ABSTRACT
Several tests exist for diagnosing coronary artery disease, with varying accuracy and cost. We sought to provide cost‐effectiveness information to aid physicians and decision‐makers in selecting the most appropriate testing strategy. We used the state‐transitions (Markov) model from the Brazilian public health system perspective with a lifetime horizon. Diagnostic strategies were based on exercise electrocardiography (Ex‐ECG), stress echocardiography (ECHO), single‐photon emission computed tomography (SPECT), computed tomography coronary angiography (CTA), or stress cardiac magnetic resonance imaging (C‐MRI) as the initial test. Systematic review provided input data for test accuracy and long‐term prognosis. Cost data were derived from the Brazilian public health system. Diagnostic test strategy had a small but measurable impact in quality‐adjusted life‐years gained. Switching from Ex‐ECG to CTA‐based strategies improved outcomes at an incremental cost‐effectiveness ratio of 3100 international dollars per quality‐adjusted life‐year. ECHO‐based strategies resulted in cost and effectiveness almost identical to CTA, and SPECT‐based strategies were dominated because of their much higher cost. Strategies based on stress C‐MRI were most effective, but the incremental cost‐effectiveness ratio vs CTA was higher than the proposed willingness‐to‐pay threshold. Invasive strategies were dominant in the high pretest probability setting. Sensitivity analysis showed that results were sensitive to costs of CTA, ECHO, and C‐MRI. Coronary CT is cost‐effective for the diagnosis of coronary artery disease and should be included in the Brazilian public health system. Stress ECHO has a similar performance and is an acceptable alternative for most patients, but invasive strategies should be reserved for patients at high risk.
Introduction
In the last decades, several noninvasive tests for diagnosing coronary artery disease (CAD) have become widely available for physicians in clinical practice. Although international guidelines based their recommendations on best strategic options,1, 2, 3 it is perceived that final decisions to order these tests also take into account other factors, such as availability, experience, and insurance coverage. In Brazil, the Unified National Health System (SUS) currently reimburses exercise electrocardiography (Ex‐ECG), stress echocardiography (ECHO), and single‐photon emission computed tomography (SPECT), but not computed tomography coronary angiography (CTA) or stress cardiac magnetic resonance imaging (C‐MRI).4
Cost‐effectiveness studies of diagnostic tests for CAD frequently focus on immediate test results and provide information on the cost per correct diagnosis. However, long‐term results analyzing impact of diagnosis on morbidity and mortality are also important and provide results that might be compared with other interventions in medicine.5 Recently, results from the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial have rekindled this discussion, showing similar results with either anatomical testing with CTA or functional testing with Ex‐ECG, SPECT, or ECHO.6
Our objective is to compare the long‐term effects of choosing between anatomic and functional testing strategies in the context of the Brazilian public health system.
Methods
Our model, developed in TreeAge Pro 2013 (TreeAge Software, Inc., Williamstown, MA) has a short‐term component and a long‐term transitional states model, built in a hypothetical cohort of patients with pretest probability of CAD between 20% and 70%; in the base‐case analysis, patients enter the model at age 60 years.
The short‐term portion is based on a decision tree with varying strategies for evaluating patients with possible stable angina. Eleven possible sequences of tests were defined (Table 1); each starts with a specific test, progressing to further testing if initial results are positive or indeterminate, with invasive coronary angiography (CA) as a final test. For example, in strategy 9, a patient presenting with chest pain could undergo CTA, have a positive test result and thus be subjected to CA. If the CA results are negative, the patient is classified as low‐risk; conversely, positive CA results would result in classification as moderate or high risk.
Table 1.
Test Sequence in Each Modeled Strategy
| Strategy | First Test | Second Test | Third Test |
|---|---|---|---|
| 1 | Ex‐ECG | Stress ECHO | CA |
| 2 | Ex‐ECG | CTA | CA |
| 3 | Ex‐ECG | SPECT | CA |
| 4 | Ex‐ECG | CA | |
| 5 | SPECT | CA | |
| 6 | Stress ECHO | CA | |
| 7 | Stress ECHO | CTA | CA |
| 8 | Stress C‐MRI | CA | |
| 9 | CTA | CA | |
| 10 | CA | Stress ECHO | |
| 11 | CA | SPECT |
Abbreviations: CA, invasive coronary angiography; C‐MRI, cardiac magnetic resonance imaging; CTA, computed tomography coronary angiography; ECHO, echocardiography; Ex‐ECG, exercise electrocardiography; SPECT, single‐photon emission computed tomography.
