ABSTRACT
Background
Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equations estimate glomerular filtration rate (GFR) more accurately than the Modification of Diet in Renal Disease (MDRD) equation.
Hypothesis
New CKD‐EPI equations improve risk stratification in patients with non‐ST‐segment elevation acute coronary syndrome (NSTE‐ACS) and provide complementary information to the Global Registry of Acute Coronary Events (GRACE) risk score.
Methods
We studied 350 subjects (mean age, 68 ± 12 years; 70% male) with NSTE‐ACS. Estimated GFR was calculated using the MDRD and new CKD‐EPI equations based on serum creatinine (SCr) and/or cystatin C (CysC) concentrations obtained within 48 hours of hospital admission. The primary endpoint was all‐cause death during follow‐up.
Results
Over the study period (median, 648 days [interquartile range, 236–1042 days]), 31 patients died (0.05% events per person‐year). Decedents had poorer renal‐function parameters (P < 0.001). Both CysC‐based CKD‐EPI equations had the highest areas under the receiver operating characteristic curve for the prediction of all‐cause mortality. After multivariate adjustment, only CysC‐based CKD‐EPI equations were independent predictors of all‐cause mortality (CKD‐EPISCr ‐ CysC, per mL/min/1.73 m2: hazard ratio: 0.975, 95% confidence interval: 0.956‐0.994, P = 0.009; CKD‐EPICysC, per mL/min/1.73 m2: hazard ratio: 0.976, 95% confidence interval: 0.959‐0.993, P = 0.005). Reclassification analyses showed that only CysC‐based CKD‐EPI equations improved predictive accuracy of the GRACE risk score.
Conclusions
In patients with NSTE‐ACS, CysC‐based CKD‐EPI equations improved clinical risk stratification for mortality and added complementary prognostic information to the GRACE risk score.
Introduction
Renal dysfunction is common in patients with non–ST‐segment elevation acute coronary syndrome (NSTE‐ACS) and is associated with adverse in‐hospital and long‐term outcomes.1 Current clinical guidelines for the management of NSTE‐ACS patients recommend the use of estimated glomerular filtration rate (eGFR) equations to assess renal function.2 One of the most widely used is the abbreviated Modification of Diet in Renal Disease (MDRD) equation, which uses only 4 variables: serum creatinine (SCr) concentration, age, sex, and race.3 The prognostic value of this equation in NSTE‐ACS patients has been validated in numerous studies.4, 5, 6 However, it is increasingly recognized that the reliability and accuracy of this equation decreases in extremes of GFR, mainly at >60 mL/min/1.73 m2.7, 8, 9
In recent years, cystatin C (CysC) has emerged as a potential alternative to SCr for estimating renal function. In previous studies, it has consistently shown to be a better mortality risk marker than SCr in NSTE‐ACS patients.10, 11, 12 However, use of CysC, both as a marker of renal function and as a risk predictor, has been sparse, partly due to a loss of standardized reference values, higher cost for the analysis, and the lack of CysC‐based equations. More recently, the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) group has proposed 3 alternative equations to estimate GFR13, 14, 15; these newer equations apply different coefficients to the same 4 variables used in the MDRD equation (CKD‐EPISCr equation), add CysC values (CKD‐EPICysC equation), or combine it with SCr (CKD‐EPISCr‐CysC equation). These new CysC‐based CKD‐EPI equations have been reported to estimate GFR more accurately than the MDRD equation,16 and its use is currently recommended as a confirmatory test for renal dysfunction, namely in patients with “normal” creatinine level, muscle wasting, or chronic illness.17 However, despite its better performance to assess renal function, studies in the area of ACS remain scarce.
Therefore, given the importance of renal function for predicting outcomes in patients with NSTE‐ACS, the aim of the present study was to evaluate the performance of the new CKD‐EPI equations in predicting all‐cause mortality during follow‐up in patients admitted for NSTE‐ACS, and to compare them with the MDRD equation. Additionally, we intended to determine the added value of these equations in risk stratification compared with the Global Registry of Acute Coronary Events (GRACE) risk score.
Methods
Between September 2006 and June 2009, a total of 544 patients were admitted to our hospital with a diagnosis of high‐risk NSTE‐ACS (defined as ischemic symptoms lasting ≥10 minutes and occurring within 72 hours before admission and either ST‐segment deviation of ≥1 mm or elevated levels of a cardiac biomarker of necrosis).2 Of them, 194 patients (36%) were excluded. Patients who refused or were unable to provide the informed consent were excluded, as well as those who were on renal replacement therapy; were taking immunosuppressive agents; had evidence of hepatic dysfunction; had concomitant neoplastic, infectious, connective tissue, or inflammatory diseases; had deep vein thrombosis or pulmonary embolism; or had recent (<1 month) surgery or trauma. Furthermore, patients with a hospitalization for ACS, acutely decompensated heart failure (HF), or pulmonary embolism in the last 3 months, or any cardiac revascularization procedure 1 month before enrollment, were also excluded. Lastly, given that all blood samples were obtained within 24 hours of hospital admission, we could not include those patients who were admitted to hospital on a weekend (Friday 3 pm to Sunday 8 am). The study protocol conforms to the ethical principles outlined in the Declaration of Helsinki, and the local ethics committee approved the study. All patients provided written informed consent at inclusion. The current study was conducted in the same population of a previous study published by our group.18
Blood samples were collected for all patients within 24 hours from admission, processed, and stored at −80°C until the study analysis. Baseline clinical characteristics and data about in‐hospital management were prospectively recorded. All patients received standard management as recommended by contemporary guidelines. During the entire hospitalization period, clinical management decisions about each patient were made by the responsible cardiologist, who was unaware of the patient's CysC concentrations.
