Abstract
Background
Premature ventricular contractions (PVCs) from the right ventricular outflow tract (RVOT) can resist conventional mapping strategies. Studies regarding optimal mapping and ablation methods for patients with noninducible RVOT‐PVCs are limited. We retrospectively evaluated the efficacy and safety of a novel mapping strategy for these cases: voltage mapping combined with pace mapping.
Hypothesis
Methods
We retrospectively included symptomatic patients (n = 148; 76 males; age, 44.5 ± 1.4 years) with drug‐refractory PVCs originating from the RVOT, who underwent radiofrequency catheter ablation (RFCA), and stratified them as Group 1 and Group 2. Group 1 patients had noninducible RVOT‐PVCs, determined after programmed stimulation, burst pacing, and isoproterenol infusion (n = 21; 12 males; age, 39.5 ± 10.8 years). Group 2 patients had inducible PVCs. Group 1 patients were subjected to voltage mapping combined with pace mapping; Group 2 underwent conventional mapping. In all patients prior to RFCA, detailed 3‐dimensional electroanatomic voltage maps of the RVOT were obtained during sinus rhythm using the CARTO system.
Results
Patients from both groups had similar success and complication rates associated with the RFCA. In Group 2, 89% (113/127) experienced the earliest and the successful ablation points in the voltage transitional zone. During the follow‐up (36 ± 8 months), patients from both groups suffered similar rates of PVC relapse (2/21 and 7/127, respectively; P = 0.826).
Conclusions
Voltage mapping combined with pace mapping is effective and safe for patients with noninducible RVOT‐PVCs determined by conventional methods.
Keywords: Radiofrequency catheter ablation, voltage mapping, pace mapping, right ventricular outflow tract, premature ventricular contractions
1. INTRODUCTION
Despite significant improvement in diagnosis and treatment of ventricular arrhythmia, premature ventricular contractions (PVCs) originating from the right ventricular outflow tract (RVOT) are among the most common ventricular arrhythmias that cause discomfort to patients.1 Clinically, frequently occurring PVCs from the RVOT (RVOT‐PVCs) not only lead to palpitations, but also to more severe clinical conditions, such as PVC‐induced cardiomyopathy.2, 3 Therefore, treatment of RVOT‐PVCs with medications or novel strategies such as catheter ablation may be of significance for the prevention of structural heart diseases in these patients.4
Indeed, a recent meta‐analysis indicated that for patients with frequent PVCs, catheter ablation improves cardiac function, especially for patients with left ventricular dysfunction.5 However, the success rates of catheter ablation have not always been satisfying in all patients with RVOT‐PVCs. This is because, in some patients, arrhythmia cannot be induced by conventional procedures such as programmed stimulation, burst pacing, or isoproterenol infusion.6 This is considered a major challenge for catheter ablation treatment. Patients with noninducible RVOT‐PVCs are also prone to require more time for both the catheter procedure and X‐ray exposure. The recurrence rate is high, even when activation mapping is applied. Therefore, the development of novel mapping strategies in the clinical setting for patients with noninducible RVOT‐PVCs is important.
Pace mapping has been suggested for patients without frequent PVCs.7 Interestingly, results of previous studies found that for ~18% of patients, successful ablation sites respond poorly to pace mapping.8 However, whether pacing mapping may be effective for patients with noninducible RVOT‐PVCs has not been determined.
In this study, we retrospectively evaluated a simple and effective novel mapping strategy using the CARTO system (Biosense Webster, Diamond Bar, California) for patients who have infrequent PVCs that cannot be induced by conventional methods during the catheter ablation. We hypothesize that, when PVCs are few during the electrophysiological study, combining the electroanatomical map and the pace‐mapping technique and focusing on the transitional zone is as safe and effective as the traditional mapping of PVCs.
2. METHODS
The institutional ethics review board of Capital Medical University approved the protocol before the study was conducted. All patients provided written informed consent for mapping and ablation.
2.1. Study Population
We retrospectively included 148 patients with recurrent paroxysmal palpitations (76 males; mean age, 45 ± 1.4 years) who underwent radiofrequency (RF) catheter ablation (RFCA) of PVCs between August 1, 2011, and December 31, 2013. The presence of RVOT‐PVCs was confirmed by 12‐lead electrocardiograms (ECGs) during palpitations showing a wide QRS complex with a left bundle branch block and an inferior axis deviation (positive QRS in leads II, III, and aVF). A clinical diagnosis of RVOT‐PVCs was made with reference to a previous international consensus.9 The patients included in this study were those who continued to be symptomatic despite the administration of active medication management. Patients were excluded who had structural heart disease based on both the echocardiographic and angiographic evaluations. Also excluded were patients who were scheduled to receive RFCA attempted from the left ventricular outflow tract, the aortic cusp, or the epicardium.