After a diagnosis is established in this initial stage, patients progress to a long‐term Markov model, in which members of the hypothetical cohort may remain stable; suffer cardiovascular (CV) events; undergo percutaneous or surgical revascularization procedures, with their inherent risks and temporary disutility; or die from CVD, cancer, or other causes.
Each diagnostic strategy pathway stratifies patients into one of several health states: low risk (represented by patients without significant CAD), medium risk (such as patients with single‐ or 2‐vessel CAD), high risk (patients with extensive CAD or associated left ventricular dysfunction), medium risk with false negative (incorrectly diagnosed as low risk), high risk with false negative (incorrectly diagnosed as low risk), and low risk with false positive (incorrectly diagnosed as medium risk).
All these health states carry inherited transition probabilities and thereby influence the probability of each clinical outcome in the long‐term model: true negative tests identify patients at low risk of CV events and determine low risk of revascularization; true positive tests identify patients at higher risk of CV events and generate more revascularization procedures. In the case of false‐negative results, patients have a high risk of CV events but are misdiagnosed as low‐risk; this further increases CV risk but results in fewer revascularization procedures. False‐positive results signal low risk of CV events, but misdiagnosis leads to more revascularization procedures, with consequent risks and costs.
We have included a simplified representation of the model structure (see Supporting Information, Figure 1, in the online version of this article). We performed our analysis from the perspective of the public health system (SUS) and measured outcomes in quality‐adjusted life‐years (QALY). The model ran yearly cycles over a lifetime horizon.
Figure 1.
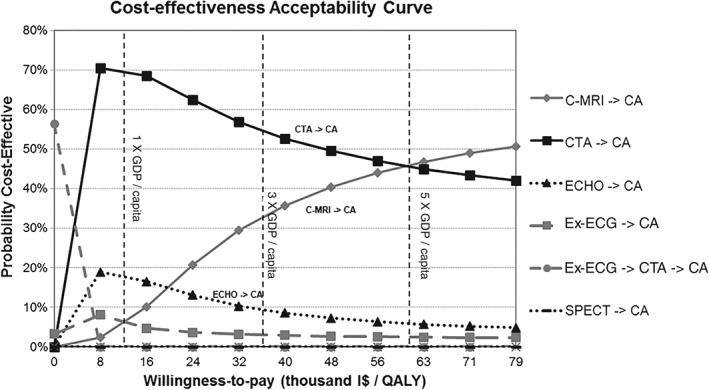
Cost‐effectiveness acceptability curve for base‐case scenario. Abbreviations: CA, invasive coronary angiography; C‐MRI, cardiac magnetic resonance imaging; CTA, computed tomography coronary angiography; ECHO, stress echocardiography; Ex‐ECG, exercise electrocardiography; GDP, gross domestic product; I$, international dollars (purchasing power parity); QALY, quality‐adjusted life‐years; SPECT, single‐photon emission computed tomography.
Input Data
We conducted a systematic review of available data on accuracy and risks of noninvasive tests and CA, as well as long‐term prognosis of patients with stable CAD. Studies identified in this review served as sources for input data, as described in Table 2 (test performance) and Table 3 (long‐term model).
Table 2.
Characteristics of Tests, Range of Values Used in Sensitivity Analysis, and Costs
| Test | Sensitivity, % (range) | Specificity, % (range) | Indeterminacy, % | Mortality, % | Cost (I$) | Sources (Article References) |
|---|---|---|---|---|---|---|
| Ex‐ECG | 65 (42–92) | 67 (43–83) | 18 | 0.05 | 16.13 | 4, 15, 21 |
| ECHO | 85 (83–87) | 77 (74–80) | 15 | 0.05 | 88.71 | 2, 4, 22 |
| SPECT | 87 (84–88) | 64 (60–76) | 6.9 | 0.05 | 425.59 | 2, 4, 22 |
| CTA | 88 (83–92) | 87 (80–92) | 2 | 0.01 | 102.67 | 3, 4, 24, 25 |
| C‐MRI | 89 (88–94) | 80 (75–87) | 5 | 0.01 | 203.76 | 2, 4, 26 |
| CA | 100 | 100 | 10 | 0.2 | 330.49 | Assumption, 4, 27, 28 |
Abbreviations: CA, invasive coronary angiography; C‐MRI, cardiac magnetic resonance imaging; CTA, computed tomography coronary angiography; ECHO, stress echocardiography; Ex‐ECG, exercise electrocardiography; SPECT, single‐photon emission computed tomography.
