ABSTRACT
Background
Contrast‐induced nephropathy (CIN) is an acute kidney injury (AKI) defined as serum creatinine (sCr) increase 48 to 72 hours after contrast administration. Because most subjects undergoing invasive cardiac procedures are discharged within 24 hours, sCr is unsuitable for CIN detection.
Hypothesis
In the present study we tested the hypothesis that neutrophil gelatinase‐associated lipocalin (NGAL) is superior compared with sCr and other established nephropathy markers in early CIN diagnosis after elective invasive cardiac procedures.
Methods
Serum creatinine, urine creatinine, serum cystatin C, urine albumin, urine NGAL (uNGAL), and plasma NGAL were measured at 0, 6, 24, and 48 hours after contrast administration in 100 elective invasive cardiac procedures. Estimated glomerular filtration rate and albumin‐to‐creatinine ratio were calculated. Changes from baseline were considered statistically significant at P < 0.05 and clinically significant when > the biomarker's reference change value. Participants were divided into those with and without clinically significant uNGAL changes (uNGAL positive and negative for AKI, respectively).
Results
Thirty‐three individuals were uNGAL positive for AKI. Serum cystatin C changes were statistically and clinically nonsignificant in both groups. Serum creatinine and plasma NGAL were statistically but not clinically elevated 48 hours postcatheterization in the AKI group. Except for contrast volume (higher in AKI group), groups were comparable at baseline (P not significant) regarding cardiovascular risk factors, coronary heart disease, coronary interventions performed, and renal biomarkers. Baseline uNGAL was significantly correlated to estimated glomerular filtration rate and albumin‐to‐creatinine ratio.
Conclusions
Urine NGAL is potentially superior compared with conventional nephropathy markers in early CIN diagnosis after elective invasive cardiac procedures. Definition of clinically significant uNGAL changes with reference change value is probably a valuable supplement to statistically defined significant variations.
Introduction
Contrast‐induced nephropathy (CIN) after invasive cardiac procedures (cardiac catheterization, coronary angiography, or/and percutaneous coronary intervention [PCI]) is a common cause of hospital‐acquired acute kidney injury (AKI).1, 2 The first 24 hours after contrast‐medium administration appears to be crucial for the development of CIN.3 However, CIN is currently defined as a ≥0.5mg/dL or ≥25% rise in serum creatinine (sCr) 48 to 72 hours after contrast exposure.3 This is because sCr is not quite sensitive to acute renal function changes; in fact, it peaks 3 to 5 days after contrast administration and returns to baseline within 1 to 3 weeks.4 Given that most subjects undergoing invasive cardiac procedures are typically discharged within 24 hours and only occasionally after 48 hours, sCr is rather unsuitable for CIN diagnosis in these individuals. Early detection of CIN after invasive cardiac procedures is important for the selection of patients needing extended hospitalization (>24–48 hours) for closer renal, metabolic, and fluid control.5
There are some additional limitations in sCr‐based CIN diagnosis. Serum Cr is highly affected by age, sex, muscle mass, diet, medications, and hydration status. Besides, it is a marker of glomerular filtration rate (GFR) and not a direct marker of tubular damage (such as occurs in CIN/AKI), and substantial increases in sCr can be observed in cases of renal hypoperfusion (resulting in prerenal azotemia) even with structurally intact kidneys.6, 7, 8, 9 For the aforementioned reasons, the use of sCr for the diagnosis of CIN/AKI is now considered imperfect because nontubular injuries may be misclassified as CIN/AKI, whereas the absence of changes in sCr does not exclude tubular damage.10
Novel biomarkers of renal tubular damage aim to facilitate the early differential diagnosis between intrinsic AKI and prerenal azotemia and help the stratification of patients at risk. In contrast to conventional markers, such as sCr or serum cystatin C (sCysC), they do not reflect kidney function but rather structural damage of the kidney cells. Of many promising molecules, neutrophil gelatinase–associated lipocalin (NGAL) has generated considerable attention.11 It is a 25‐kDa protein, a member of the lipocalin family, and was originally isolated from the supernatant of activated human neutrophils.12 As a low‐molecular‐weight protein (LMWP) and due to its resistance to degradation, plasma NGAL (pNGAL) is readily excreted and can be detected in urine. Filtered NGAL is normally reabsorbed by megalin‐faciliated endocytosis in the proximal tubules.13 Consequently, the proximal tubular injury occurring in CIN/AKI reduces reabsorption and increases NGAL concentration in urine. Moreover, NGAL is produced in renal tubules in response to nephrotoxic or ischemic stimuli and it is secreted in urine.14, 15 Thus, urine NGAL (uNGAL) might serve as an early marker of CIN/AKI after invasive cardiac procedures. However, standard reference values have not been defined, as its concentration can be elevated in patients with chronic kidney disease and/or albuminuria.16, 17 These limitations can be bypassed by defining clinically significant uNGAL changes taking into account baseline renal function and calculating the reference change value (RCV) based on the biological and analytical variation of uNGAL measurement.18
Building on the previous reports, the present study sought to assess the role of NGAL as an early marker of CIN/AKI after elective invasive cardiac procedures, compared with traditional markers (sCr, sCysC, estimated GFR [eGFR], and urine albumin‐to‐Cr ratio [ACR]). The effect of baseline renal function on uNGAL levels and the incidence of uNGAL RCV‐based tubular damage were also examined.
