ABSTRACT
Background
Following successful cavotricuspid isthmus (CTI) ablation during typical atrial flutter (AFL), anticoagulation therapy is usually withdrawn. However, potential subsequent atrial fibrillation (AF) in these patients may increase embolic risk in the long term. Embolic rates in this setting have not been clearly established. Our aim was to determine the incidence of stroke/systemic embolism following radiofrequency ablation of AFL, particularly in those without a prior history of AF.
Hypothesis
After succesful AFL ablation, patients may suffer embolic complications in the long‐term follow‐up, mainly due to asymptomatic AF episodes.
Methods
We conducted a retrospective analysis of all patients who underwent CTI ablation due to AFL in our center between 2006 and 2009.
Results
During the study period, 188 patients (mean age, 62.9 ± 8.6 years) underwent CTI ablation; 120 without prior AF were included in the study. At the end of the follow‐up period (mean, 5.0 ± 2.4 years), 56.7% of patients (68/120) remained in sinus rhythm, 7/120 experienced a recurrence of AFL, and 45/120 (38%) developed AF. Ischemic stroke occurred in 11 patients and systemic embolism in 1. Of these patients, 5 had documented AF following AFL ablation. In the remaining 7 cases, previously undiagnosed AF was subsequently diagnosed at the time of stroke/embolism.
Conclusions
Patients with AFL who undergo successful ablation are by no means free from embolic complications during long‐term follow‐up, mainly due to a high rate of AF development. Given the difficulties in detecting AF and the uncertainty about the temporal relation of AF and stroke, oral anticoagulation may need to be continued in those patients with underlying stroke risk factors.
Introduction
Typical atrial flutter (AFL) remains a common arrhythmia, closely related to atrial fibrillation (AF).1 Until recently, however, clinical trials and observational studies grouped patients with both AFL and AF together, and the signs identifying flutter remained hidden, being much less frequent than AF. Although information on the prognostic significance and pharmacological treatment of AF and its systemic complications is abundant, data remain scarce regarding AFL; clinical recommendations are thus largely based on clinical observations and expert consensus rather than on evidence.
The identification of the cavotricuspid isthmus (CTI) as a key arrhythmic substrate within the lower right atrium allows for successful catheter‐based ablation therapy. In recent times, catheter ablation of the CTI has been increasingly used as first‐line therapy, offering a high acute success rate coupled with a low complication rate in experienced electrophysiology laboratories.2, 3, 4
The current recommendation following successful ablation of the CTI for AFL is to withdraw anticoagulation therapy if there is no evidence of recurrence or remote history of AF.5 However, there is increasing evidence of the late development of AF following successful AFL ablation, which may increase embolic risk in the long term. Data regarding the incidence of such embolic events are scarce and seldom reported.
The aim of our study was to assess the frequency of embolic events after AFL ablation, especially in patients without previously documented AF.
Methods
We conducted a retrospective analysis of all patients who underwent CTI ablation due to AFL in our center between 2006 and 2009. Patients with non–CTI‐dependent AFL were excluded.
Data Collection
Data regarding ablation procedure were collected prospectively within a dedicated database providing details of arrhythmia substrate and demographic information. Prior medical history was obtained from the clinical records of each patient.
Following the procedure, the referring cardiologist in the outpatient service followed patients at 2 months post‐ablation and then again at yearly intervals. Data regarding new medical events were obtained from individual reports and charts available at the hospital. Phone contact with patients or their relatives and consulting the databases of the health service, admission, and clinical documentation services completed the follow‐up information.
Atrial Flutter Ablation
Written informed consent was obtained before each procedure. A multipolar recording Halo catheter (Biosense Webster, Diamond Bar, CA) positioned around the tricuspid annulus was used for pacing and electrogram interpretation. Involvement of the CTI was confirmed by entrainment mapping. Success was defined as flutter termination with bidirectional isthmus block and no inducibility afterward.
Drug Management After Procedure
Pharmacological management post‐ablation was at discretion of the referring cardiologist. As a general rule, all antiarrhythmic drugs were stopped unless prior history of AF was present. Chronotropic drugs were maintained when patients had indications other than AFL for β‐blockers or calcium antagonists. Oral anticoagulation (OAC) was maintained in patients with a prior history of AF, according to embolic risk, and in patients with prosthetic cardiac valves when indicated.