Table 3.
Input Data for Long‐Term Model
| Base Case | Range for SA, % | Article Reference(s) | |
|---|---|---|---|
| CV mortality, % | |||
| Low risk | 1.1 | 1.0–1.3 | 7 |
| Medium risk | 2.3 | 2.1–2.5 | 7 |
| High risk | 3.9 | 2.7–5.0 | 7 |
| MI, % | |||
| Low risk | 0.6 | 0.5–0.7 | 7 |
| Medium risk | 1.1 | 1.0–1.3 | 7 |
| High risk | 1.8 | 1.0–2.6 | 7 |
| Probability of new revascularization, % | |||
| Low risk | 1.4 | 1.1–1.8 | 7 |
| Medium risk | 3.6 | 3.2–3.9 | 7 |
| High risk | 5.1 | 4.6–5.6 | 7 |
| Proportion of CABG if revascularized, % | |||
| Low risk | 10 | 5–20 | Assumption |
| Medium risk | 25 | 12.5–50 | Assumption |
| High risk | 50 | 25–90 | Assumption |
| Procedure‐related mortality, % | |||
| PCI | 0.68 | 0.1–1.0 | 29 |
| CABG | 1.75 | 1.0–3.0 | 29 |
| Utilities | |||
| Stable patients | 0.74 | 0.61–0.86 | 8, 10 |
| Disutility for PCI | 0.04 | 0.0–0.07 | 10, 30, 31 |
| Disutility for CABG | 0.08 | 0.0–0.07 | 10, 30, 31 |
| Disutility for MI | 0.04 | 0.02–0.07 | 10, 30, 32 |
| Costs | |||
| PCI | I$834 | I$417–1667 | 4 |
| Stent | I$1076 | I$538–2153 | 4 |
| Annual cost‐stable patients | I$732 | I$366–1463 | 9 |
| CABG | I$3575 | I$1787–7150 | 4 |
| MI | I$1500 | I$749–2995 | 4 |
Abbreviations: CABG, coronary artery bypass grafting; CV, cardiovascular; Disutility, negative utility; I$, international dollars; MI, myocardial infarction; PCI, percutaneous coronary intervention; SA, sensitivity analysis.
Because of its large sample size and long‐term follow‐up, we considered the Reduction of Atherothrombosis for Continued Health (REACH) Registry7 to be a valid study to retrieve long‐term probability of CV events, stratified by initial risk group.
We based long‐term costs and utilities for CAD patients on local cohorts,8, 9 because these data are prone to wide variation in different countries and settings. Health‐related quality of life was measured using the Short Form 6D (SF‐6D), and disutility resulting from complications and procedures was assumed as the difference between utility immediately before and 3 months after events.10
Impact of radiation exposure on the lifetime risk of cancer was based on available studies11, 12 and assumes an average dose of 10 mSv for CTA, SPECT, and CA.12 After a 10‐year latency period, risk of fatal cancer due to radiation increases exponentially, reaching 0.5 per 1000 in the second decade after exposure, 1.5 per 1000 in the fourth decade after exposure, and 3.5 per 1000 in the sixth decade after exposure.
Brazil's SUS 2013 reimbursement rates were the source of costs for diagnostic tests for currently reimbursed tests4; costs of CTA and MRI were estimated based on rates for currently reimbursed tests (chest CT and rest C‐MRI), inflated proportionally to cost differences among these tests in the private sector13 (Table 2). Costs have been converted from the Brazilian real to international dollars (I$), at a rate of 1.89, corresponding to the World Bank's latest purchasing power parity conversion factor.14
Assumptions
Because CA is the gold standard for diagnosing CAD, it was assumed to have 100% sensitivity and specificity in the model. An alternative scenario, with CA sensitivity and specificity lower than 100% (arbitrarily defined as 95%), has been explored in sensitivity analysis. Additionally, the last test in any strategy never generates indeterminate results.