Methods
Participants and Study Design
All adults (age >18 years) undergoing elective invasive cardiac procedures at our institution over a 6‐month period were enrolled. Written informed consent was obtained from all, and the study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of our institution. Serial morning spot urine and blood samples were collected at baseline (prior to cardiac procedure) and at 6, 24, and 48 hours after contrast administration.
Biochemical Analyses
Serum and urine Cr were measured with a modified Jaffe method on an ARCHITECT ci16200 analyzer (Abbott, Chicago, IL). The analytical coefficient of variation (CVa) of the assay in our laboratory is 2.8%.
Serum CysC was measured with a turbidimetric assay (Sentinel, Milan, Italy) on the same analyzer. The CVa of the assay in our laboratory is 2.0%.
Estimated GFR was calculated with the Modification of Diet in Renal Disease (MDRD) formula (eGFR_MDRD = 175 × sCr−1.154 × age−0.203 [× 0.742 if female]) and the CysC‐based formula (eGFR_CysC = 71/sCysC1.28).
Urine albumin was measured with a turbidimetric assay on the same analyzer and ACR was calculated.
Urine NGAL was also measured on the same analyzer using a 2‐step sandwich immunoassay with chemiluminescent signal detection. This assay utilizes high‐affinity mouse anti‐NGAL antibodies generated toward distinct, nonoverlapping NGAL epitopes. The functional sensitivity of the assay is 2 ng/mL with a range that extends up to 1500 ng/mL. The CVa of the assay in our laboratory is 4.5%.
Plasma NGAL was measured with a manual enzyme‐linked immunosorbent assay (Bioporto, Gentofte, Denmark).
Statistical Analysis
Data were analyzed using SPSS statistical software, version 19 (IBM Corp, Armonk, NY). Continuous variables are presented as mean ± SD and categorical variables as n (%). The t test (paired or unpaired, as appropriate) was used to evaluate differences in continuous variables among or between groups once normality was demonstrated (Kolmogorov‐Smirnov or Shapiro‐Wilk test); otherwise, a nonparametric test (Wilcoxon or Mann‐Whitney, respectively) was used. Differences in categorical variables were analyzed using the χ2 test (or Fisher exact test, if applicable). A general linear model for repeated measures or a nonparametric test (2‐way ANOVA) was used to identify differences within serial measurements of the continuous variables at the 4 time points. Correlations between continuous variables were determined using the Pearson r coefficient. Differences were considered statistically significant for a 2‐sided P value <0.05.
Nevertheless, statistically significant changes in renal biomarkers may not be of clinical significance. In general, changes in measured biomarkers are considered clinically significant (with 95% probability) when they are greater than the combined analytical (CVa) and biological (CVi) variation of the measured variable. This total variation (named RCV) is calculated with the following formula: RCV = 21/2 × 1.96 × (CVa 2 + CVi 2)1/2, where CVa is the analytical variation of the biomarker in the laboratory and CVi is its within‐subjects biological variation.19 For sCr, sCysC, and uNGAL, the CVa in our laboratory is 2.8%, 2.0%, and 4.5%, respectively, and the CVi is 5.95%, 5.0%, and 84%, respectively.20, 21 This means that changes from baseline in sCr, sCysC, and uNGAL must be greater than 18.1%, 14.9%, and 233%, respectively (which is their RCV) to be considered clinically significant (with 95% probability). Based on uNGAL changes from baseline, our population was divided in 2 groups: those with clinically significant changes (>233%), characterized as “uNGAL positive for AKI,” and those with clinically nonsignificant changes (<233%), characterized as “uNGAL negative for AKI.”