Statistical Analysis
SPSS version 15.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. A P value < 0.05 was accepted as statistically significant. Continuous data are presented as mean ± SD or median (range). Comparisons between groups were completed with Student t test or the Mann‐Whitney U test. Categorical variables were assessed with the Fisher exact test or χ2 test. For survival analysis, the log‐rank test and Kaplan‐Meier survival analysis were performed.
Results
During the study period, 188 patients (145 men, 77.1%; mean age, 62.9 ± 8.6 years) underwent CTI ablation and 68 patients had previous known history of AF. Patients of interest were thus the remaining 120 patients with no prior AF episodes documented pre‐AFL ablation, thereby comprising the chief study population (Figure 1). Mean follow‐up was 5 ± 2.4 years (95% confidence interval: 4.9‐5.8). Table 1 describes baseline characteristics and comorbidities.
Figure 1.
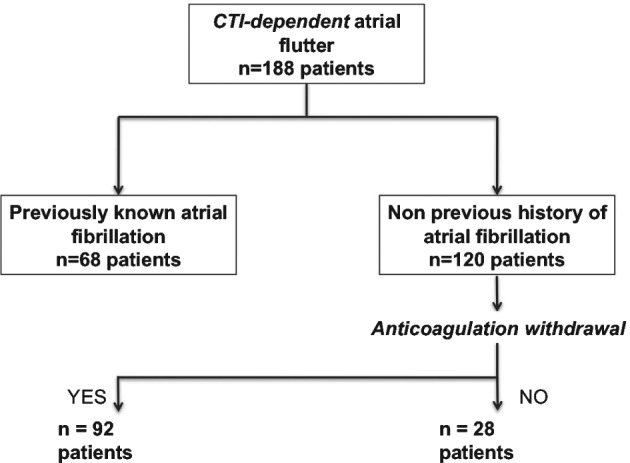
Study flowchart. Abbreviations: CTI, cavotricuspid isthmus.
Table 1.
Patient Characteristics and Comorbidities
| Total, N = 120 | Group 1, n = 92a | Group 2, n = 28b | P Value | |
|---|---|---|---|---|
| Male sex | 99 (82.5) | 77 (83.7) | 22 (78.6) | 0.71 |
| Age, y | 63.2 ± 1.1 | 67.3 ± 9.8 | 62.2 ± 7.6 | 0.62 |
| LVEF, % | 52.8 ± 9 | 53.3 ± 11.1 | 50.2 ± 14.6 | 0.68 |
| LVEF <35% | 11 (9.2) | 5 (5.4) | 6 (21.4) | |
| CHADS2 score | ||||
| Mean | 1.7 | 1.34 | 2.23 | 0.04 |
| 0 | 25 (20.8) | 25 (27.2) | 0 (0) | |
| 1 | 42 (35) | 34 (36.9) | 8 (28.6) | |
| 2 | 28 (23.3) | 18 (19.5) | 10 (35.7) | |
| 3 | 18 (15) | 13 (14.1) | 5 (17.9) | |
| 4 | 6 (5.0) | 1 (1.1) | 5 (17.9) | |
| 5 | 1 (0.8) | 1 (1.1) | 0 (0) | |
| HTN | 76 (63.3) | 54 (58.7) | 22 (78.6) | 0.10 |
| DM | 17 (14.2) | 7 (7.6) | 10 (35.7) | 0.20 |
| COPD | 21 (17.5) | 13 (14.1) | 8 (28.6) | 0.05 |
| Renal failure | 17 (14.2) | 10 (10.9) | 7 (25) | 0.12 |
| Previous stroke | 10 (8.0) | 4 (4.3) | 6 (21.4) | 0.05 |
| Arteriopathy | 11 (9.1) | 8 (8.7) | 3 (10.7) | 0.7 |
| CAD | 20 (16.7) | 14 (15.2) | 6 (21.4) | 0.54 |
| AMI | 14 (11.7) | 8 (8.7) | 6 (21.4) | |
| PCI‐stent | 8 (6.6) | 5 (5.4) | 3 (10.7) | |
| CABG | 7 (5.8) | 4 (4.3) | 3 (10.7) | |
| DCM | 7 (5.8) | 4 (4.3) | 3 (10.7) | 0.21 |
| Medications | ||||
| ACEI/ARB | 61 (50.8) | 44 (47.8) | 17 (60.7) | 0.2 |
| β‐Blocker | 32 (26.7) | 20 (21.7) | 12 (42.8) | 0.08 |
| Statin | 56 (46.6) | 49 (53.2) | 17 (60.7) | 0.44 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; AFL, atrial flutter; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHADS2, congestive HF, HTN, age ≥75 y, DM, prior stroke/TIA/TE; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; SD, standard deviation; TE, thromboembolism; TIA, transient ischemic attack.