We calculated eGFR using the MDRD equation3 and the CKD‐EPI equations.13, 14, 15 Determination of CysC levels was performed using a BN ProSpec analyzer (Dade Behring GmbH, Liederbach, Germany). The intra‐assay and interassay coefficients of variation for CysC were 2.5% and 2.0%, respectively. For each patient, the GRACE 6‐month postdischarge mortality risk score was calculated.19
All patients were clinically followed during a median of 648 days (interquartile range, 236–1042 days), and a common final date for all was used as the criterion for study termination. The study endpoint was all‐cause mortality during follow‐up. Death was ascertained from available medical records and death certificates. If hospital records were ambiguous or unavailable, patients' families were interviewed through telephone contact.
Statistical Analysis
Continuous variables were tested for a normal distribution by the Kolmogorov‐Smirnov test. Normally distributed data are presented as mean ± SD, and non–normally distributed data are presented as median (interquartile range). Categorical variables are expressed as percentages. Categorized analyses were performed according to the occurrence of death during the follow‐up. Differences in baseline characteristics were compared using the t test or the Mann‐Whitney U test for continuous variables and the χ2 test for categorical variables.
To compare different accuracy of each equation for predicting all‐cause death, we performed discrimination and calibration analyses. The capacity for discrimination was analyzed by calculating the area under the receiver operating characteristic curve (AUC), and statistical comparisons of AUCs were performed using the DeLong method.20 A model with an AUC between 0.8 and 0.9 is considered to be a model with a good capacity for discrimination.21 To assess the calibration of both scores, we used the Hosmer‐Lemeshow goodness‐of‐fit test.22, 23 A P value > 0.05 indicates that the model is well adjusted to the data and therefore is a good predictor of patients' probability of death. The independent effect of eGFR equations on prognosis was calculated using a Cox multivariable regression analysis, incorporating as covariates age, left ventricular ejection fraction (LVEF), and GRACE 6‐month mortality risk score. Linearity assumption was tested using martingale residuals. Log‐cumulative hazard plots, time‐dependent covariates, and Schoenfeld residuals were used to evaluate adherence of the Cox proportional hazard assumptions. Moreover, the improvement in predictive accuracy of using the new CKD‐EPI equations over the MDRD equation and the GRACE risk score was evaluated by calculating the net reclassification improvement (NRI) as described by Pencina et al.24 The cumulative incidence of all‐cause death was estimated according to the Kaplan‐Meier method, and the log‐rank statistic was used for comparisons. All P values <0.05 were accepted as statistically significant. Statistical analysis was performed using SPSS version 15.0 (SPSS Inc., Chicago, IL).
Results
Clinical characteristics of the study population are shown in Table 1. The mean eGFR was 79 ± 23 mL/min/1.73 m2, 83 ± 26 mL/min/1.73 m2, 74 ± 21 mL/min/1.73 m2, and 78 ± 24 mL/min/1.73 m2 according to the CKD‐EPISCr‐CysC, CKD‐EPICysC, CKD‐EPISCr, and MDRD equations, respectively.
Table 1.