Results
A total of 100 subjects (80% males) were included in the analysis. Their baseline characteristics are presented in Table 1. Clinically (and statistically) significant increases in uNGAL from baseline (“uNGAL positive for AKI”) were noted in 33 patients (33.0%). The remaining 67 patients (67%) had only minor changes in uNGAL (“uNGAL negative for AKI”), as shown in Table 2. With the exception of the administered contrast volume (significantly higher in the AKI group), the 2 groups were comparable at baseline (P value not significant) with regard to the prevalence of cardiovascular risk factors (age, sex, arterial hypertension, diabetes mellitus, dyslipidemia, smoking, obesity) and significant coronary heart disease, the number of PCIs performed, as well as the levels of all renal biomarkers (Table 1).
Table 1.
Baseline Characteristics of the Study Population
| uNGAL Positive for AKI, n = 33 | uNGAL Negative for AKI, n = 67 | P Value | Total Population, N = 100 | |
|---|---|---|---|---|
| Clinical Parameters | ||||
| Age, y, mean (SD) | 64.3 (7.7) | 63.3 (9.8) | 0.598 | 63.6 (9.2) |
| Sex, M/F, n | 25/8 | 55/12 | 0.457 | 80/20 |
| Arterial hypertension, % | 69.7 | 67.8 | 0.851 | 68.0 |
| DM, % | 30.3 | 23.4 | 0.462 | 26.0 |
| Dyslipidemia, % | 63.6 | 51.7 | 0.266 | 56.0 |
| Smoking, % | 39.4 | 42.9 | 0.500 | 41.0 |
| BMI, kg/m2, mean (SD) | 28.2 (3.4) | 29.6 (5.0) | 0.264 | 29.0 (4.4) |
| Angiographic parameters | ||||
| Contrast volume, mL, mean (SD) | 427 (257) | 300 (178) | 0.005 | 343 (215) |
| Significant CHD, % | 72.8 | 74.6 | 0.734 | 73.0 |
| PCI, % | 60.6 | 55.2 | 0.439 | 57.0 |
| Renal biomarkers | ||||
| uNGAL, ng/mL, mean (SD) | 15.16 (9.98) | 17.20 (12.84) | 0.419 | 16.53 (11.81) |
| Range | 3.10–50.13 | 6.10–66.50 | — | 3.10–66.50 |
| sCr, mg/dL, mean (SD) | 0.92 (0.18) | 0.96 (0.26) | 0.393 | 0.95 (0.23) |
| Range | 0.67–1.46 | 0.62–2.35 | — | 0.62–2.35 |
| sCysC, mg/L, mean (SD) | 0.95 (0.24) | 0.94 (0.23) | 0.814 | 0.94 (0.23) |
| Range | 0.65–1.65 | 0.61–1.81 | — | 0.61–1.81 |
| pNGAL, ng/mL, mean (SD) | 137.67 (68.28) | 109.32 (52.93) | 0.025 | 118.68 (58.12) |
| Range | 28.24–301.45 | 33.78–262.07 | — | 28.24–301.45 |
| eGFR_MDRD, mL/min/m2, mean (SD) | 86.8 (18.8) | 85.1 (21.0) | 0.704 | 85.7 (20.3) |
| Range | 50.3–125.3 | 30.2–145.1 | — | 30.2–145.1 |
| eGFR_cysC, mL/min/m2, mean (SD) | 81.1 (20.8) | 82.4 (21.4) | 0.776 | 82.0 (21.2) |
| Range | 37.4–123.2 | 33.2–133.7 | — | 33.2–133.7 |
| ACR, mg/g, mean (SD) | 42.0 (129.2) | 28.5 (61.7) | 0.479 | 33.0 (94.0) |
| Range | 3.3–739.9 | 2.3–419.6 | — | 2.3–739.9 |
Abbreviations: ACR, albumin‐to‐creatinine ratio; AKI, acute kidney injury; BMI, body mass index; CHD, coronary heart disease; CysC, cystatin C; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; F, female; M, male; MDRD, Modification of Diet in Renal Disease; PCI, percutaneous coronary intervention; pNGAL, plasma neutrophil gelatinase–associated lipocalin; sCr, serum creatinine; sCysC, serum cystatin C; SD, standard deviation; uNGAL, urine neutrophil gelatinase–associated lipocalin.