Data are presented as n (%) or mean ± SD.
Group 1: No previously documented AF; anticoagulation stopped after successful AFL ablation.
Group 2: No previously documented AF; anticoagulation continued after successful AFL ablation.
Atrial Fibrillation in Follow‐up
At the end of the follow‐up period, 68 patients remained in sinus rhythm without AFL recurrence (7 patients) or de novo AF development (45 patients; 38%). Two patients underwent pulmonary‐vein isolation due to highly symptomatic episodes of paroxysmal AF, with no AF recurrences during follow‐up. Another patient refused trans‐septal puncture, so catheter ablation was not attempted.
Anticoagulation Management After Atrial Flutter Ablation
After a mean post‐ablation time of 12.3 ± 3.5 months (95% confidence interval: 8‐17), OAC was suspended in 92 patients. In the remaining 28, OAC was continued because of recurrent AFL (7 patients refused a new ablation procedure), documentation of AF during early follow‐up (11) and CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke/transient ischemic attack/thromboembolism) score >1, or valvular heart disease (6). In the remaining 4 patients, reasons for OAC continuation were not clearly specified.
Embolic Events in Patients After Atrial Flutter Ablation
Stroke (n = 11) or systemic embolism (n = 1) occurred in 12 patients (10%) following AFL ablation (Figure 2). Of all patients who suffered stroke/systemic embolism following AFL ablation, 5 of the 12 had documented AF after their AFL ablation. These patients harbored risk factors for stroke (CHADS2 score >1) and were receiving OAC. The embolic events occurred during subtherapeutic international normalized ratio (INR) levels (rank, 1.1‐1.9).
Figure 2.
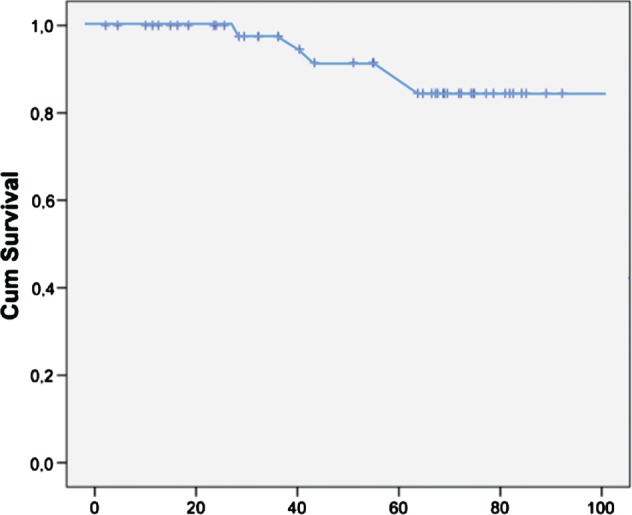
Kaplan‐Meier estimates for stroke‐free survival after anticoagulation withdrawal in patients without previous history of AF. The x‐axis shows the time in months after AFL ablation. The y‐axis shows the percentage of patients who are stroke‐free after AFL ablation. Abbreviations: AF, atrial fibrillation; AFL, atrial flutter; Cum, cumulative.
Interestingly, the other 7 ischemic strokes happened in patients without a prior documented history of AF, with a discontinuation of OAC post‐AFL ablation. Mean time (for stroke) following discontinuation of OAC was 35.7 ± 19.2 months. The CHADS2 score was 1 in all these patients but 1 (CHADS2 score = 2). Atrial fibrillation was diagnosed at admission in all patients but 2. All these patients were started on OAC with acenocoumarol following the diagnosis of stroke. Acenocoumarol was switched to dabigatran 150 mg bid in 1 patient after a new ischemic stroke despite a therapeutic INR of 2.6.
A further patient suffered a serious hemorrhagic event while receiving anticoagulant therapy (in the setting of an INR of 7.1 at admission) initiated after AF documentation.
Mortality
Death occurred in 19 patients (15.8%). Causes of death are summarized in Table 2.
Table 2.
