ABSTRACT
The Russian Acute Coronary Syndrome Registry (RusACSR) is a retrospective, continuous, nationwide, Web‐based registry of patients with acute coronary syndromes (ACS). The RusACSR is a database that uses a secure Web‐based interface for data entry by individual users. Participation in the RusACSR is voluntary. Any clinical center that provides health care to ACS patients can take part in the RusACSR. The RusACSR enrolls ACS patients who have undergone care in Russian hospitals from February 2008 to the present. Key data elements and methods of data analysis in the RusACSR are presented in this article. Up to 2015, 213 clinical centers from 36 regions of Russia had participated in the RusACSR. Currently, the database contains data on more than 250 000 ACS patients who underwent care from 2008 to 2015. Some current problems are highlighted in this article. The RusACSR is a perspective project for different epidemiologic studies in Russian ACS patients.
Introduction
There are a number of registries from various countries that include patients with acute coronary syndromes (ACS).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 The main goal of these registries is to fill the gap between probative data of randomized controlled trials and real clinical practice.12, 13 The main functions of the ACS registries are (1) to report on clinical and demographic characteristics of patients with ACS in covered cohorts; and (2) to assess the treatment of ACS in accordance with the current guidelines.
The registries can also be used for epidemiologic studies, original studies, risk modeling, improving the quality of health care, and other objectives. The registry is an ideal tool for studying the real clinical practice, especially when it is necessary to improve the quality of health care.
It is known that the quality of health care in ACS patients, especially revascularization in ACS patients with ST‐segment elevation on electrocardiogram (STE‐ACS), requires improvement in many countries.14, 15, 16 Within the framework of the Russian Federal Target Program Prevention and Control of Social Diseases (2007–2011) and the All‐Russian Vascular Program, in 2008 the Russian Acute Coronary Syndrome Registry (RusACSR; https://federalregistry.ru) was established. This registry is still functioning.
The aim of this article is to describe the objectives and design of the RusACSR. The presented results may be interesting to a wide audience, as the RusACSR is currently the largest registry of ACS patients in Russia.
Description of the Russian Acute Coronary Syndrome Registry
Objectives
The RusACSR has the following main objectives: (1) to create a national database with information on health care in ACS patients treated in Russia; (2) to obtain data on the demographic, clinical, and laboratory characteristics of ACS patients treated in Russia; (3) to identify the national features of associations between the characteristics of ACS and clinical outcomes, including mortality, complications, length of hospital stay, and quality of health care; and (4) to propose a practical guide for improving the quality and efficiency of ACS treatment in each clinical center participating in the RusACSR.
Developers
The Russian Cardiology Research and Production Complex (Moscow, Russia) was responsible for the development of the RusACSR and centralized data analysis at a federal level. The RusACSR was established in 2007 and 2008 by researchers, cardiologists, and information technology specialists from the Saratov Research Institute of Cardiology (Saratov, Russia). The current support of the RusACSR is carried out by the staff of both above‐mentioned organizations.
Participation
Participation in the RusACSR is voluntary and free of charge. Any clinical center that provides health care for ACS patients can participate in the RusACSR by sending a request to the technical support staff of the RusACSR. Starting in 2008, most of the main centers participating in the All‐Russian Vascular Program were invited to take part in the RusACSR by the Russian Cardiology Research and Production Complex. Up to 2015, 213 clinical centers from 36 regions of Russia had participated in the RusACSR (Figure 1) .17
Figure 1.
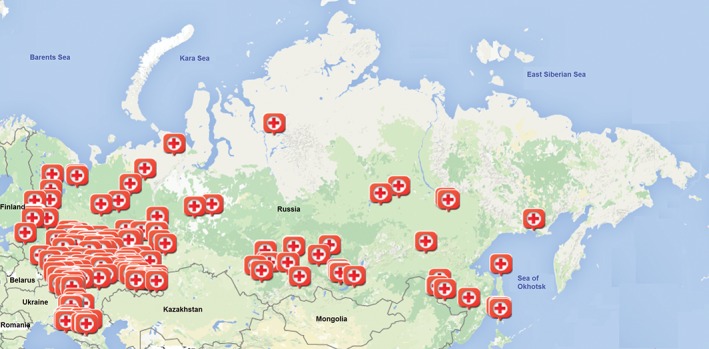
Maps with clinical centers participated in the RusACSR. Abbreviations: RusACSR, Russian Acute Coronary Syndrome Registry.
The majority of centers participating in the RusACSR are located in the Central Federal Region of the Russian Federation, and several centers are located in the North‐West Federal Region, South Federal Region, Ural Federal Region, and Siberian Federal Region. Thus, registry centers represent federal districts where the majority of the Russian population lives. Of course, these regions are socioeconomically heterogeneous, but an annual public report contains the data on each region separately.17
Design of the Russian Acute Coronary Syndrome Registry
The RusACSR is established as a retrospective, continuous, nationwide, Web‐based registry operating online (https://federalregistry.ru). The design of the RusACSR is based on the main points of the clinical guidelines on diagnosis and treatment of ACS.18, 19, 20, 21
Access to the Registry is given to registered members. Each user has a unique identification number and password to log in to the database. The Web forms are designed to be interactive. They limit or exclude certain options to avoid entry of conflicting or spurious data. Wherever possible, the data are entered by selection from drop‐down menus to minimize the number of keyboard errors. The purpose of all these measures is to maximize the accuracy of data.