Table 2.
Contrast‐Induced Changes in Renal Biomarkers Over Time
| uNGAL Positive for AKI, n = 33 | uNGAL Negative for AKI, n = 67 | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | P1 Valuea | Mean (SD) | P1 Valuea | P2 Valueb | ||
| uNGAL, ng/mL | Baseline | 15.16 (9.98) | — | 17.20 (12.84) | — | 0.419 |
| 6 hours | 43.18 (24.44) | 0.022 | 18.02 (12.42) | 0.942 | <0.001 | |
| 24 hours | 72.54 (62.16) | <0.001 | 19.35 (14.82) | 0.616 | <0.001 | |
| 48 hours | 62.60 (50.66) | <0.001 | 17.64 (15.15) | 0.985 | <0.001 | |
| P3 Value < 0.001c | P3 Value = 0.780c | |||||
| sCr, mg/dL | Baseline | 0.92 (0.18) | — | 0.96 (0.26) | — | 0.393 |
| 6 hours | 0.93 (0.22) | 0.979 | 0.88 (0.23) | 0.109 | 0.241 | |
| 24 hours | 1.01 (0.22) | 0.160 | 0.93 (0.22) | 0.761 | 0.078 | |
| 48 hours | 1.04 (0.22) | 0.049 | 0.95 (0.23) | 0.982 | 0.055 | |
| P3 Value = 0.049c | P3 Value = 0.196c | |||||
| sCysC, mg/L | Baseline | 0.95 (0.24) | — | 0.94 (0.23) | — | 0.814 |
| 6 hours | 0.96 (0.24) | 1.000 | 0.89 (0.21) | 0.369 | 0.151 | |
| 24 hours | 0.99 (0.25) | 0.824 | 0.91 (0.22) | 0.845 | 0.104 | |
| 48 hours | 1.03 (0.26) | 0.418 | 0.93 (0.23) | 0.944 | 0.063 | |
| P3 Value = 0.517c | P3 Value = 0.527c | |||||
| pNGAL, ng/mL | Baseline | 137.67 (68.28) | — | 109.32 (52.93) | — | 0.025 |
| 6 hours | 152.57 (66.73) | 0.712 | 107.57 (60.29) | 0.997 | 0.001 | |
| 24 hours | 171.64 (69.31) | 0.120 | 123.48 (66.63) | 0.414 | 0.001 | |
| 48 hours | 178.86 (71.66) | 0.047 | 123.07 (68.48) | 0.476 | 0.001 | |
| P3 Value = 0.072c | P3 Value = 0.299c |
Abbreviations: AKI, acute kidney injury; pNGAL, plasma neutrophil gelatinase–associated lipocalin; sCr, serum creatinine; sCysC, serum cystatin C; SD, standard deviation; uNGAL, urine neutrophil gelatinase–associated lipocalin.
Comparison between each time point and baseline.
Comparison between biomarker‐positive and ‐negative subjects at each time point.
Comparison within repeated measurements.
Baseline uNGAL was significantly negatively correlated to eGFR_MDRD and eGFR_CysC (r = −0.604 and r = −0.575, respectively; Figure 1) and significantly positively correlated to ACR (r = 0.496).
Figure 1.