Clinical Characteristics of the Whole Study Population and as a Function of the Occurrence of Death
| Variables | Whole Population, N = 350 | Events, n = 31 | No Events, n = 318 | P Value |
|---|---|---|---|---|
| Age, y | 68 ± 12 | 80 ± 7 | 67 ± 11 | <0.001 |
| Male sex | 244 (70) | 17 (55) | 227 (71) | 0.055 |
| BMI, kg/m2 | 29 ± 4 | 30 ± 7 | 29 ± 4 | 0.442 |
| Medical history | ||||
| Current smoking | 91 (26) | 3 (10) | 88 (28) | 0.029 |
| HTN | 277 (79) | 28 (90) | 249 (78) | 0.114 |
| DM | 167 (48) | 19 (61) | 148 (47) | 0.117 |
| Hyperlipidemia | 219 (63) | 21 (68) | 198 (62) | 0.547 |
| LVEF, % | 60 (56–65) | 54 (40–60) | 60 (53–65) | 0.004 |
| Previous ACS | 127 (37) | 15 (48) | 112 (35) | 0.212 |
| Previous PCI | 107 (31) | 13 (42) | 94 (30) | 0.154 |
| Previous CABG | 27 (8) | 5 (16) | 22 (7) | 0.078 |
| Chronic HF | 15 (4) | 5 (16) | 10 (3) | 0.006 |
| AF/flutter | 36 (10) | 4 (13) | 32 (10) | 0.544 |
| Previous stroke | 33 (10) | 2 (6) | 31 (10) | 0.753 |
| PAD | 29 (8) | 4 (13) | 25 (8) | 0.309 |
| COPD | 31 (9) | 3 (10) | 28 (9) | 0.871 |
| Clinical status at admission | ||||
| SBP, mm Hg | 145 ± 28 | 145 ± 27 | 142 ± 33 | 0.966 |
| Heart rate, bpm | 80 ± 20 | 88 ± 21 | 79 ± 19 | 0.029 |
| Killip class II | 41 (12) | 10 (32) | 31 (10) | <0.001 |
| In‐hospital procedures and treatments | ||||
| Coronary angiography | 309 (89) | 25 (81) | 284 (89) | 0.146 |
| LM or 3‐vessel diseasea | 86 (28) | 10 (40) | 76 (27) | 0.157 |
| Femoral accessa | 120 (39) | 11 (44) | 109 (38) | 0.580 |
| Radial accessa | 220 (71) | 17 (68) | 203 (71) | 0.713 |
| Use of GP IIb/IIIa inhibitors | 17 (5) | 0 (0) | 17 (5) | 0.382 |
| PCI | 213 (61) | 16 (52) | 197 (62) | 0.260 |
| DES | 159 (45) | 12 (39) | 147 (46) | 0.373 |
| BMS | 73 (21) | 7 (23) | 66 (21) | 0.845 |
| CABG | 27 (8) | 2 (6) | 25 (8) | 0.780 |
| Medical treatment | 109 (31) | 13 (42) | 96 (30) | 0.178 |
| GRACE risk score | 110 ± 29 | 139 ± 22 | 107 ± 28 | <0.001 |
| Laboratory parameters | ||||
| Hgb, g/dL | 14 ± 2 | 13 ± 2 | 14 ± 2 | 0.010 |
| SCr, mg/dL | 0.95 (0.83–1.11) | 1.16 (0.90–1.46) | 0.94 (0.82–1.10) | <0.001 |
| CysC, mg/dL | 0.87 (0.73–1.08) | 1.14 (0.98–1.76) | 0.86 (0.72–1.04) | <0.001 |
| BUN, mg/dL | 39 (32–49) | 49 (41–81) | 38 (31–47) | <0.001 |
| eGFR, mL/min/1.73 m2 | ||||
| CKD‐EPIScr‐CysC | 79 ± 23 | 54 ± 21 | 83 ± 24 | <0.001 |
| CKD‐EPICysC | 83 ± 26 | 55 ± 24 | 89 ± 28 | <0.001 |
| CKD‐EPIScr | 74 ± 21 | 53 ± 19 | 78 ± 20 | <0.001 |
| MDRD | 78 ± 24 | 58 ± 20 | 80 ± 23 | <0.001 |
| Medical treatment at dischargeb | ||||
| β‐Blocker | 307 (88) | 25 (89) | 282 (89) | 1.000 |
| ACEI/ARB | 301 (87) | 24 (86) | 277 (87) | 0.768 |
| Aldosterone antagonist | 22 (6) | 2 (7) | 20 (6) | 0.679 |
| Statin | 334 (97) | 26 (93) | 308 (97) | 0.563 |
| ASA | 335 (97) | 27 (96) | 308 (97) | 0.825 |
| P2Y12 inhibitor | 290 (84) | 22 (79) | 268 (84) | 0.419 |
| Anticoagulation | 25 (7) | 2 (7) | 23 (7) | 1.000 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ACS, acute coronary syndrome; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ASA, aspirin; BMI, body mass index; BMS, bare‐metal stent; CABG, coronary artery bypass grafting; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; CysC, cystatin C; DES, drug‐eluting stent; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; GP, glycoprotein; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; Hgb, hemoglobin; HTN, hypertension; IQR, interquartile range; LM, left main; LVEF, left ventricular ejection fraction; MDRD, Modification of Diet in Renal Disease; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SCr, serum creatinine; SD, standard deviation.
Data are expressed as n (%), mean ± SD, or median (IQR), as appropriate.
Referred to patients undergoing coronary angiography. b Referred to patients alive at discharge (347 patients).
Over the study period, 31 patients died (0.05% events per person‐year). Table 1 presents the distribution of clinical characteristics and laboratory parameters in accordance with the occurrence of death. Decedents were older, less likely to be smokers, and more often had chronic HF. Moreover, they had higher SCr and CysC concentrations, lower hemoglobin concentrations, and lower LVEF. The GRACE 6‐month mortality risk scores were higher among deceased patients (139 ± 22 vs 107 ± 28; P < 0.001). There were no differences between groups with respect to the in‐hospital management and treatment at discharge. Regarding renal function, patients who died had lower eGFR, regardless of the equation used.