Cost of serious short‐term test complications (including death) were assumed to be equivalent to SUS average national costs for myocardial infarction (MI) admissions in 2012, I$1500.4
As in previously published models,15 the base‐case assumption is that 10% of initially misdiagnosed cases are correctly rediagnosed in the first year, with increasing numbers until the tenth year, by which all have been properly rediagnosed. This accounts for the possibility of correcting diagnostic errors in subsequent office visits or with further testing.
As per current recommendation by the Brazilian Ministry of Health, we used a 5% discount rate for costs and utilities in the base‐case analysis, as well as 0% and 10% rates in sensitivity analysis.
There is no official willingness‐to‐pay (WTP) threshold for health care interventions in Brazil; the World Health Organization (WHO) recommends a WTP threshold between 1× and 3× a nation's gross domestic product (GDP) per capita16 for middle‐income countries, which would represent figures between I$11 909 and I$35 727 per QALY in Brazil.
Sensitivity and Statistical Analysis
Aiming to test the robustness of the model and the weight of individual parameters on results, during sensitivity analysis we varied test accuracies and rates of complications and indeterminacy around their 95% confidence intervals (CIs). Alternative costs of tests ranged from half the original values to double those values.
In addition to 1‐ and 2‐way sensitivity analyses, we performed probabilistic sensitivity analysis with 10 000 samples, with simultaneous variation of model parameters around their CIs. We used β distributions for test accuracies and gamma distributions for costs.
Regarding rediagnosis, we considered alternative scenarios in which time until all patients are correctly diagnosed varies between 5 years and 20 years.
Results
Considering the base case scenario of patients at age 60 years and 50% pretest probability of CAD, life expectancy shows little variation with different diagnostic strategies: between 11.19 and 11.26 years with moderate pretest probability. Changing assumption to low pretest probability results in life expectancy 0.5 years higher, and high pretest probability results in loss of 0.5 years in life expectancy.
Main results are represented in Table 4 (see also Supporting Information, Figure 2, in the online version of this article). Strategies 1 and 2, based on Ex‐ECG as a “gatekeeper” test, followed if positive by either ECHO or CTA, and then by CA if the second test is positive, were the least costly, least effective strategies, with mean lifetime cost of I$9500 and expected 8.06 QALY gained. Strategy 4, also including Ex‐ECG as the initial test, but proceeding directly to CA if positive, was marginally more costly and more effective, with an incremental cost‐effectiveness ratio (ICER) vs strategy 2 of I$2200 per QALY gained.
Table 4.
Base‐Case Cost‐Utility Results
| Strategy | Adjusted Lifetime Cost (I$) | Adjusted Life Expectancy, y | Adjusted QALY | ICER (I$/QALY) |
|---|---|---|---|---|
| Low pretest probability of CAD | ||||
| 2 (Ex‐ECG → CTA → CA) | 9560 | 11.777 | 8.604 | |
| 7 (ECHO → CTA → CA) | 9601 | 11.790 | 8.614 | 8100 |
| 9 (CTA → CA) | 9650 | 11.801 | 8.622 | 11 300 |
| 8 (C‐MRI → CA) | 9765 | 11.802 | 8.623 | 286 000 |
| Moderate pretest probability of CAD | ||||
| 2 (Ex‐ECG → CTA → CA) | 9465 | 11.194 | 8.059 | |
| 4 (Ex‐ECG → CA) | 9496 | 11.213 | 8.073 | 2200 |
| 9 (CTA → CA) | 9589 | 11.254 | 8.103 | 3100 |
| 8 (C‐MRI → CA) | 9698 | 11.257 | 8.105 | 50 500 |
| High pretest probability of CAD | ||||
| 2 (Ex‐ECG → CTA → CA) | 9401 | 10.802 | 7.696 | |
| 4 (Ex‐ECG → CA) | 9414 | 10.832 | 7.716 | 610 |
| 10 (CA → ECHO) | 9590 | 10.920 | 7.779 | 2800 |
Abbreviations: CA, invasive coronary angiography; CAD, coronary artery disease; C‐MRI, cardiac magnetic resonance imaging; CTA, computed tomography coronary angiography; ECHO, stress echocardiography; Ex‐ECG, exercise electrocardiography; ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life‐years.
Results are not meant to be highly precise; decimal places are shown to make small differences apparent.
Strategy 9, with CTA as the initial test, fared better and yielded additional 0.3 QALY, in average, at an ICER of I$3100. Strategy 6, based on ECHO and CA, had almost identical results to strategy 9 (QALY difference, 0.0022; cost difference, I$14).