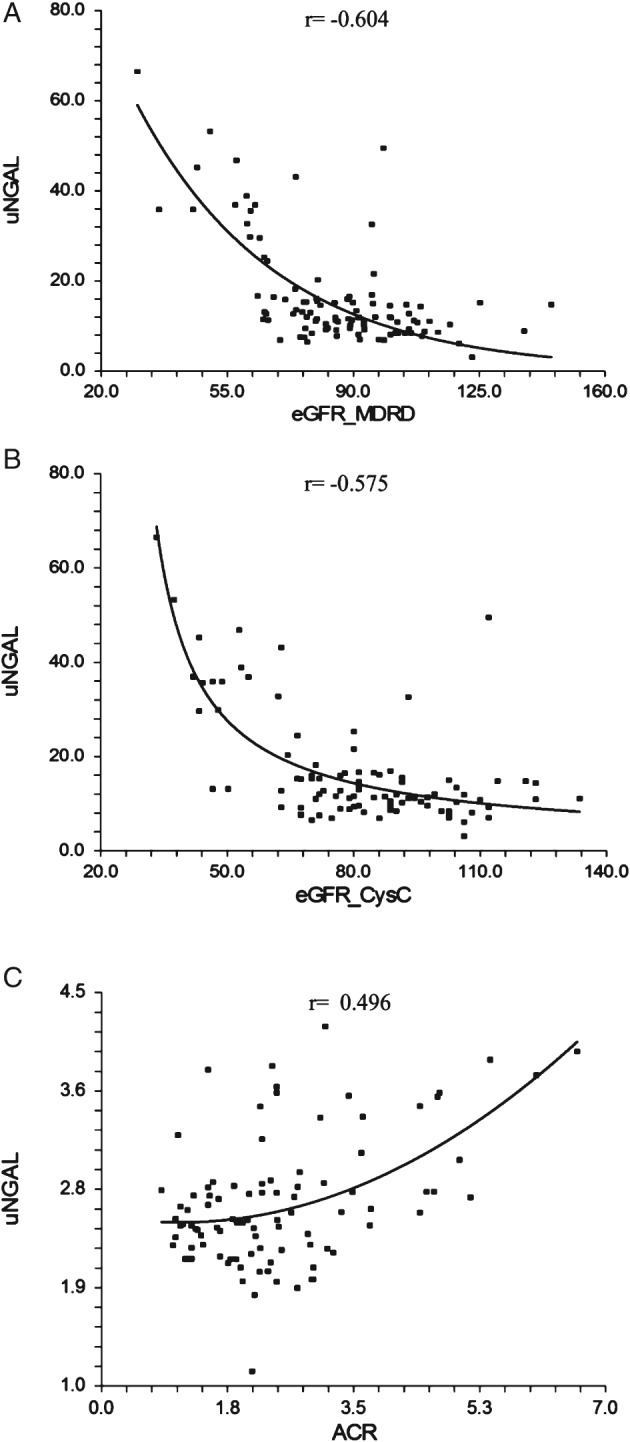
Correlation of baseline uNGAL with (A) eGFR_MDRD and (B) eGFR_CysC‐based formula and (C) ACR. Abbreviations: ACR, albumin‐to‐creatinine ratio; CysC, cystatin C; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; uNGAL, urine neutrophil gelatinase–associated lipocalin.
Serial changes of NGAL, sCr, and sCysC during the 48‐hour follow‐up are presented in Table 2 and Figure 2. Urine NGAL showed significant statistical and clinical elevations as early as 6 hours postcatheterization in the uNGAL positive for AKI group with only minor changes in the uNGAL negative for AKI group. Serum CysC did not show any significant statistical or clinical change at any time point of the follow‐up in either of the 2 groups. Serum Cr and pNGAL were statistically significantly elevated in the AKI group, but only 48 hours postcatheterization. However, these changes were not clinically significant because they were lower than the threshold of RCV.
Figure 2.
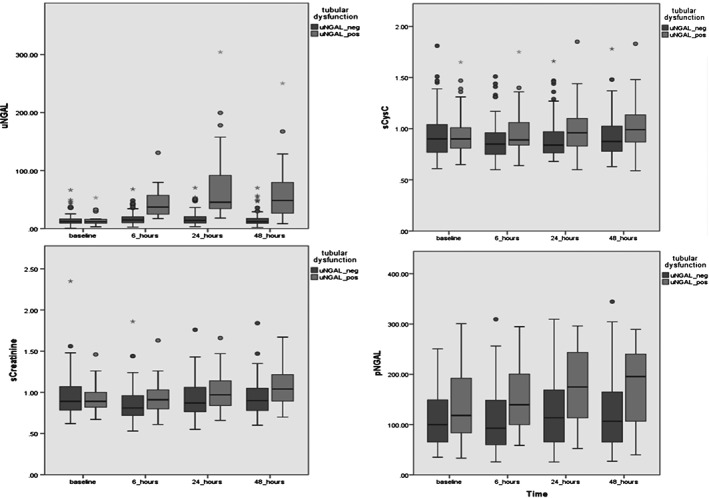
Serial changes of (top to bottom) uNGAL, sCr, sCysC, and pNGAL over time. Abbreviations: pNGAL, plasma neutrophil gelatinase–associated lipocalin; sCr, serum creatinine; sCysC, serum cystatin C; uNGAL, urine neutrophil gelatinase–associated lipocalin.