2.2. Electrophysiological Testing
The electrophysiological tests were performed after the discontinuation of any antiarrhythmic medications for a period ≥5 half‐lives after the last dose. All patients underwent local anesthesia without general anesthesia or sedation during the electrophysiological study. A 6‐Fr quadripolar catheter was introduced from the right femoral vein and placed at the right ventricular apex for pacing. Surface and intracardiac ECGs were continuously recorded with an electrophysiology workstation (Cardiolab; GE, Freiburg, Germany). Intracardiac signals were filtered at a band pass of 30 to 500 Hz. When spontaneous PVCs were absent, or few PVCs were observed (<10 PVC/h), induction was attempted by burst pacing up to 210 bpm at the RVOT or the right ventricular apex, before and after an isoproterenol infusion (1–4 µg/min).
The patients who had undergone programmed stimulation, burst pacing, and isoproterenol infusion but still showed <10 PVC/h during the procedure were assigned to Group 1, as having noninducible PVCs. These patients were subsequently examined with voltage mapping combined with pace mapping. Patients with >10 PVC/h after conventional inducing methods were assigned to Group 2 as having inducible PVCs; these patients were assessed with conventional mapping strategies (only activation mapping or combined with pace mapping).
2.3. Voltage Mapping
Prior to RFCA, detailed 3‐dimensional (3D) electroanatomic voltage maps of the RVOT were obtained during sinus rhythm using the CARTO system. Mapping and pacing in the RVOT were performed using an 8‐Fr externally saline‐irrigated ThermoCool ablation catheter (NaviStar ThermoCool; Biosense Webster). All patients underwent complete endocardial mapping to ensure the reconstruction of the 3D geometry of the RVOT. The area around the RVOT was densely mapped (≥90 points).
We estimated the location of the pulmonic valve based on the guidance provided by biaxial fluoroscopy and right ventriculography. Adequate catheter contact was confirmed by the motion of the catheter tip that was observed as silhouettes on fluoroscopy (Figure 1). The color display for the voltage on bipolar ECGs of the myocardium ranged from red (indicating the low‐voltage zone, generally with an amplitude of <0.5 mV) to purple (the high‐voltage zone, generally >1.5 mV). Intermediate colors represented the location of the transitional‐voltage zone, with amplitudes between 0.5 and 1.5 mV (Figure 2).
Figure 1.
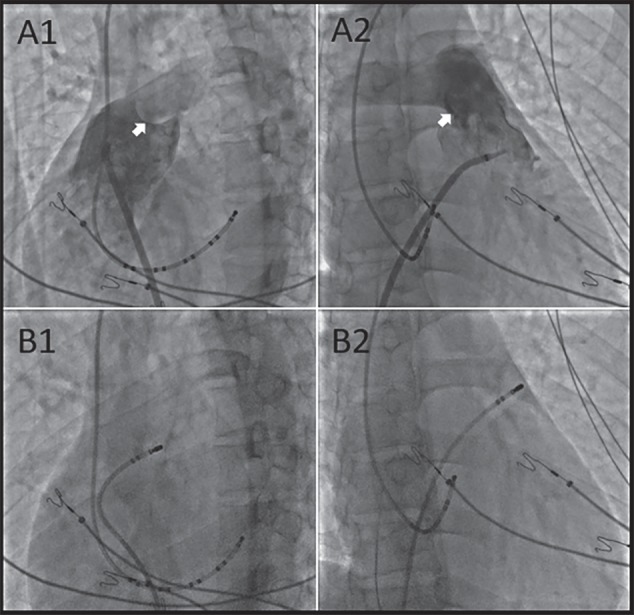
(A1, A2) Right ventriculography (LAO 45, RAO 30). The white arrows show the PV. Biaxial fluoroscopy (B1, B2) showed that the catheter was located at the RVOT (LAO 45, RAO 30). The catheter location was confirmed to be near the PV. Abbreviations: LAO, left anterior oblique; PV, pulmonary valve; RAO, right anterior oblique; RVOT, right ventricular outflow tract.