Strategy 8, based on C‐MRI as the initial test, emerged as the most effective strategy, with an average of 8.11 QALY; but it came at a higher cost, so that the ICER vs strategy 9 is I$50 500.
Strategies 3 and 5, based on SPECT, performed marginally better than equivalent strategies with ECHO or CTA, but they resulted in much higher costs and therefore were dominated in all scenarios.
Varying pretest probability between 20% and 70% resulted in variation of overall costs, survival, and QALY, but relative performance of each strategy was not changed. In all cases, Ex‐ECG–based strategies were the least costly/least effective; ECHO‐ or CTA‐based strategies were more effective than Ex‐ECG at relatively low ICERs; C‐MRI strategies were the most effective with a high ICER; and SPECT strategies were dominated.
When CA was included as an acceptable initial test, the invasive strategies became the most effective because of the “perfect accuracy” of CA assumed by the model. When pretest probability is ≥65%, strategy 10, based on CA as the initial test, dominated all other strategies except for Ex‐ECG strategies, which remained as the least costly/least effective alternative. However, with such high pretest probabilities, Ex‐ECG–based strategies generated a >20% false‐negative initial diagnosis, a performance that could be considered unacceptable in clinical practice.
One‐way sensitivity analysis of relevant parameters did not affect overall results significantly, with one exception: because CTA‐based and ECHO‐based strategies showed almost identical base‐case results, variation in cost or in accuracy of these tests changed preference between the 2.
Sensitivity analysis of other parameters, including rates of test indeterminacy and complications, influenced average costs and QALY but did not alter the relative performance of strategies.
Specifically comparing the performance of currently available imaging tests ECHO and SPECT, the results showed that ECHO‐based strategies are consistently more cost‐effective in our model. The SPECT‐based strategies were only dominant if SPECT cost was reduced to 110% of the costs of ECHO in the base case, or to 125% of the cost of ECHO when accuracy of SPECT was raised to the upper limit of the CI.
We also explored threshold analysis regarding cost of C‐MRI and found that a reduction to values below I$160 (79% of base‐case cost) resulted in an ICER vs CTA‐based strategies below I$35 700 (3× GDP per capita). A C‐MRI cost < I$110 (54% of base‐case cost) was necessary for an ICER below I$11 900 (1× GDP per capita).
Probabilistic sensitivity analysis with 10 000 iterations resulted in test performances consistent with base‐case results. In the setting of a WTP threshold of I$11 909, probability of being cost‐effective was 69% for strategy 9, based on CTA, and 18% for strategy 6, based on ECHO. At a higher WTP threshold of I$35 700, the probability of being cost‐effective was 55% for strategy 9, based on CTA; 32% for strategy 8, based on C‐MRI; and 10% for strategy 6, based on ECHO. Other strategies had <5% probability of being optimal.
Cost‐effectiveness acceptability curves (Figure 1) show that Ex‐ECG–based strategies were optimal only in very constrained budgets, with WTP thresholds close to zero. The CTA‐based and ECHO‐based strategies quickly emerged as the ones most likely to be cost‐effective, and a C‐MRI–based strategy showed a high likelihood of being cost‐effective only with WTP threshold >5× Brazilian GDP per capita.
Discussion
Recent data suggest that clinical outcomes are similar for anatomic and functional testing strategies for patients with suspected stable CAD. In the PROMISE trial, patients randomized to CTA as the initial test were more likely to undergo an invasive CA with no evidence of CAD, and coronary revascularization was more likely in this group.6
The long‐term clinical significance of these findings is still a matter of debate, but it seems clear that the finding of comparable clinical performance of anatomic and functional strategies increases the usefulness of cost‐effectiveness data for decision‐makers and physicians.
We used the large body of evidence available on the relative performance of noninvasive tests for diagnosing CAD and performed a broad analysis on the long‐term consequences of selecting one test over another.
Our results suggest that, for patients at moderate pretest probability, ECHO‐based strategies are the most cost‐effective among currently available tests in the public health system, and that inclusion of CTA in the role of available tests would bring an effective additional strategy at a very attractive ICER. In scenarios of high pretest probability (close to 70%), invasive strategies are dominant and seem to be the most reasonable alternative when there is diagnostic uncertainty.