Figure 1 shows the all‐cause mortality rate according to eGFR categories using the different equations. A stepwise increase in mortality rate was seen with declining eGFR category for all equations. Both CysC‐based CKD‐EPI equations provided the highest all‐cause mortality rate in patients with eGFR <30 mL/min/1.73 m2. Moreover, the MDRD equation did not show difference between the all‐cause mortality rates in the 2 lowest eGFR categories.
Figure 1.
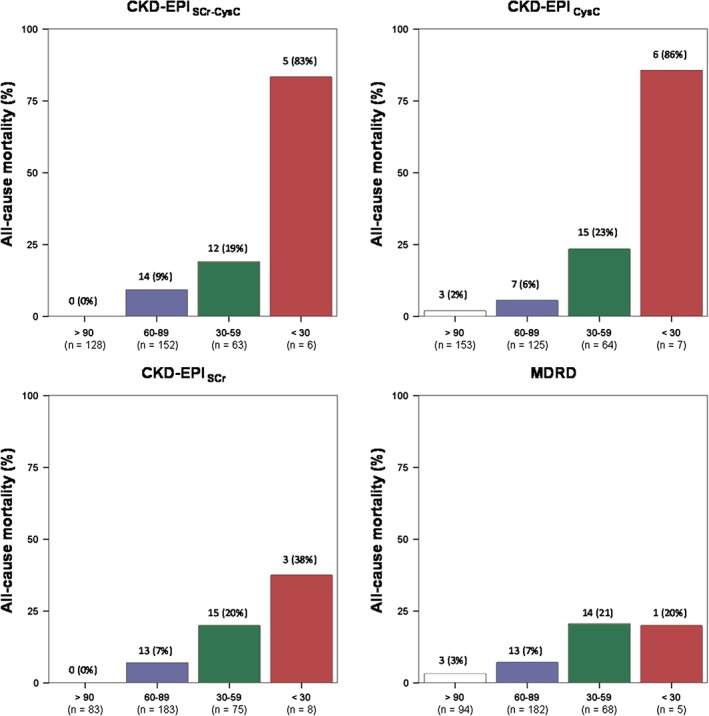
Rate of all‐cause mortality according to estimated GFR categories using different equations. Abbreviations: CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CysC, cystatin C; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; SCr, serum creatinine.
As detailed in Table 2, only CysC‐based CKD‐EPI equations showed a good discrimination capacity (AUC >0.80) for predicting all‐cause death in the whole population. Moreover, AUCs for all CKD‐EPI equations were higher (or trend to be higher) than AUC for the MDRD equation, both in the whole population and in patients with eGFR ≥60 mL/min/1.73 m2. By contrast, all eGFR equations showed similar discrimination capacity in the subgroup of patients with eGFR <60 mL/min/1.73 m2. The analyses showed acceptable calibration for both scores in the whole population and in all ACS subgroups (Hosmer‐Lemeshow P values were >0.05 in all cases).
Table 2.
Performance of eGFR Equations for Prediction of Death According to Kidney Function Status
| Variable | AUC (95% CI) | P Valuea | H‐L P Value |
|---|---|---|---|
| CKD‐EPISCr‐CysC | |||
| All patients | 0.82 (0.77‐0.86) | <0.05 | 0.247 |
| eGFR ≥60 mL/min/1.73 m2 | 0.78 (0.72‐0.82) | <0.05 | 0.294 |
| eGFR <60 mL/min/1.73 m2 | 0.71 (0.59‐0.81) | 0.18 | 0.212 |
| CKD‐EPICysC | |||
| All patients | 0.82 (0.77‐0.86) | <0.05 | 0.361 |
| eGFR ≥60 mL/min/1.73 m2 | 0.71 (0.66‐0.77) | 0.10 | 0.308 |
| eGFR <60 mL/min/1.73 m2 | 0.66 (0.53‐0.77) | 0.26 | 0.120 |
| CKD‐EPISCr | |||
| All patients | 0.77 (0.74‐0.83) | 0.08 | 0.590 |
| eGFR ≥60 mL/min/1.73 m2 | 0.74 (0.68‐0.79) | <0.05 | 0.576 |
| eGFR <60 mL/min/1.73 m2 | 0.65 (0.54‐0.75) | 0.22 | 0.875 |
| MDRD | |||
| All patients | 0.75 (0.70‐0.79) | — | 0.423 |
| eGFR ≥60 mL/min/1.73 m2 | 0.69 (0.63‐0.74) | — | 0.470 |
| eGFR <60 mL/min/1.73 m2 | 0.72 (0.60‐0.81) | — | 0.624 |
Abbreviations: AUC, area under the curve; CI, confidence interval; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CysC, cystatin C; eGFR, estimated glomerular filtration rate; H‐L, Hosmer‐Lemeshow; MDRD, Modification of Diet in Renal Disease; SCr, serum creatinine.