Causes of Death
| Total | Group 1a | Group 2b | |
|---|---|---|---|
| CV causes | 6 | 2 | 4 |
| SCD | 1 | — | 1 |
| Stroke | 2 | 1 | 1 |
| HF | 1 | 1 | |
| AMI | 1 | — | 1 |
| Infective endocarditis | 1 | — | 1 |
| Non‐CV causes | 13 | 8 | 5 |
| Solid‐organ carcinoma | 5 | 3 | 2 |
| Infectious diseases | 1 | — | 1 |
| Acute abdominal illness | 1 | 1 | — |
| Others | 3 | 1 | 2 |
| Unknown | 3 | 3 | — |
Abbreviations: AMI, acute myocardial infarction; CV, cardiovascular; HF, heart failure; SCD, sudden cardiac death.
Group 1: No previously documented AF; anticoagulation stopped after successful AFL ablation.
Group 2: No previously documented AF; anticoagulation continued after successful AFL ablation.
Discussion
The main finding of this study is that following successful AFL ablation, patients are by no means free from suffering embolic complications in the long‐term follow‐up, even if no AF was detected prior to the procedure. During an average follow‐up period of 5 years after AFL ablation, 10% of our patients experienced embolic events, mainly stroke. This fact raises some important questions regarding contemporary management of anticoagulation therapy in patients with CTI‐dependent AFL.
Until recently, patients with both AFL and AF were grouped together in clinical trials and observational studies, and the signs identifying flutter remained hidden, being much less frequent than AF.1 Although we have direct information regarding the natural history, prognostic significance, and pharmacological treatment of AF, data are scarce regarding AFL, and extremely scarce on the incidence of systemic embolism in patients with AFL alone.6 It has been estimated that the incidence of systemic embolism during AFL is approximately one‐third that of AF.7 Although there are no studies allowing a formal assessment of the risk‐benefit ratio of various anticoagulation strategies in patients with lone AFL, in general the same guidelines have been adopted regarding anticoagulation therapy as in patients with AF.
In contrast to AF, identification of the CTI as key passage and low turning point of AFL spawned the development of catheter‐based approaches that substantially improve the disappointing results seen with antiarrhythmic medications. In more recent times, catheter ablation of CTI has been increasingly used as first‐line therapy.2, 3, 4
The risk of stroke and systemic embolism and their prevention following CTI ablation for AFL is an important unresolved issue. As the long‐term recurrence of AFL alone is estimated at about 5% to 10%, occurring primarily during the first 3 months post‐ablation, anticoagulant therapy can usually be ceased 4 to 6 weeks later if sinus rhythm is still present and there are no other indications for its continuation.8 However, the increasing evidence of subsequent development of AF following the procedure has hampered this success. The relationship between AF and AFL has long been recognized in clinical practice, and recent experimental and clinical studies have suggested an even closer mechanistic relationship, with similar pulmonary‐vein‐based and non–pulmonary‐vein‐based triggers responsible for onset of the great majority of AFL episodes via transitional AF.9, 10
In our series, the incidence of new‐onset AF following successful AFL ablation is approximately 40% of patients after a mean follow‐up of 5 years. These data confirm prior observations. Although the incidence of AF late post–AFL ablation is not clearly established, it seems to be time dependent, with reported rates of 17% to 22% at 6 months increasing to 50% at 2 years, and even 60% to 80% at 4 years.11, 12, 13 Discrepancies between published data, including the current study, could also likely reflect the intensity of monitoring, being even higher when loop recorders are implanted.14
Several risk factors for AF occurrence after AFL ablation have been identified in prior studies, with pre‐ablation AF being the most consistently described.15 The spectrum of risk factors (left ventricular ejection fraction, significant mitral regurgitation, increased left atrial size) is in fact associated with adverse left atrial remodeling and an elevated risk of incident AF regardless of AFL ablation.11, 16, 17, 18
Interestingly, it seems that patients with AF occurrence after AFL ablation, and in whom OAC is generally stopped following ablation, are at heightened risk for stroke, even prior to the formal diagnosis of AF occurrence. Atrial fibrillation may be asymptomatic and consequently subclinical, posing difficulties for diagnosis. The prevalence and prognostic value of subclinical AF have been difficult to assess, but there is increasing evidence that patients with this condition, in different clinical scenarios, are at higher risk of embolic events.19 Even more striking is the uncertainty about the temporal relationship between AF and stroke in these patients.20 Thus, given the embolic risk of patients referred for AFL ablation (mean CHADS2 score >1.5 in our entire population, with 45% ≥2), we suspect a meaningful risk for stroke is present that likely associates with these asymptomatic AF episodes.