Discussion
To our knowledge, this is one of the few studies in the literature assessing the role of NGAL as an early marker of CIN after invasive cardiac procedures compared with established markers of nephropathy. The main findings of this study denote that no CIN cases could be diagnosed early based on sCr changes, whereas uNGAL emerges as superior in the early‐CIN diagnosis within 24 hours after elective invasive cardiac procedures. Thus, uNGAL is useful for the selection of patients needing extended hospitalization (>24–48 hours) for closer renal follow‐up. The use of RCV for the definition of clinically significant uNGAL changes is probably a valuable supplement to the statistically defined significant variations. Moreover, baseline renal function (eGFR, ACR) seems to greatly influence uNGAL levels.
These results seem to be in agreement with the current literature. Liebetrau et al recently reported that uNGAL is a biomarker for predicting contrast‐induced AKI when measured 24 hours after PCI.22 Padhy et al found that even pNGAL and sCysC may act as early markers of contrast‐induced AKI in patients undergoing PCI.23 However, AKI diagnosis was sCr‐based in this study; also, RCV was not used for the definition of clinically significant biomarker changes, but specific cutoff values were determined instead.
The reported incidence of CIN varies widely in the literature, depending not only on the specific population and the baseline risk factors, but also on the definition of this clinical event.1, 3, 4, 24 In the general population, the incidence of CIN is reported to be 0.6% to 2.3%.3 However, in several patient subsets it is significantly higher (up to 20%).3 This is especially true for patients with cardiovascular pathology.3 Ozcan et al recently reported a 9.3% incidence of CIN after elective PCI in nondiabetic individuals with metabolic syndrome, compared with 4.9% in the control group (nondiabetic subjects without metabolic syndrome).24
According to most definitions, slight sCr changes at 48 to 72 hours after exposure to a contrast agent establish CIN diagnosis.3, 25 However, sCr‐based CIN diagnosis turns out to be rather difficult. The laboratory analytical variation and the within‐subjects biological variation are the most important sources of variation in its measurement, frequently leading to misdiagnosis. To keep the total variation minimal and to achieve a uniform measurement between laboratories, the analytical variation must be <50% of the biological variation.26 As previously reported, this goal was achieved in our laboratory. Even at this case, with a 18.1% RCV for sCr, only changes >18.1% in serial sCr measurements were able to be discriminated. During the 48‐hour follow‐up, none of the participants exhibited such a sCr change (sCr‐based incidence of CIN, 0%). Meantime, a significant proportion of the same population (33.3%) exhibited clinically significant elevations of uNGAL, which is a tubular damage–specific biomarker (uNGAL‐based incidence of CIN, 33%). This is probably because we actually diagnosed a “subclinical acute kidney injury,” previously described as “biomarker‐positive/sCr‐negative kidney injury” representing the first stage of AKI and characterized by biomarker positivity only.5, 27, 28, 29 Our data support the idea of a “mild tubular injury” because peak uNGAL levels found in the present study (72.54 ± 62.16 ng/mL) are much lower than those observed in severe AKI cases (1113.4 ± 88.8 ng/mL in a study with subjects undergoing cardiac surgery).30
The correlation between baseline renal function (eGFR, ACR) and uNGAL levels found in the present study practically means that subjects with lower eGFR or/and higher ACR exhibit higher baseline uNGAL levels; and, therefore, greater uNGAL increase is required after contrast administration to be characterized as CIN/AKI. In other words, the presence of chronic kidney disease and/or albuminuria may increase the threshold for the detection of CIN/AKI using NGAL. McIlroy et al have previously reported that the relationship between uNGAL and AKI after cardiac surgery varies with baseline renal function, with optimal discriminatory performance in patients with normal preoperative function.31 In intact kidneys, albumin is filtered through the glomerulus and is reabsorbed by megalin‐cubulin receptor–mediated endocytosis.32, 33 In the presence of proteinuria, competition for the same receptor between albumin, uNGAL (produced by neutrophils or/and kidney epithelial cells), and other LMWPs could account for increased uNGAL levels, independently of tubular injury.17, 34 Other LMWPs absorbed by receptor‐mediated endocytosis include some of the newly discovered biomarkers of AKI, such as urine cystatin C (uCysC), liver fatty‐acid binding protein, α1‐microglobulin, and β2‐microglobulin.35, 36, 37, 38, 39 Nejat et al have shown that repeated protein loading could induce transient albuminuria, leading to increased uCysC levels that decreased again when albuminuria returned to baseline.17 Nishida et al found that the extent of proteinuria is correlated with uNGAL levels in children with chronic renal diseases.40 These observations enhance the hypothesis that albuminuria decreases the absorption of LMWPs by competition for a common transport mechanism. For these reasons, a standard NGAL cutoff value would probably be problematic. Instead, the interpretation of uNGAL changes from baseline in the base of RCV is probably a better approach.