Comparison between MDRD Study equation and the other equations.
Table 3 details the univariate and multivariate Cox regression analysis for the prediction of the study endpoint. All equations were associated with all‐cause mortality. However, after multivariate adjustment (incorporating age, hemoglobin concentration, LVEF, and GRACE 6‐month mortality risk score), only both CysC‐based CKD‐EPI equations remained as independent predictors of the study endpoint.
Table 3.
Cox Regression Risk Analysis for Prediction of Death
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| CKD‐EPIScr‐CysC (per mL/min/1.73 m2) | 0.952 (0.937‐0.968) | <0.001 | 0.975 (0.956‐0.994) | 0.009 |
| CKD‐EPICysC (per mL/min/1.73 m2) | 0.957 (0.943‐0.971) | <0.001 | 0.976 (0.959‐0.993) | 0.005 |
| CKD‐EPISCr (per mL/min/1.73 m2) | 0.955 (0.939‐0.972) | <0.001 | 0.989 (0.967‐1.011) | 0.054 |
| MDRD (per mL/min/1.73 m2) | 0.960 (0.944‐0.977) | <0.001 | 0.992 (0.971‐1.013) | 0.064 |
Abbreviations: CI, confidence interval; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CysC, cystatin C; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio; LVEF, left ventricular ejection fraction; MDRD, Modification of Diet in Renal Disease; SCr, serum creatinine.
Adjusted for age, LVEF (%), and GRACE 6‐month mortality risk score.
The CKD‐EPI and MDRD equations were tested separately and multivariable.
Reclassification analyses show that the addition of all CKD‐EPI equations to the MDRD equation was associated with a significant improvement in the predictive accuracy (NRI: 24%, P < 0.001, for CKD‐EPISCr‐CysC; NRI: 26%, P = 0.001, for CKD‐EPICysC; and NRI: 20%, P = 0.032, for CKD‐EPISCr; see Supporting Information, tables 1, 2, 3, in the online version of this article). It was particularly reflected in the percentage of no‐events correctly reclassified (27%, 26%, and 23%, respectively). Moreover, the addition of CysC‐based CKD‐EPI equations (but not SCr‐based CKD‐EPI or MDRD equations) to the GRACE risk score improved the predictive accuracy (NRI: 19%, P < 0.052, for CKD‐EPISCr‐CysC; and NRI: 19%, P = 0.048, for CKD‐EPICysC; see Supporting Information, tables 4 and 5, in the online version of this article).
Finally, Kaplan‐Meier survival analyses showed the complementary prognostic value of all equations and the GRACE 6‐month mortality risk score for the prediction of all‐cause mortality. As detailed in Figure 2, patients with a GRACE risk score <118 (low and intermediate risk) and an eGFR ≥60 mL/min/1.73 m2 had the lowest risk of experiencing adverse outcomes, regardless of the equation used (all log‐rank test P < 0.001). Interestingly, patients with GRACE risk score ≥119 (high risk) and eGFR <60 mL/min/1.73 m2, using both CysC‐based CKD‐EPI equations, showed the lowest survival rate.
Figure 2.
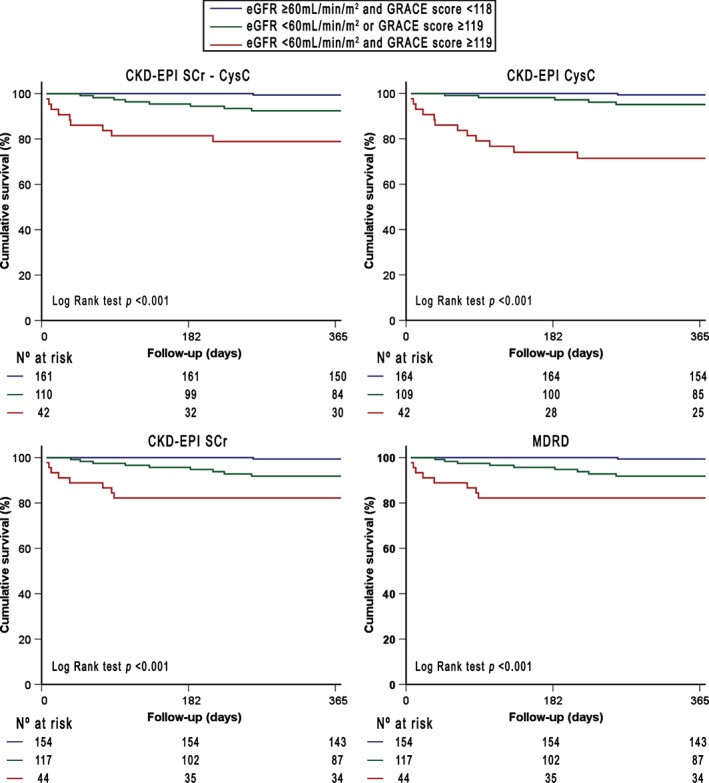
Kaplan‐Meier survival curves for death as a function of GFR estimated using different equations and GRACE 6‐month postdischarge risk score. Abbreviations: CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CysC, cystatin C; GFR, glomerular filtration rate; GRACE, Global Registry of Acute Coronary Events; MDRD, Modification of Diet in Renal Disease; SCr, serum creatinine.