Figure 2.
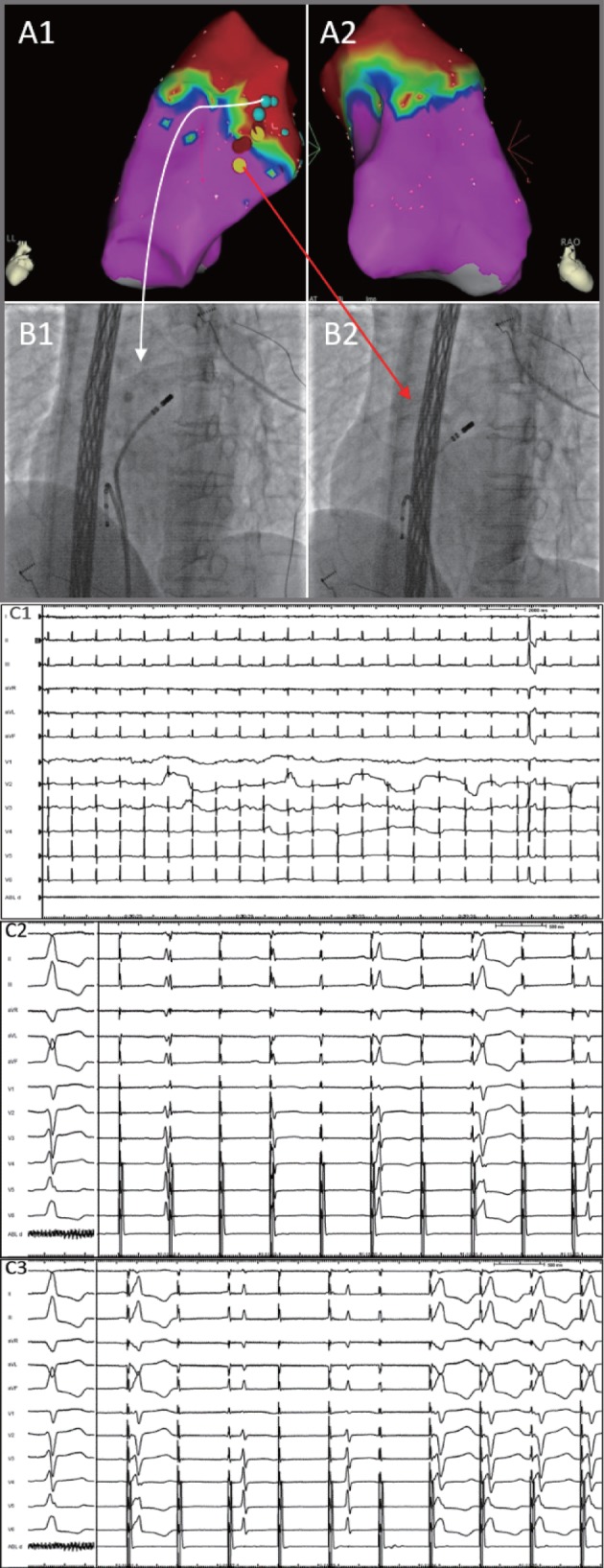
A patient with few PVCs occurring during the procedure (C1), who was subsequently evaluated with voltage mapping combined with pace mapping. Electroanatomical voltage mapping (A1, A2) in the RVOT (LL, RAO 30). The pacing ablation catheter was pulled down along the septum of the RVOT from the upper position to the lower position. (B1) When the pacing ablation catheter was placed in the upper RVOT (LAO 45, white arrow), the pacing ECG showed that the ventricle was not captured (C2). However, in (B2) the lower RVOT and sites near the transitional‐voltage zone (LAO 45, red arrow), the ventricle was fully captured, and the morphology of the pacing ECG was similar to the intrinsic PVCs (C3). Abbreviations: ECG, electrocardiogram; LAO, left anterior oblique; LL, left lateral; PA, posteroanterior; PVCs, premature ventricular contractions; RAO, right anterior oblique; RVOT, right ventricular outflow tract.