In our study, investigation of stable chest pain patients with C‐MRI resulted in slightly higher QALY in the long term than other noninvasive tests, but at a higher cost as compared with ECHO or CTA results, leading to ICERs above the proposed WTP threshold at the estimated base‐case cost. This result is sensitive to the cost of the C‐MRI test, and this alternative could become cost‐effective if reimbursement value upon inclusion in SUS remains < I$160.
In current practice, it is common for physicians to have to choose between ECHO and SPECT when patients have contraindications to Ex‐ECG. Our results suggest that, in this particular scenario, ECHO is economically more attractive and should be preferred in the majority of cases.
With the steady increase in health care costs, evaluating the financial impact of health care policies has become a worldwide concern.2, 16, 17, 18, 19, 20, 21, 22, 23 However, economic analyses of noninvasive tests from other countries' perspectives are not directly transferable to our context, mainly because of the wide variation in the costs of tests and procedures. Still, despite differences in total costs and QALY, previous works have generally shown similar results in terms of choice among tests, with either ECHO or CTA usually emerging as the recommended strategy.2, 18, 19, 20
Significant controversy still exists regarding translation of cost‐effectiveness results to clinical practice and widespread recommendation. In their latest guidance, England's National Institute for Health and Care Excellence (NICE) suggests patients with possible stable angina and a positive coronary calcium score should undergo CTA for diagnosis if pretest probability is low. Additionally, NICE specifically states that Ex‐ECG should not be used to diagnose or exclude stable angina, due to its inferior performance in their analysis.3
The American Heart Association's latest guidelines for diagnosis and management of stable angina made more traditional recommendations, maintaining Ex‐ECG as preferred first‐line test (class I) and giving weaker recommendations to ECHO and SPECT (class IIa) or CTA (class IIb).1
In Brazil, operators and administrators in centers performing Ex‐ECG or ECHO often complain that an adjustment in SUS reimbursement values is overdue. This may lead to reduced availability of tests, and could explain, at least in part, the greater emphasis on SPECT than ECHO observed in the Brazilian public health system: 114 000 SPECT exams were performed in 2013, and 18 000 ECHO tests.4
A comparison among SUS reimbursement values of tests supports this notion: SPECT value is nearly 5× higher than ECHO, 25× higher than Ex‐ECG, and 1.3× higher than CA.4 Meanwhile, in the United Kingdom's public health system, for example, SPECT cost is only 1.5× higher than ECHO and 5× higher than Ex‐ECG, and it is 3× less costly than CA.3 Addressing these distortions could promote broader availability of cost‐effective diagnostic alternatives.
One frequent concern regarding noninvasive tests is the radiation exposure associated with CTA, SPECT, and CA. We have attempted to include this disadvantage in our model by including an increase in the probability of cancer after exposure. However, the impact of radiation‐related cancer was very small in our study, because of the low risk attributable to a single exposure and the long latency before the risk arises. Still, radiation‐related risks may be more pronounced for younger patients, or after repetitive testing, leading to the recommendation of keeping exposure As Low as Reasonably Achievable (ALARA).1
Study Limitations
A possible limitation of our study is that input data regarding the relative performance of tests came from multiple sources obtained from the available literature. We have tried to minimize the effects of this by performing a systematic review and using large previous meta‐analyses or registries as our sources of input data, but some degree of publication bias cannot be excluded.
Our model does not include all possible test sequences as potential strategies, because the results would become excessively lengthy and impractical to report. Included strategies were selected by a group of experts who judged them to be the most likely to occur in current practice.
In addition, our model did not account for costs of acquisition and implementation. These costs could have a significant additional budget impact, particularly in the case of CTA and C‐MRI, currently unavailable in many—if not most—public health centers.
Our results are applicable to the choice among tests for diagnostic purposes, or, in other words, to cases in which there is uncertainty whether chest pain is due to stable angina or to an alternative diagnosis. A different use of noninvasive tests—not considered in our analysis—is to estimate the prognosis of patients with an established diagnosis of CAD, seeking to aid in the choice of treatment modality or to assess response to therapy. In this case, some modalities that were less cost‐effective for diagnosis, like Ex‐ECG, may perform well enough to warrant widespread use.
Additionally, even though cost‐effectiveness results are useful to determine the “standard” test for clinical use, there must be flexibility in the list of available tests, allowing physicians to select the most useful test in atypical situations.