Discussion
Accurate assessment of renal function plays a major role in NSTE‐ACS patients. In the present study, renal dysfunction, regardless of the eGFR equation used, was associated with increased all‐cause mortality in patients with NSTE‐ACS. Interestingly, only both CysC‐based CKD‐EPI equations showed good discriminative power and outperformed the most widely used eGFR equation, the MDRD equation. This higher discriminative power was mainly related to a better performance of both CysC‐based equations in patients with eGFR >60 mL/min/1.73 m2; that corresponds to the eGFR range where the MDRD equation shows less accuracy.7, 8, 9 Compared with the MDRD equation, all CKD‐EPI equations accurately reclassified a significant percentage of patients into a more appropriate risk category. However, only both CysC‐based CKD‐EPI equations are independent predictors after multivariate adjustment and add predictive value to the most widely used prognostic score in these patients, the GRACE risk score. Therefore, we provide evidence that the use of CysC‐based CKD‐EPI equations improves the role of eGFR in risk stratification, as judged by the risk of death from any cause. Our results may be useful to clinicians to guide risk‐adjusted management and to clinical trialists alike and support the need to reconsider current standards for how kidney function is assessed in NSTE‐ACS patients.
The SCr has long been the basis for calculating the eGFR. However, despite standardization, eGFR based on SCr remains relatively imprecise owing to variation in non‐GFR determinants of SCr.25 In recent years, CysC has arisen as an interesting marker of renal function. Cystatin C is a low‐molecular‐weight, nonglycosylated protein that is synthesized by all nucleated cells and released into the blood at a relatively constant rate. It is freely filtered by the glomerulus, is reabsorbed and catabolized in the proximal tubular cells without secretion, and does not appear in the urine.26 Compared with SCr, it is less affected by age, sex, muscle mass, or diet.27 Liver disease, hyperthyroidism, and high doses of corticosteroids have been described to increase its production.28, 29, 30, 31 In our study, patients with these characteristics were excluded. Therefore, given its properties, CysC has been proposed as a more reliable marker of renal function than SCr.26
The prognostic role of CysC has been studied specifically in NSTE‐ACS patients. Although the precise mechanism remains to be clarified, elevated levels of plasma CysC have been associated with adverse outcomes in these patients, including in‐hospital cardiovascular adverse outcomes,32 long‐term mortality,10, 11, 12 and bleeding complications.33 In these studies, the accuracy in predicting adverse outcomes of elevated CysC values outperformed other widely used parameters for estimating renal function (SCr or SCr‐based equations). However, despite the favorable properties of CysC, the lack of CysC‐based eGFR equations has limited its use in clinical practice. More recently, the CKD‐EPI group has developed newer eGFR equations based on CysC values.13, 14, 15 Compared with SCr‐based equations, CysC‐based equations provide a more precise and accurate eGFR in patients with near‐normal renal function.16 Therefore, the use of CysC‐based equations is currently recommended to confirm the presence of renal dysfunction.17
Recent studies have focused on evaluating the prognostic value of these new equations in different clinical scenarios. Our group has previously demonstrated that both CysC‐based CKD‐EPI equations outperformed the MDRD equation and added complementary information to natriuretic peptides for predicting adverse outcomes in acute HF patients.34, 35 In patients with acute myocardial infarction, Abu‐Assi et al36 reported that both CysC‐based CKD‐EPI equations were the most accurate for predicting in‐hospital mortality, rather than the MDRD and CKD‐EPISCr equations. Almeida et al37 found that the CKD‐EPICysC equation revealed the highest discriminative performance in predicting long‐term mortality in patients with ACS and added predictive value to the GRACE risk score. However, unlike this study, we have focused on patients with NSTE‐ACS. These patients tend to be older, have more comorbidity (including worse renal function), and have worse long‐term prognosis compared with ST‐segment elevation ACS patients.38 Therefore, accurate long‐term risk stratification of NSTE‐ACS patients is even more crucial. In contrast with these results, Åkerblom et al12 failed to demonstrate a benefit in predicting long‐term mortality with a CysC‐based equation in the large ACS population enrolled in the Platelet Inhibition and Patient Outcomes (PLATO) study. The possible explanation may be related to the use of a different CysC‐based equation in the PLATO study. In fact, mean eGFR using CysC in the PLATO study (104 mL/min/1.73 m2) differs significantly from mean eGFR in the Almeida et al study (84 mL/min/1.73 m2) and in our study (83 mL/min/1.73 m2).