There are very few references pertaining to stroke following long‐term AFL ablation in the literature. Beatrice et al reported on a series of 1121 patients who underwent AFL ablation. Two strokes (mean follow‐up, 2.1 years) were described, both occurring in patients with AF history (0.6%) who were not anticoagulated at the time of stroke despite atrial arrhythmias being documented post‐ablation.16
Further data of stroke following AFL ablation were reported by Thomson et al.15 After a mean follow‐up period of 40 months, stroke occurred in 8 of the 126 patients (6%; 7 ischemic and 1 hemorrhagic). Once again, 6 of these 8 patients (75%) had documented AF following their AFL ablation, but in the remaining 2 cases AF was diagnosed at admission. Most patients in this study remained on OAC, including 71% of those who experienced thromboembolic strokes, probably related to subtherapeutic INR. These data are corroborated in our present study, with subtherapeutic INR levels documented in the vast majority of our patients receiving anticoagulation who presented with stroke.
In light of these findings, several authors have advocated for the long‐term continuation of anticoagulants in certain patients following AFL ablation,15, 21 as there is strong evidence to maintain anticoagulants in patients after AF ablation, irrespective of symptoms.22
The usefulness of the CHADS2 and CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–74 years, sex category [women]) scores as tools for assessing the risk of stroke in patients following AFL ablation merits further exploration, although these scores have been shown to predict embolic events in patients other than those with established AF.23 Whether lifestyle and risk‐factor modification after AFL ablation may delay or obviate AF occurrence remains unanswered.24
Study Limitations
This study has several limitations, including its single‐center, observational, nonrandomized, and retrospective design. The main limitation is the relatively low number of patients for a study with a low frequency endpoint (systemic embolism and stroke).
Management of OAC varied according to referring‐physician preference; but, in accordance with guideline recommendations, it was withdrawn in patients without an AF history when no recurrence of AFL was documented. The wide range of time intervals may reflect the doubts regarding stopping OAC in the real world.
Methodology used to detect atrial arrhythmias post‐ablation, limited mainly to electrocardiography in the routinely programmed visits to the cardiology outpatient clinic, perhaps resulted in a low probability to detect asymptomatic episodes of AF. This can be extended to asymptomatic episodes of AF prior to AFL ablation.
Conclusion
Stroke and systemic embolism prevention following CTI ablation for AFL remains an unresolved issue. Patients with AFL history have a high rate of AF development during long‐term follow‐up and are by no means free from embolic complications. Although OAC withdrawal is recommended following successful CTI ablation in the absence of AF, these patients require close follow‐up to detect rhythms conducive to systemic embolism. Given the difficulties in detecting AF and the uncertainty about the temporal relationship between AF and stroke, patients with risk factors for stroke may need to continue OAC. The usefulness of the CHADS2 and CHA2DS2‐VASc scores as tools for assessing the risk of stroke in this population merits further exploration. Further studies are needed to clarify the best treatment strategy in these patients.