2.4. Radiofrequency Catheter Ablation
For patients in Group 1, the origin position of the PVCs was preliminarily judged according to the QRS morphology from the ECGs; then, the pacing ablation catheter was pulled down along the septum of the RVOT from the upper to the lower positions. When the pacing ablation catheter was placed in the upper RVOT, the pacing ECG showed that the ventricle was not captured. In the lower RVOT and near the transitional‐voltage zone, the ventricle was fully captured and the morphology of the pacing ECG was similar to the intrinsic PVCs (Figure 2). The target myocardium for ablation was determined by pace mapping in the transitional‐voltage zone, which provided ≥11 of 12 matches between the paced and spontaneous PVCs (pace score >92%), as indicated by a previous study.7 In Group 2, the site of origination of the PVCs was determined by activation mapping alone or combined with pace mapping.
Radiofrequency energy was delivered through a Cordis‐Stockert generator (Biosense Webster) during irrigation, at a rate of 17 mL/min via the Cool Flow pump (Biosense Webster). The RF power output was limited to 30 W, with a target maximum temperature of 43°C. The RF delivery was applied for 30 to 60 seconds. The Holter examination was performed routinely 1 to 2 months after the procedure, and then at 3‐ to 6‐month intervals after ablation. The RFCA treatment was considered successful if it met the following criteria: the spontaneous and induced arrhythmias were eliminated by radiofrequency ablation (acute success), no recurrence of a symptomatic arrhythmia was detected (If the patients had symptoms of palpitation or self‐testing pulse was irregular, then PVC recurrence was detected with ECG or Holter), and the PVC burden decreased >70% with no antiarrhythmic drug therapy during the follow‐up.10
2.5. Statistical Analysis
Continuous variables are presented as mean ± SD, calculated with the unpaired t test. Discrete variables were compared using the χ2 test or Fisher exact test. Statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, Illinois). A P value < 0.05 was considered statistically significant.
3. RESULTS
3.1. Patient Characteristics
The study population consisted of 148 patients with recurrent paroxysmal palpitations and confirmed RVOT‐PVCs, with no evidence of structural heart disease (Table 1). Twenty‐one patients (12 males, age 39.5 ± 10.8 years) with few PVCs were assigned as Group 1, and the remaining 127 patients (64 males, age 42 ± 11.5 years) were assigned as Group 2. Patients from the 2 groups were similar in age, sex, prevalence of hypertension and diabetes, and burden of PVCs.
Table 1.
Patient characteristics
| Group 1 | Group 2 | P Value | |
|---|---|---|---|
| Subjects, n | 21 | 127 | — |
| Male sex, n (%) | 12 (57.1) | 64 (50.4) | 0.566 |
| Mean age, y | 39.5 ± 10.8 | 42 ± 11.5 | 0.194 |
| HTN, n (%) | 4 (19.0) | 16 (12.6) | 0.648 |
| DM, n (%) | 3 (14.3) | 11 (8.7) | 0.679 |
| PVC burden, %1 | 14.4 ± 4.9 | 14.1 ± 5.1 | 0.421 |
| LVEF, % | 62 ± 3.3 | 61.5 ± 3.6 | 0.089 |
Abbreviations: DM, diabetes mellitus; HTN, hypertension; LVEF, left ventricular ejection fraction; PVC, premature ventricular contraction.
24‐hour Holter monitor.
3.2. Electrophysiological Studies and Mapping
Clinical arrhythmia occurred spontaneously in 96 patients during the electrophysiological test. In the remaining 52 patients, clinical arrhythmia was induced in 31 patients by burst pacing and isoproterenol infusion. However, the remaining 21 patients had few PVCs (<10 PVC/h) after conventional induction and could not be assessed using activation mapping.
Nineteen patients decided to terminate the electrophysiological tests without ablation, and 11 of these (52.4%) experienced PVC relapse when they returned to the ward. The other 8 patients experienced recurrence in 1 to 2 days. These patients required a second electrophysiology test, and again <10 PVCs/h were induced. These 21 patients (Group 1) were evaluated with voltage mapping combined with pace mapping. The other 127 patients for whom PVCs could occur spontaneously or could be induced with conventional procedures were assigned to Group 2.
Electroanatomic voltage maps were successfully acquired during sinus rhythm in all patients (Figure 3). Overall, the mean number of sites sampled during RVOT electroanatomic voltage mapping was 104 ± 11, with an average mapping time of 16 ± 5 minutes.
Figure 3.