Given the well‐established prognostic role of renal dysfunction in NSTE‐ACS patients, our results may be explained by better assessment of renal function using the CysC‐based CKD‐EPI equations. However, though less defined than those of SCr, non‐GFR determinants of CysC also exist and the possibility that CysC‐based equations are capturing prognosis information from these nonrenal factors should be considered. In this perspective, previous studies found a significant correlation between the levels of inflammatory markers and CysC.32 The hypothesized mechanism is that CysC also may be a marker of an ongoing inflammatory process, which could explain its role in cardiovascular‐risk prediction. This concurs with studies that found a relationship between CysC levels and cardiovascular outcomes in patients without kidney disease.39, 40 In addition, the confounding effect of non‐GFR determinants of SCr (such as muscle mass, diet, and physical activity) can also contribute to the best performance of CysC‐based equations in risk stratification, given that SCr concentrations could be lower than expected for level of renal dysfunction in frail patients, who are more likely to die.41 In this way, Sanchis et al found that CysC outperformed SCr as a frailty marker in patients after ACS.42 Unfortunately, we cannot distinguish among these possibilities for the enhanced association between CysC‐based equation and the risk of death in NSTE‐ACS patients, because the strength of our study is in establishing associations, with limited ability to determine causal mechanisms.
Study Limitations
Our study has several limitations. The relatively small size of our single‐center sample and the lack of direct measure of GFR should be considered the main limitations of this research, especially because these new eGFR equations have not been completely validated in the setting of ACS. The blood samples used to measure SCr and CysC were collected during the first 48 hours after hospital admission and may not reflect a steady state of renal function. Furthermore, we did not have serial renal‐function measurements during follow‐up or a group of patients with which to externally validate our results.
Conclusion
The new CKD‐EPI equations based on CysC provide an apparently improved method for assessing long‐term all‐cause mortality risk in patients admitted with NSTE‐ACS, compared with the SCr‐based eGFR equations. Moreover, these new equations improved the predictive value of the GRACE risk score. These results further highlight the value of CysC‐based equations as a risk‐stratification tool in NSTE‐ACS patients and support future research to achieve a better understanding of the mechanisms of renal dysfunction on cardiovascular outcomes and provide additional therapeutic options to reduce the risk in these patients.
Supporting information
Table S1
Table S2
Table S3
Table S4
Table S5
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Al Suwaidi J, Reddan DN, Williams K, et al; PARAGON‐A Investigators. Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation. 2002;106:974–980. [DOI] [PubMed] [Google Scholar]
- 2. Hamm CW, Bassand JP, Agewall S, et al; ESC Committee for Practice Guidelines . ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 3. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate [published correction appears in Ann Intern Med. 2008;149:519]. Ann Intern Med. 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 4. Reddan DN, Szczech L, Bhapkar MV, et al. Renal function, concomitant medication use and outcomes following acute coronary syndromes. Nephrol Dial Transplant. 2005;20:2105–2112. [DOI] [PubMed] [Google Scholar]
- 5. Gibson CM, Dumaine RL, Gelfand EV, et al. Association of glomerular filtration rate on presentation with subsequent mortality in non–ST‐segment elevation acute coronary syndrome: observations in 13 307 patients in five TIMI trials. Eur Heart J. 2004;25:1998–2005. [DOI] [PubMed] [Google Scholar]
- 6. Mielniczuk LM, Pfeffer MA, Lewis EF, et al. Estimated glomerular filtration rate, inflammation, and cardiovascular events after an acute coronary syndrome. Am Heart J. 2008;155:725–731. [DOI] [PubMed] [Google Scholar]
- 7. Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the Modification of Diet in Renal Disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–2757. [DOI] [PubMed] [Google Scholar]
- 8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. [DOI] [PubMed] [Google Scholar]
- 9. Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the Modification of Diet in Renal Disease and Cockcroft‐Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. [DOI] [PubMed] [Google Scholar]
- 10. Jernberg T, Lindahl B, James S, et al. Cystatin C: a novel predictor of outcome in suspected or confirmed non–ST‐segment elevation acute coronary syndrome. Circulation. 2004;110:2342–2348. [DOI] [PubMed] [Google Scholar]
- 11. Manzano‐Fernández S, López‐Cuenca A, Januzzi JL, et al. Usefulness of β‐trace protein and cystatin C for the prediction of mortality in non–ST‐segment elevation acute coronary syndromes. Am J Cardiol. 2012;110:1240–1248. [DOI] [PubMed] [Google Scholar]