Study Limitations
It is important to consider potential limitations. First, this was a narrow‐scale study with a rather limited number of participants. Larger‐scale studies are necessary to confirm our results. Second, the CVi used for RCV calculation is the one reported in literature and has been calculated in apparently healthy individuals. However, RCV derived from “healthy CVi” may be inappropriate for monitoring patients in certain diseases.41 Although it is difficult to measure biological variation in the setting of specific diseases, it has been reported that, for the majority of markers that are routinely measured in clinical laboratories, only minor differences in CVi are present between healthy subjects and populations with chronic diseases.41 In the present study, patients with acute coronary syndrome (ACS) were excluded and only stable patients undergoing elective procedures were enrolled. Thus, the use of “healthy CVi” has probably minimal impact on the accuracy of the results. However, the exclusion of patients with ACS is another limitation of the study. Future studies on ACS patients are necessary to examine the role of NGAL as a marker of CIN.
Conclusion
Urine NGAL emerges as potentially superior compared with established markers of nephropathy in the early diagnosis of CIN/AKI after elective invasive cardiac procedures and the selection of patients needing extended hospitalization (>24–48 hours) for closer renal and fluid follow‐up. The use of RCV for the definition of clinically significant uNGAL changes is probably a valuable supplement to the statistically defined significant variations. The definition of standard cutoff values for uNGAL is rather improper given that its levels are highly dependent on eGFR and ACR. Future larger‐scale studies are necessary to confirm our results and extend them to ACS patients.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. McCullough PA. Contrast‐induced acute kidney injury [published correction appears in J Am Coll Cardiol. 2008;51:2197]. J Am Coll Cardiol. 2008;51:1419–1428. [DOI] [PubMed] [Google Scholar]
- 2. Sudarsky D, Nikolsky E. Contrast‐induced nephropathy in interventional cardiology. Int J Nephrol Renovasc Dis. 2011;4:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehran R, Nikolsky E. Contrast‐induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006:S11–S15. [DOI] [PubMed] [Google Scholar]
- 4. McCullough PA, Sandberg KR. Epidemiology of contrast‐induced nephropathy. Rev Cardiovasc Med. 2003;(4 suppl 5):S3–S9. [PubMed] [Google Scholar]
- 5. Perrin T, Descombes E, Cook S. Contrast‐induced nephropathy in invasive cardiology. Swiss Med Wkly. 2012;142:w13608. [DOI] [PubMed] [Google Scholar]
- 6. Haase M, Devarajan P, Haase‐Fielitz A, et al. The outcome of neutrophil gelatinase–associated lipocalin‐positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clerico A, Galli C, Fortunato A, et al. Neutrophil gelatinase–associated lipocalin (NGAL) as biomarker of acute kidney injury: a review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med. 2012;50:1505–1517. [DOI] [PubMed] [Google Scholar]
- 8. Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30:33–37. [DOI] [PubMed] [Google Scholar]
- 9. Bellomo R, Ronco C, Kellum JA, et al; Acute Dialysis Quality Initiative workgroup . Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waikar SS, Betensky RA, Emerson SC, et al. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Geus HR, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin Kidney J. 2012;5:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase–associated lipocalin from humans. Genomics. 1997;45:17–23. [DOI] [PubMed] [Google Scholar]
- 13. Schmidt‐Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase–associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. [DOI] [PubMed] [Google Scholar]
- 14. Singer E, Markó L, Paragas N, et al. Neutrophil gelatinase–associated lipocalin: pathophysiology and clinical applications. Acta Physiol (Oxf). 2013;207:663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin‐siderophore‐iron complex rescues the kidney from ischemia‐reperfusion injury. J Clin Invest. 2005;115:610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase–associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nejat M, Hill JV, Pickering JW, et al. Albuminuria increases cystatin C excretion: implications for urinary biomarkers. Nephrol Dial Transplant. 2012;27(suppl 3):iii96–iii103. [DOI] [PubMed] [Google Scholar]
- 18. Fraser CG. Making better use of differences in serial laboratory results. Ann Clin Biochem. 2012;49(part 1):1–3. [DOI] [PubMed] [Google Scholar]
- 19. Fraser CG, ed. Biological Variation: From Principles to Practice. Washington, DC: AACC Press; 2001. [Google Scholar]
- 20. Minchinela J, Ricós C, Perich C, et al. Biological variation database, and quality specifications for imprecision, bias and total error (desirable and minimum): the 2012 update. Available at: http://www.westgard.com/biodatabase‐2012‐update.htm. Accessed June 20, 2012.