The activation map and voltage map for 2 patients. (A1, A2) The earliest point at the posterior septal side of the RVOT (PA, RAO 30). (A3) The red dot shows the successful ablation site at the earliest point. (A4) An electroanatomic voltage map of the RVOT obtained using the CARTO system (PA). The areas outlined in red indicate the low‐voltage zone (<0.5 mV), those in green indicate the transitional‐voltage zone (0.5–1.5 mV), and those in purple indicate the high‐voltage zone (>1.5 mV). Compared with (A1) and (A3), the earliest point and successful ablation site were at the transitional‐voltage zone. (B1, B2) The earliest points at the anterior septal side of the RVOT (LL, PA). Similar to the observations in (A1) and (A3), the earliest point and successful ablation site were also at the transitional‐voltage zone (B3). Abbreviations: LL, left lateral; PA, posteroanterior; RAO, right anterior oblique; RVOT, right ventricular outflow tract.
3.3. Results of Catheter Ablation
The prevalence of successful ablation sites in Group 2 was as follows: 4 (3.1%) patients were ablated in the low‐voltage zone, 113 (89.0%) patients in the transitional‐voltage zone, and 10 (7.9%) patients in the high‐voltage zone (Figure 3). On the other side, in Group 1, the target for ablation was determined by pace mapping in the transitional‐voltage zone, which provided ≥11 of 12 matches between the paced and spontaneous PVCs (Figure 2). The best pace mapping point (pace score >92%) of 1 (4.8% cf 3.1%; P = 0.785) patient was targeted in the low‐voltage zone, 18 (85.7% cf 89.0%; P = 0.948) patients in the transitional‐voltage zone, and 2 (9.5% cf 7.9%; P = 0.861) patients in the high‐voltage zone.
During the follow‐up of 36 ± 13 months, 93.9% of all the included patients remained free of ventricular arrhythmia in the absence of antiarrhythmic drug therapy, and their PVC burden was significantly reduced (Group 1: 14.4 ± 4.9 cf 0.55 ± 0.4, P < 0.0001; Group 2: 14.1 ± 5.1 cf 0.40 ± 0.4, P < 0.001). Nine patients had late recurrence of PVCs, 2 (9.5%) in Group 1 and 7 (5.5%) in Group 2 (P > 0.05; Table 2). No significant difference regarding the success rate and ventricular arrhythmia relapses were detected between the 2 groups. The QRS complexes of the recurrent PVCs showed minimal differences compared with the findings before RFCA. Two patients with recurrent PVCs in Group 2 remained symptomatic despite the administration of amiodarone, and they underwent a second ablation procedure. These 2 patients were free of arrhythmia after 13 months and 21 months of follow‐up, respectively. The other 7 patients continued to have drug therapy and regular visits to the clinic, because both the frequency and duration of the PVC attacks decreased and their symptoms were relieved.
Table 2.
Results and complications
| Group 1 | Group 2 | P Value | |
|---|---|---|---|
| Subjects, n | 21 | 127 | — |
| Follow‐up | |||
| Meantime, min | 42.5 ± 8.5 | 37.5 ± 7.6 | 0.332 |
| PVC burden, %1 | 0.55 ± 0.4 | 0.40 ± 0.4 | 0.210 |
| PVC relapse, n (%) | 2 (9.5) | 7 (5.5) | 0.826 |
| Complications | |||
| Local vascular | 1 (4.8)2 | 5 (3.9)3 | 0.922 |
| Cardiac perforation | — | — | — |
Abbreviations: PVC, premature ventricular contraction.
24‐hour Holter monitor.
Femoral vein hematoma.
Four cases of femoral vein hematoma, 1 case with femoral artery pseudoaneurysms.
One patient in Group 1 had a femoral vein hematoma; in Group 2, 4 patients had a femoral vein hematoma and 1 patient had femoral artery pseudoaneurysms. No hemopneumothorax, arteriovenous fistula, or cardiac perforation developed in either group. Regarding complications, there were no significant differences between the 2 groups.
4. DISCUSSION
4.1. Induction Program
Evidence from previous studies indicated that one of the major challenges in the catheter ablation of PVCs is that some PVCs were resistant to conventional induction procedures during the electrophysiological test. Even when programmed stimulation, burst pacing, and isoproterenol infusion have been performed to facilitate induction, some patients still have few PVCs during the procedure.1, 6 In our study, 21 of the 148 included patients had noninducible PVCs. Makhija et al11 showed that variations of many pathophysiological factors seemed to contribute to resistance to the conventional induction of PVCs, such as temporal variations, autonomic influences, sedation, and hormonal factors. However, the exact mechanisms underlying the difficulties of PVC induction during the procedure are still unknown.