The Web interface of the RusACSR contains 11 Web forms with the following titles: (1) personal data of ACS patients; (2) history of present event of ACS; (3) past history; (4) results of physical examination; (5) results of instrumental examinations; (6) results of laboratory tests; (7) invasive intervention; (8) prior therapy; (9) drug treatment of ACS; (10) recommendations at discharge; and (11) complications and outcomes.
Patients
The RusACSR enrolls patients who were admitted for ACS (ie, unstable angina or myocardial infarction [MI]) and underwent care in hospitals in Russia. Enrollment of patients started in February 2008 and has continued to the present day.
Inclusion criteria comprise any type of ACS (ie, unstable angina or MI) as a presumptive diagnosis, age ≥18 years, finished patient hospital chart, and absence of any exclusion criteria.22
The RusACSR exclusion criteria are the following: (1) symptoms considered as consistent with acute cardiac ischemia are absent within the last 24 hours prior to admission; (2) patient was transferred into a registry hospital >24 hours after admission to the initial hospital; (3) patient was transferred out of a registry hospital <24 hours after admission; (4) symptoms considered as ACS at admission were not consistent with acute cardiac ischemia; and (5) ACS accompanied by a significant comorbidity, such as a motor vehicle accident, trauma, or severe gastrointestinal bleeding, and operation or procedure directly before admission.22
A flow diagram of patient selection for inclusion in the RusACSR is presented in Figure 2.
Figure 2.
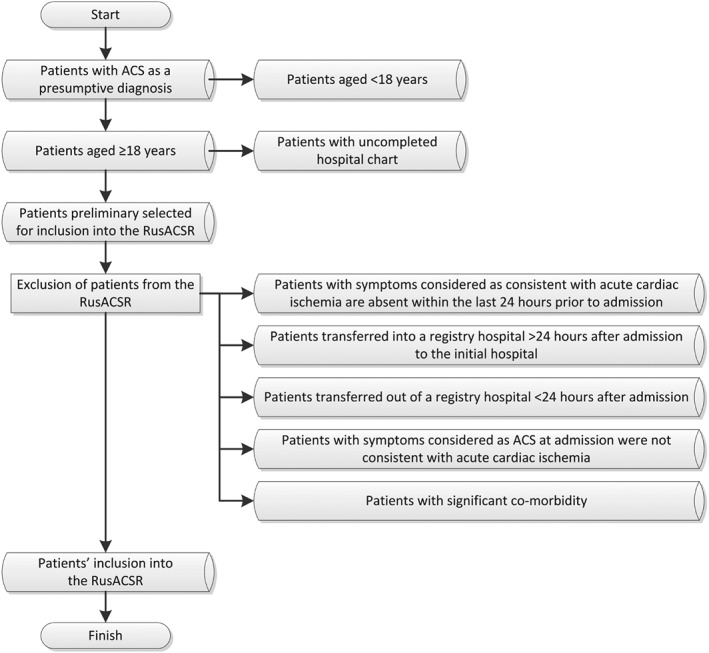
Flow diagram of patients' selection for inclusion to the RusACSR. Abbreviations: ACS, acute coronary syndrome; RusACSR, Russian Acute Coronary Syndrome Registry.
Data Elements
The key data elements and definitions of the RusACSR database were developed using the American College of Cardiology (ACC) 2001 Key Data Elements and Definitions for Measuring the Clinical Management and Outcomes of Patients with ACS23 and the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) 2011 Key Data Elements and Definitions of a Base Cardiovascular Vocabulary for Electronic Health Records.24 After 2013, we adopted the RusACSR to the 2013 ACCF/AHA Key Data Elements and Definitions for Measuring the Clinical Management and Outcomes of Patients with ACS and Coronary Artery Disease.25
Data on patient demographics, clinical characteristics, previous and hospital drug treatment, and reperfusion therapy are collected. Data on post‐hospital treatment of ACS patients are not included in the Registry. There is no capacity to follow patients after hospital discharge. (For details on the key data elements of the RusACSR database, see Supporting Information, Appendix A, in the online version of this article).
Data Collection
The centers participating in the RusACSR were asked to include all patients following inclusion/exclusion criteria treated for ACS during the year prior to the year of participation. The source of patient data is the hospital chart. To help the participants, a detailed user manual was developed.22 This user manual is available on the RusACSR Web site.
The centers are interested in accurate data collection because these data are analyzed further by experts separately for each center and used on‐site for health care quality management. In each center, ≥1 physicians have been trained to log patient data into the Registry. The content of data‐entry Web forms is intuitive for 88% of untrained users.26 Each year, experts from the RusACSR check the validity of entered data by reconciling randomly selected records with the data of patients' hospital charts. Currently, the RusACSR contains data on >250 000 ACS patients treated from 2008 to 2015. The main baseline characteristics of patients included in the RusACSR from February 1, 2008, to October 22, 2015, are presented in the Table 1.