Conclusion
For patients presenting with chest pain and low or moderate pretest probability, coronary CT is cost‐effective for the diagnosis of CAD, and its inclusion in the list of tests available in SUS is warranted. Making C‐MRI available in the public health system could be an attractive option, depending on what reimbursement value can be defined upon its inclusion. Stress ECHO is a viable alternative, and performed better than SPECT from the economic perspective. Invasive strategies, using CA as the initial test, should be reserved for patients with high pretest probability.
Supporting information
FigureS1. Schematic representation of model structure.Prob. = probability; MI = myocardial infarction; revasc. = revascularization procedures; PCI = percutaneous coronary intervention; CABG = coronary artery bypass graft
FigureS2. Base‐case cost‐utility results (starting age 60 years, pretest probability 50%). I$ = international dollars (PPP); QALY = quality adjusted life‐years; Ex‐ECG = exercise electrocardiogram; ECHO = stress echocardiogram; SPECT = single‐photon emission computed tomography; CTA = computed tomography coronary angiogram; MRI = cardiac magnetic resonance; CA = invasive coronary angiography
This study was supported by the CNPq (MCT/MS), under the Program of the National Institute for Science and Technology (INCT) for Health Technology Assessment (IATS). Drs. Rohde Polanczyk received research scholarship from CNPq.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons [published correction appears in Circulation. 2014;129:e463]. Circulation. 2012;126:e354–e471. [DOI] [PubMed] [Google Scholar]
- 2. Toronto Health Economics and Technology Assessment (THETA) . Relative Cost‐Effectiveness of Five Non‐invasive Cardiac Imaging Technologies for Diagnosing Coronary Artery Disease in Ontario. http://lgdata.s3‐website‐us‐east‐1.amazonaws.com/docs/3744/928383/TR_2010.2_theta_cardiac_imaging_report.pdf. Accessed June, 2014. [Google Scholar]
- 3. Cooper A, Calvert N, Skinner J, et al. Chest Pain of Recent Onset: Assessment and Diagnosis of Recent Onset Chest Pain or Discomfort of Suspected Cardiac Origin. London, UK: National Institute for Health and Clinical Excellence (NICE); 2010. [Google Scholar]
- 4.Ministério da Saúde do Brasil. DATASUS. 2014. http://www.datasus.gov.br [website in Portuguese].
- 5. Kind P, Lafata JE, Matuszewski K, et al. The use of QALYs in clinical and patient decision‐making: issues and prospects. Value Health. 2009;(12 suppl 1):S27–S30. [DOI] [PubMed] [Google Scholar]
- 6. Douglas PS, Hoffmann U, Patel MR, et al; PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhatt DL, Eagle KA, Ohman EM, et al; REACH Registry Investigators . Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 8. Cruz LN, Fleck MP, Polanczyk CA. Depression as a determinant of quality of life in patients with chronic disease: data from Brazil. Soc Psychiatry Psychiatr Epidemiol. 2010;45:953–961. [DOI] [PubMed] [Google Scholar]
- 9. Ribeiro RA, Mello RG, Melchior R, et al. Annual cost of ischemic heart disease in Brazil: public and private perspective [article in Portuguese]. Arq Bras Cardiol. 2005;85:3–8. [DOI] [PubMed] [Google Scholar]
- 10. Cruz LN, Camey SA, Hoffmann JF, et al. Estimating the SF‐6D value set for a population‐based sample of Brazilians. Value Health. 2011;14(5 suppl 1):S108–S114. [DOI] [PubMed] [Google Scholar]
- 11. Einstein AJ, Moser KW, Thompson RC, et al. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290–1305. [DOI] [PubMed] [Google Scholar]
- 12. Gerber TC, Carr JJ, Arai AE, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119:1056–1065. [DOI] [PubMed] [Google Scholar]
- 13. Associação Médica Brasileira . Classificação Brasileira Hierarquizada de Procedimentos Médicos (CBHPM). São Paulo, Brazil: Associação Médica Brasileira; 2013. [Google Scholar]
- 14. World Bank . World Development Indicators & Global Development Finance. http://data.worldbank.org/data‐catalog/world‐development‐indicators/wdi‐2012. Accessed February, 2015.
