Scholar]
- 24. Khatib R, Joseph P, Briel M, et al. Blockade of the renin‐angiotensin‐aldosterone system (RAAS) for primary prevention of non‐valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2013;165:17–24. [DOI] [PubMed] [Google Scholar]
- 25. Vaidya A, Williams JS. The relationship between vitamin D and the renin‐angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2012;61:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li YC. Vitamin D regulation of the renin‐angiotensin system. J Cell Biochem. 2003;88:327–331. [DOI] [PubMed] [Google Scholar]
- 27. Liu T, Li L, Korantzopoulos P, et al. Meta‐analysis of association between C‐reactive protein and immediate success of electrical cardioversion in persistent atrial fibrillation. Am J Cardiol. 2008;101:1749–1752. [DOI] [PubMed] [Google Scholar]
- 28. Liu T, Li G, Li L, et al. Association between C‐reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta‐analysis. J Am Coll Cardiol. 2007;49:1642–1648. [DOI] [PubMed] [Google Scholar]
- 29. Shea MK, Booth SL, Massaro JM, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eleftheriadis T, Antoniadi G, Liakopoulos V, et al. Inverse association of serum 25‐hydroxyvitamin D with markers of inflammation and suppression of osteoclastic activity in hemodialysis patients. Iran J Kidney Dis. 2012;6:129–135. [PubMed] [Google Scholar]
- 31. Korantzopoulos P, Kolettis TM, Galaris D, et al. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115:135–143. [DOI] [PubMed] [Google Scholar]
- 32. Canpolat U, Yayla Ç, Akboğa MK, et al. Effect of vitamin D replacement on atrial electromechanical delay in subjects with vitamin D deficiency. J Cardiovasc Electrophysiol. 2015;26:649–655. [DOI] [PubMed] [Google Scholar]
- 33. Ozer N, Aytemir K, Atalar E, et al. P wave dispersion in hypertensive patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2000;23(11 part 2):1859–1862. [DOI] [PubMed]
- 34. Cui QQ, Zhang W, Wang H, et al. Assessment of atrial electromechanical coupling and influential factors in nonrheumatic paroxysmal atrial fibrillation. Clin Cardiol. 2008;31:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Z, Li G, Liu T. Psoriasis and risk of atrial fibrillation. Int J Cardiol. 2015;185:301–303. [DOI] [PubMed] [Google Scholar]
- 36. Hanafy DA, Chang SL, Lu YY, et al. Electromechanical effects of 1,25‐dihydroxyvitamin D with antiatrial fibrillation activities. J Cardiovasc Electrophysiol. 2014;25:317–323. [DOI] [PubMed] [Google Scholar]
- 37. Rizzoli R, Boonen S, Brandi ML, et al. Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr Med Res Opin. 2013;29:305–313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure. Funnel plot of meta‐analysis. Left panel for the studies that used vitamin D levels as a categorical variable; right panel for the studies that used vitamin D levels as a continuous variable. SE = standard error.
Supplement table 1. Reference of vitamin D deficiency and the magnitude of vitamin D change that the OR refers to.
Supplement table 2. Confounding factors used in multivariate analysis in the 6 included studies.