Many animal experiments and human studies have suggested that for patients with RVOT tachycardia by high‐frequency stimulation of the extravascular sympathetic nerves within the proximal pulmonary artery innervating the RVOT may be effective for induction of RVOT‐PVCs.12, 13 Therefore, this strategy may be a useful protocol for patients with noninducible RVOT‐PVCs.
4.2. Voltage Mapping Combined With Pace Mapping
We hypothesized that the transitional‐voltage zone may be an important and potential substrate for the development of RVOT‐PVCs and could serve as a landmark for pace mapping. Results of the present study confirmed this hypothesis. We successfully located the target site with pace mapping in patients resistant to conventional mapping procedures, and the mapping time was comparable with patients with voltage mapping. Yamashina et al14 found some similar results. They evaluated the distribution of successful ablation sites within the RVOT using a 3D electroanatomic mapping system and demonstrated that almost 90% of the successful ablation sites were located in the transitional‐voltage zone beneath the pulmonary valve (PV). Therefore, we propose that pacing mapping around the transitional‐voltage zone may be feasible, and it is a safe and effective protocol for patients with RVOT‐PVCs. For patients with idiopathic RVOT‐initiated noninducible tachycardia, a combined mapping strategy with voltage and pacing may allow fewer and shorter hospital stays, and lower healthcare costs. Although the mechanism of this method and the anatomic and histological foundation of the voltage transition zone are not clear yet, we think that the transitional‐voltage zone reflects a heterogeneously distributed arrhythmogenic myocardium around the PV, which may contribute most to the pathogenesis of RVOT‐derived PVCs.
An investigation by Hasdemir et al15 found that because ventricular myocardial extensions could extend into the pulmonary artery beyond the PV, factors such as incomplete myocardial regression may be responsible for the presence of a myocardial sleeve in the main stem of the pulmonary artery, which was connected to the rest of the related ventricles. Interestingly, Liu et al16 found that 46% of the RVOT arrhythmia foci were localized beyond the valve in the pulmonary artery (median, 8.2 mm above the PV). Moreover, these locations were confirmed as supravalvular by direct intracardiac echocardiography visualization. However, myocardial voltage extension into the pulmonary artery in humans is ubiquitous. These extensions frequently serve as origins of presumed RVOT arrhythmias. Based on the above findings, we hypothesized that the heterogeneously distributed myocardium beyond the PV may contribute to arrhythmogenesis for arrhythmias such as PVCs, providing the substrate for the pathogenesis of arrhythmia and a subsequent ablation target for treatment of the related arrhythmia. We intend to conduct animal experiments to explore this problem further.
4.3. Study Limitations
The present study has several limitations which should be noted when interpreting the results. First, because neither magnetic resonance imaging of the RVOT nor biopsies of the RVs were performed, we could not investigate as association between electrophysiological characteristics of the transitional‐voltage zones and the anatomic or structural disorders in the pathological regions of our patients. Secondly, the bipolar voltage threshold of >1.5 mV was based on the normal myocardium in the ventricle proper. Therefore, more data are needed regarding the RVOT voltage for patients with RVOT‐PVCs, to determine whether this is an appropriate cutoff.
Finally, only 21 patients with noninducible RVOT‐PVCs were included in Group 1, which may be underpowered statistically for some clinical outcomes. Therefore, larger, prospective, controlled studies are needed to confirm the safety and efficacy of combined mapping strategy with voltage and pacing in patients with noninducible RVOT‐PVCs. In particular, the effects of this novel mapping strategy on clinical outcomes, such as the recurrence of arrhythmia, should be further evaluated.
5. CONCLUSION
An ablation approach based on voltage mapping combined with pace mapping is a safe and effective treatment for patients with noninducible RVOT‐PVCs. The safety and efficacy of the combined mapping strategy in patients with RVOT‐PVCs deserves further study.
5.1. Conflicts of interest
The authors declare no potential conflicts of interest.
Wang Z, Zhang H, Peng H, Shen X, Sun Z, Zhao C, Dong R, Gao H and Wu Y. Voltage combined with pace mapping is simple and effective for ablation of noninducible premature ventricular contractions originating from the right ventricular outflow tract, Clin Cardiol 2016;39(12):733–738.