Acknowledgments
The authors gratefully acknowledge Rishi Puri, MBBS, PhD, FRACP (interventional cardiology and structural heart disease, Quebec Heart and Lung Institute, Laval Hospital) for his language editing and grammatical assistance.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Granada J, Uribe W, Chyou PH, et al. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol. 2000;36:2242–2246. [DOI] [PubMed] [Google Scholar]
- 2. Wellens HJ. Contemporary management of atrial flutter. Circulation. 2002;106:649–652. [DOI] [PubMed] [Google Scholar]
- 3. Shah DC, Haïseguarre M, Jaïs P, et al. Atrial flutter: contemporary electrophysiology and catheter ablation. Pacing Clin Electrophysiol. 1999;22:344–359. [DOI] [PubMed] [Google Scholar]
- 4. Da Costa A, Zarqane‐Sliman N, Romeyer‐Bouchard C, et al. Safety and efficacy of radiofrequency ablation of common atrial flutter in elderly patients: a single‐center prospective study. Pacing Clin Electrophysiol. 2003;26:1729–1734. [DOI] [PubMed] [Google Scholar]
- 5. Seidl K, Hauer B, Schwick NG, et al. Risk of thromboembolic events in patients with atrial flutter. Am J Cardiol. 1998;82:580–583. [DOI] [PubMed] [Google Scholar]
- 6. Ghali WA, Wasil BI, Brant R, et al. Atrial flutter and the risk of thromboembolism: a systematic review and meta‐analysis. Am J Med. 2005;118:101–107. [DOI] [PubMed] [Google Scholar]
- 7. Wood KA, Eisenberg SJ, Kalman JM, et al. Risk of thromboembolism in chronic atrial flutter. Am J Cardiol. 1997;79:1043–1047. [DOI] [PubMed] [Google Scholar]
- 8. García Cosío F, Pastor A, Núñez A, et al. Atrial flutter: an update [article in Spanish]. Rev Esp Cardiol. 2006;59:816–831. [PubMed] [Google Scholar]
- 9. Waldo AL, Feld GK. Inter‐relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779–786. [DOI] [PubMed] [Google Scholar]
- 10. Josephson ME. Atrial flutter and fibrillation In: Josephson ME, ed. Clinical Cardiac Electrophysiology: Techniques and Interpretations. 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2008:285–338. [Google Scholar]
- 11. Da Costa A, Romeyer C, Mourot S, et al. Factors associated with early atrial fibrillation after ablation of common atrial flutter: a single‐centre prospective study. Eur Heart J. 2002;23:498–506. [DOI] [PubMed] [Google Scholar]
- 12. Bertaglia E, Zoppo F, Bonso A, et al; Northeastern Italian Study on Flutter Ablation Investigators. Long‐term follow‐up of radiofrequency catheter ablation of atrial flutter: clinical course and predictors of atrial fibrillation occurrence. Heart. 2004;90:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post‐cavotricuspid isthmus ablation in patients with typical atrial flutter: left‐atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799–802. [DOI] [PubMed] [Google Scholar]
- 14. Mittal S, Pokushalov E, Romanov A, et al. Long‐term ECG monitoring using an implantable loop recorder for the detection of atrial fibrillation after cavotricuspid isthmus ablation in patients with atrial flutter. Heart Rhythm. 2013;10:1598–1604. [DOI] [PubMed] [Google Scholar]
- 15. Tomson TT, Kapa S, Bala R, et al. Risk of stroke and atrial fibrillation after radiofrequency catheter ablation of typical atrial flutter. Heart Rhythm. 2012;9:1779–1784. [DOI] [PubMed] [Google Scholar]
- 16. Brembilla‐Perrot B, Girerd N, Sellal JM, et al. Risk of atrial fibrillation after atrial flutter ablation: impact of AF history, gender, and antiarrhythmic drug medication. J Cardiovasc Electrophysiol. 2014;25:813–820. [DOI] [PubMed] [Google Scholar]
- 17. Reithmann C, Dorwarth U, Dugas M, et al. Risk factors for recurrence of atrial fibrillation in patients undergoing hybrid therapy for antiarrhythmic drug‐induced atrial flutter. Eur Heart J. 2003;24:1264–1272. [DOI] [PubMed] [Google Scholar]
- 18. Della Bella P, Riva S, Galimberti P. Should ablation of atrial flutter be discouraged in patients with documented atrial fibrillation? Cardiologia. 1999;44:439–442. [PubMed] [Google Scholar]
- 19. Healey J, Connolly SJ, Gold MR, et al; for the ASSERT Investigators . Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 20. Anastasio N, Frankel DS, Deyell MW, et al. Nearly uniform failure of atrial flutter ablation and continuation of antiarrhythmic agents (hybrid therapy) for the long‐term control of atrial fibrillation. J Interv Card Electrophysiol. 2012;35:57–61. [DOI] [PubMed] [Google Scholar]
- 21. Daoud EG, Glotzer TV, Wyse DG, et al; TRENDS Investigators . Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm. 2011;8:1416–1423. [DOI] [PubMed] [Google Scholar]
- 22. Camm AJ, Kirchhof P, Lip GY, et al; ESC Committee for Practice Guidelines . Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). European Heart Rhythm Association; European Association for Cardio‐Thoracic Surgery [published correction appears in Europace. 2011;13:1058]. Europace. 2010;12:1360–1420. [DOI] [PubMed] [Google Scholar]
- 23. Saliba W, Rennert G. CHADS2, CHA2DS2‐VASc, and long‐term stroke outcome in patients without atrial fibrillation. Neurology. 2013;80:1009–1017. [DOI] [PubMed] [Google Scholar]
- 24. Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]