- 21. Delanaye P, Rozet E, Krzesinski JM, et al. Urinary NGAL measurement: biological variation and ratio to creatinine. Clin Chim Acta. 2011;412:390. [DOI] [PubMed] [Google Scholar]
- 22. Liebetrau C, Gaede L, Doerr O, et al. Neutrophil gelatinase–associated lipocalin (NGAL) for the early detection of contrast‐induced nephropathy after percutaneous coronary intervention. Scand J Clin Lab Invest. 2014;74:81–88. [DOI] [PubMed] [Google Scholar]
- 23. Padhy M, Kaushik S, Girish MP, et al. Serum neutrophil gelatinase–associated lipocalin (NGAL) and cystatin C as early predictors of contrast‐induced acute kidney injury in patients undergoing percutaneous coronary intervention. Clin Chim Acta. 2014;435:48–52. [DOI] [PubMed] [Google Scholar]
- 24. Ozcan OU, Adanir Er H, Gulec S, et al. Impact of metabolic syndrome on development of contrast‐induced nephropathy after elective percutaneous coronary intervention among nondiabetic patients. Clin Cardiol. 2015;38:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris EK. Statistical principles underlying analytic goal‐setting in clinical chemistry. Am J Clin Pathol. 1979;72(2 suppl):374–382. [PubMed] [Google Scholar]
- 27. Ronco C, Stacul F, McCullough PA. Subclinical acute kidney injury (AKI) due to iodine‐based contrast media. Eur Radiol. 2013;23:319–323. [DOI] [PubMed] [Google Scholar]
- 28. Haase M, Kellum JA, Ronco C. Subclinical AKI—an emerging syndrome with important consequences. Nat Rev Nephrol. 2012;8:735–739. [DOI] [PubMed] [Google Scholar]
- 29. Bagshaw SM. Subclinical acute kidney injury: a novel biomarker‐defined syndrome. Crit Care Resusc. 2011;13:201–203. [PubMed] [Google Scholar]
- 30. McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase–associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amsellem S, Gburek J, Hamard G, et al. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol. 2010;21:1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gekle M. Renal tubule albumin transport. Annu Rev Physiol. 2005;67:573–594. [DOI] [PubMed] [Google Scholar]
- 34. Thielemans N, Lauwerys R, Bernard A. Competition between albumin and low‐molecular‐weight proteins for renal tubular uptake in experimental nephropathies. Nephron. 1994;66:453–458. [DOI] [PubMed] [Google Scholar]
- 35. Nejat M, Pickering JW, Walker RJ, et al. Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit Care. 2010;14:R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Christensen EI, Nielsen R. Role of megalin and cubilin in renal physiology and pathophysiology. Rev Physiol Biochem Pharmacol. 2007;158:1–22. [DOI] [PubMed] [Google Scholar]
- 37. Hvidberg V, Jacobsen C, Strong RK, et al. The endocytic receptor megalin binds the iron transporting neutrophil gelatinase–associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579:773–777. [DOI] [PubMed] [Google Scholar]
- 38. Oyama Y, Takeda T, Hama H, et al. Evidence for megalin‐mediated proximal tubular uptake of L‐FABP, a carrier of potentially nephrotoxic molecules. Lab Invest. 2005;85:522–531. [DOI] [PubMed] [Google Scholar]
- 39. Dieterle F, Perentes E, Cordier A, et al. Urinary clusterin, cystatin C, β2‐microglobulin and total protein as markers to detect drug‐induced kidney injury. Nat Biotechnol. 2010;28:463–469. [DOI] [PubMed] [Google Scholar]
- 40. Nishida M, Kawakatsu H, Okumura Y, et al. Serum and urinary neutrophil gelatinase–associated lipocalin levels in children with chronic renal diseases. Pediatr Int. 2010;52:563–568. [DOI] [PubMed] [Google Scholar]
- 41. Ricós C, Iglesias N, García‐Lario JV, et al. Within‐subject biological variation in disease: collated data and clinical consequences. Ann Clin Biochem. 2007;44(part 4):343–352. [DOI] [PubMed] [Google Scholar]