Funding Information The present study was supported by the Basic‐Clinical Cooperation Program from Capital Medical University (No.16JL23).
REFERENCES
- 1. Nakagawa M, Takahashi N, Nobe S, et al. Gender differences in various types of idiopathic ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:633–638. [DOI] [PubMed] [Google Scholar]
- 2. Saurav A, Smer A, Abuzaid A, et al. Premature ventricular contraction‐induced cardiomyopathy. Clin Cardiol. 2015;38:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee AK, Deyell MW. Premature ventricular contraction–induced cardiomyopathy. Curr Opin Cardiol. 2016;31:1–10. [DOI] [PubMed] [Google Scholar]
- 4. Morady F, Kadish AH, DiCarlo L, et al. Long‐term results of catheter ablation of idiopathic right ventricular tachycardia. Circulation. 1990;82:2093–2099. [DOI] [PubMed] [Google Scholar]
- 5. Zang M, Zhang T, Mao J, et al. Beneficial effects of catheter ablation of frequent premature ventricular complexes on left ventricular function. Heart. 2014;100:787–793. [DOI] [PubMed] [Google Scholar]
- 6. Jesuraj ML, Rao BH, Sharada K, et al. Idiopathic right ventricular tract outflow tachycardia induced by high‐frequency stimulation. J Cardiovasc Electrophysiol. 2013;24:221–223. [DOI] [PubMed] [Google Scholar]
- 7. Calvo N, Jongbloed M, Zeppenfeld K. Radiofrequency catheter ablation of idiopathic right ventricular outflow tract arrhythmias. Indian Pacing Electrophysiol J. 2013;13:14–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boukens BJ, Christoffels VM, Coronel R, et al. Developmental basis for electrophysiological heterogeneity in the ventricular and outflow tract myocardium as a substrate for life‐threatening ventricular arrhythmias. Circ Res. 2009;104:19–31. [DOI] [PubMed] [Google Scholar]
- 9. Badhwar N, Scheinman MM. Idiopathic ventricular tachycardia: diagnosis and management. Curr Prob Cardiol. 2007;32:7‐43. [DOI] [PubMed] [Google Scholar]
- 10. Flemming MA, Oral H, Kim MH, et al. Electrocardiographic predictors of successful ablation of tachycardia or bigeminy arising in the right ventricular outflow tract. Am J Cardiol. 1999;84:1266–1268. [DOI] [PubMed] [Google Scholar]
- 11. Makhija A, Sharada K, Hygriv Rao B, et al. Hormone sensitive idiopathic ventricular tachycardia associated with pregnancy: successful induction with progesterone and radiofrequency ablation. J Cardiovasc Electrophysiol. 2011;22:95–98. [DOI] [PubMed] [Google Scholar]
- 12. Hasdemir C, Alp A, Aydin M, et al. Human model simulating right ventricular outflow tract tachycardia by high‐frequency stimulation in the left pulmonary artery: autonomics and idiopathic ventricular arrhythmias. J Cardiovasc Electrophysiol. 2009;20:759–763. [DOI] [PubMed] [Google Scholar]
- 13. Zhou J, Scherlag BJ, Yamanashi W, et al. Experimental model simulating right ventricular outflow tract tachycardia: a novel technique to initiate RVOT‐VT. J Cardiovasc Electrophysiol. 2006;17:771–775. [DOI] [PubMed] [Google Scholar]
- 14. Yamashina Y, Yagi T, Namekawa A, et al. Distribution of successful ablation sites of idiopathic right ventricular outflow tract tachycardia. Pacing Clin Electrophysiol. 2009;32:727–733. [DOI] [PubMed] [Google Scholar]
- 15. Hasdemir C, Aktas S, Govsa F, et al. Demonstration of ventricular myocardial extensions into the pulmonary artery and aorta beyond the ventriculo‐arterial junction. Pacing Clin Electrophysiol. 2007;30:534–539. [DOI] [PubMed] [Google Scholar]
- 16. Liu CF, Cheung JW, Thomas G, et al. Ubiquitous myocardial extensions into the pulmonary artery demonstrated by integrated intracardiac echocardiography and electroanatomic mapping: changing the paradigm of idiopathic right ventricular outflow tract arrhythmias. Circ Arrhythm Electrophysiol. 2014;7:691–700. [DOI] [PubMed] [Google Scholar]