- 12. Akerblom Å, Wallentin L, Siegbahn A, et al. Cystatin C and estimated glomerular filtration rate as predictors for adverse outcome in patients with ST‐elevation and non–ST‐elevation acute coronary syndromes: results from the Platelet Inhibition and Patient Outcomes study. Clin Chem. 2012;58:190–199. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011;155:408]. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C [published corrections appear in N Engl J Med, 2012;367:681 and N Engl J Med. 2012;367:2060]. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Disease Kidney: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 18. Flores‐Blanco PJ, López‐Cuenca Á, Januzzi JL, et al. Major bleeding risk prediction using Chronic Kidney Disease Epidemiology Collaboration and Modification of Diet in Renal Disease equations in acute coronary syndrome. Eur J Clin Invest. 2015;45:385–393. [DOI] [PubMed] [Google Scholar]
- 19. Eagle KA, Lim MJ, Dabbous OH, et al; GRACE Investigators . A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6‐month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. [DOI] [PubMed] [Google Scholar]
- 20. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 21. López de Ullibarri Galparsoro I, Pita Fernández S. Curvas ROC [article in Spanish]. Cad Aten Primaria. 1998;5:229–235. [Google Scholar]
- 22. Lemeshow S, Hosmer DW Jr. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. [DOI] [PubMed] [Google Scholar]
- 23. Lemeshow S, Klar J, Teres D. Outcome prediction for individual intensive care patients: useful, misused, or abused? Intensive Care Med. 1995;21:770–776. [DOI] [PubMed] [Google Scholar]
- 24. Pencina MJ, d'Agostino RB Sr, d'Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 25. Verhave JC, Gansevoort RT, Hillege HL, et al. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol. 2004;15:1316–1322. [PubMed] [Google Scholar]
- 26. Taglieri N, Koenig W, Kaski JC. Cystatin C and cardiovascular risk. Clin Chem. 2009;55:1932–1943. [DOI] [PubMed] [Google Scholar]
- 27. Newman DJ. Cystatin C. Ann Clin Biochem. 2002;39(part 2):89–104. [DOI] [PubMed] [Google Scholar]
- 28. Chu SC, Wang CP, Chang YH, et al. Increased cystatin C serum concentrations in patients with hepatic diseases of various severities. Clin Chim Acta. 2004;341:133–138. [DOI] [PubMed] [Google Scholar]
- 29. Fricker M, Wiesli P, Brändle M, et al. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63:1944–1947. [DOI] [PubMed] [Google Scholar]
- 30. Filler G, Bökenkamp A, Hofmann W, et al. Cystatin C as a marker of GFR—history, indications, and future research. Clin Biochem. 2005;38:1–8. [DOI] [PubMed] [Google Scholar]
- 31. Bökenkamp A, Laarman CA, Braam KI, et al. Effect of corticosteroid therapy on low‐molecular‐weight protein markers of kidney function. Clin Chem. 2007;53:2219–2221. [DOI] [PubMed] [Google Scholar]
- 32. García Acuña JM, González‐Babarro E, Grigorian Shamagian L, et al. Cystatin C provides more information than other renal function parameters for stratifying risk in patients with acute coronary syndrome [article in English, Spanish]. Rev Esp Cardiol. 2009;62:510–519. [DOI] [PubMed] [Google Scholar]
- 33. López‐Cuenca Á, Manzano‐Fernández S, Marín F, et al. β‐Trace protein and cystatin C as predictors of major bleeding in non–ST‐segment elevation acute coronary syndrome. Circ J. 2013;77:2088–2096. [DOI] [PubMed] [Google Scholar]
- 34. Manzano‐Fernández S, Flores‐Blanco PJ, Pérez‐Calvo JI, et al. Comparison of risk prediction with the CKD‐EPI and MDRD equations in acute decompensated heart failure. J Card Fail. 2013;19:583–591. [DOI] [PubMed] [Google Scholar]
- 35. Flores‐Blanco PJ, Manzano‐Fernández S, Pérez‐Calvo JI, et al. Cystatin C–based CKD‐EPI equations and N‐terminal pro‐B‐type natriuretic peptide for predicting outcomes in acutely decompensated heart failure. Clin Cardiol. 2015;38:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abu‐Assi E, Raposeiras‐Roubin S, Riveiro‐Cruz A, et al. Creatinine‐ or cystatin C‐based equations to estimate glomerular filtration rate in acute myocardial infarction: a disparity in estimating renal function and in mortality risk prediction. Int J Cardiol. 2013;168:4300–4301. [DOI] [PubMed] [Google Scholar]
- 37. Almeida I, Caetano F, Barra S, et al. Estimating glomerular filtration rate in acute coronary syndromes: different equations, different mortality risk prediction. Eur Heart J Acute Cardiovasc Care. 2015. doi: 10.1177/2048872615576219. [DOI] [PubMed] [Google Scholar]
- 38. Chan MY, Sun JL, Newby LK, et al. Long‐term mortality of patients undergoing cardiac catheterization for ST‐elevation and non–ST‐elevation myocardial infarction [published correction appears in Circulation. 2009;120:e28]. Circulation. 2009;119:3110–3117. [DOI] [PubMed] [Google Scholar]
- 39. Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. [DOI] [PubMed] [Google Scholar]
- 40. Koenig W, Twardella D, Brenner H, et al. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem. 2005;51:321–327. [DOI] [PubMed] [Google Scholar]
- 41. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanchis J, Núñez E, Ruiz V, et al. Usefulness of clinical data and biomarkers for the identification of frailty after acute coronary syndromes. Can J Cardiol. 2015;31:1462–1468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Table S5