- 15. Mowatt G, Vale L, Brazzelli M, et al. Systematic review of the effectiveness and cost‐effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. Health Technol Assess. 2004;8:iii–iv, 1–207. [DOI] [PubMed] [Google Scholar]
- 16. WHO Commission on Macroeconomics and Health . Macroeconomics and Health: Investing in Health for Economic Development. Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization; December 2001. http://apps.who.int/iris/bitstream/10665/42435/1/924154550X.pdf. [Google Scholar]
- 17. Gold MR, Siegel JE, Russell LB, et al, eds. Cost‐Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 18. Kuntz KM, Fleischmann KE, Hunink MG, et al. Cost‐effectiveness of diagnostic strategies for patients with chest pain. Ann Intern Med. 1999;130:709–718. [DOI] [PubMed] [Google Scholar]
- 19. Garber AM, Solomon NA. Cost‐effectiveness of alternative test strategies for the diagnosis of coronary artery disease. Ann Intern Med. 1999;130:719–728. [DOI] [PubMed] [Google Scholar]
- 20. Bedetti G, Pasanisi EM, Pizzi C, et al. Economic analysis including long‐term risks and costs of alternative diagnostic strategies to evaluate patients with chest pain. Cardiovasc Ultrasound. 2008;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patterson RE, Eisner RL, Horowitz SF. Comparison of cost‐effectiveness and utility of exercise ECG, single‐photon emission computed tomography, positron emission tomography, and coronary angiography for diagnosis of coronary artery disease. Circulation. 1995;91:54–65. [DOI] [PubMed] [Google Scholar]
- 22. Fleischmann KE, Hunink MG, Kuntz KM, et al. Exercise echocardiography or exercise SPECT imaging? A meta‐analysis of diagnostic test performance. JAMA. 1998;280:913–920. [DOI] [PubMed] [Google Scholar]
- 23. Brunetti ND, Dellegrottaglie G, Lopriore C, et al. Prehospital telemedicine electrocardiogram triage for a regional public emergency medical service: is it worth it? A preliminary cost analysis. Clin Cardiol. 2014;37:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arbab‐Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre‐test probability of coronary artery disease and severity of coronary arterial calcification. The CORE‐64 (Coronary Artery Evaluation Using 64‐Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol. 2012;59:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mowatt G, Cummins E, Waugh N, et al. Systematic review of the clinical effectiveness and cost‐effectiveness of 64‐slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. Health Technol Assess. 2008;12:iii–iv, ix–143. [DOI] [PubMed] [Google Scholar]
- 26. Hamon M, Fau G, Née G, et al. Meta‐analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson. 2010;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cooper A, Timmis A, Skinner J; for the Guideline Development Group . Assessment of recent onset chest pain or discomfort of suspected cardiac origin: summary of NICE guidance. BMJ. 2010;340:c1118. [DOI] [PubMed] [Google Scholar]
- 28. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography [published correction appears in N Engl J Med. 2010;363:498]. N Engl J Med. 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hannan EL, Racz MJ, Walford G, et al. Long‐term outcomes of coronary‐artery bypass grafting versus stent implantation. N Engl J Med. 2005;352:2174–2183. [DOI] [PubMed] [Google Scholar]
- 30. Bischof M, Briel M, Bucher HC, et al. Cost‐effectiveness of drug‐eluting stents in a US Medicare setting: a cost‐utility analysis with 3‐year clinical follow‐up data. Value Health. 2009;12:649–656. [DOI] [PubMed] [Google Scholar]
- 31. Bagust A, Grayson AD, Palmer ND, et al. Cost effectiveness of drug eluting coronary artery stenting in a UK setting: cost‐utility study. Heart. 2006;92:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bravo Vergel Y, Palmer S, Asseburg C, et al. Is primary angioplasty cost effective in the UK? Results of a comprehensive decision analysis. Heart. 2007;93:1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigureS1. Schematic representation of model structure.Prob. = probability; MI = myocardial infarction; revasc. = revascularization procedures; PCI = percutaneous coronary intervention; CABG = coronary artery bypass graft
FigureS2. Base‐case cost‐utility results (starting age 60 years, pretest probability 50%). I$ = international dollars (PPP); QALY = quality adjusted life‐years; Ex‐ECG = exercise electrocardiogram; ECHO = stress echocardiogram; SPECT = single‐photon emission computed tomography; CTA = computed tomography coronary angiogram; MRI = cardiac magnetic resonance; CA = invasive coronary angiography