Table 1.
Baseline Characteristics of ACS Patients Included in the RusACSR From February 1, 2008 to October 22, 2015
| Parameters | Values |
|---|---|
| Demographic and clinical characteristics (n = 254 584) | |
| Male sex | 49.8 |
| Age, y | 67 (58–77) |
| Prior MI | 28.6 |
| Prior chest pain | 55.5 |
| Prior PCI | 4.5 |
| Prior CABG | <0.1 |
| Prior CHF | 43.8 |
| Prior stroke and/or TIA | 7.6 |
| PVD | 6.3 |
| Family history of CAD | 19.9 |
| Hypertension | 79.3 |
| Smoking | 22.8 |
| DM | 15.3 |
| STE‐ACS patients | 33.6 |
| NSTE‐ACS patients | 49.2 |
| Heart rate at admission, bpm | 76 (68–86) |
| SBP at admission, mm Hg | 140 (120–160) |
| DBP at admission, mm Hg | 85 (80–90) |
| Killip class at admission (n = 169 007) | |
| I | 71.4 |
| II | 21.3 |
| III | 4.5 |
| IV | 2.8 |
| TC, mmol/L | 5.0 (4.2–6.0) |
| TG, mmol/L | 1.4 (1.0–2.0) |
| Cr, µmol/L | 92 (76–111) |
| Blood glucose, mmol/L | 5.6 (4.9–7.0) |
| ACS patients who underwent PCI | 16.6 |
| Prior medical therapy (n = 197 428) | |
| ASA | 45.6 |
| Clopidogrel | 9.5 |
| Nitrates | 34.6 |
| ACEIs | 47.8 |
| ARBs | 7.1 |
| β‐Blockers | 43.2 |
| Dihydropyridine CCBs | 9.1 |
| Non‐dihydropyridine CCBs | 2.0 |
| Warfarin | 1.9 |
| Reperfusion therapy in STE‐ACS patients (n = 85 496) | |
| Fibrinolitic therapy | 27.6 |
| Fibrinolitic therapy before hospital arrival | 8.0 |
| Time from chest pain (or its equivalent) to fibrinolitic therapy, min (n = 20 544) | 180 (120–300) |
| PCI (n = 85 496) | 29.4 |
| Primary PCI (n = 85 496) | 24.0 |
| Time from chest pain (or its equivalent) to PCI, min (n = 20 436) | 330 (200–750) |
| Drug therapy in ACS patients (STE‐ACS patients, n = 85 496; NSTE‐ACS patients, n = 125 228) | |
| Antiplatelet agents | |
| STE‐ACS patients | 97.8 |
| NSTE‐ACS patients | 97.3 |
| ASA | |
| STE‐ACS patients | 96.4 |
| NSTE‐ACS patients | 95.5 |
| Clopidogrel | |
| STE‐ACS patients | 84.4 |
| NSTE‐ACS patients | 76.4 |
| Anticoagulants | |
| STE‐ACS patients | 95.3 |
| NSTE‐ACS patients | 94.0 |
| β‐Blockers | |
| STE‐ACS patients | 89.0 |
| NSTE‐ACS patients | 89.8 |
| Statins | |
| STE‐ACS patients | 85.4 |
| NSTE‐ACS patients | 87.1 |
| ACEIs or ARBs | |
| STE‐ACS patients | 82.8 |
| NSTE‐ACS patients | 87.0 |
| Outcomes (STE‐ACS patients, n = 85 496; NSTE‐ACS patients, n = 125 228) | |
| In‐hospital cardiogenic shock | |
| STE‐ACS patients | 5.6 |
| NSTE‐ACS patients | 1.2 |
| Death during hospital stay | |
| STE‐ACS patients | 7.3 |
| NSTE‐ACS patients | 2.1 |
| Death during first day after admission | |
| STE‐ACS patients | 3.4 |
| NSTE‐ACS patients | 0.8 |
| GRACE risk score for in‐hospital death (STE‐ACS patients, n = 85 496; NSTE‐ACS patients, n = 125 228) | |
| STE‐ACS patients with data for calculating GRACE risk of in‐hospital death, n (%) | 40 214 (47.0) |
| NSTE‐ACS patients with data for calculating GRACE risk of in‐hospital death, n (%) | 27 896 (22.3) |
| STE‐ACS patients (n = 40 214) | |
| Low risk | 22.2 |
| Medium risk | 32.9 |
| High risk | 44.9 |
| NSTE‐ACS patients (n = 27 896) | |
| Low risk | 26.6 |
| Medium risk | 29.8 |
| High risk | 43.6 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitors; ACS, acute coronary syndrome; ARB, angiotensin II receptor blocker; ASA, aspirin; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCB, calcium channel blocker; CHF, chronic heart failure; Cr, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; GRACE, Global Registry of Acute Coronary Events; IQR, interquartile range; MI, myocardial infarction; NSTE‐ACS, non–ST‐segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; RusACSR, Russian Acute Coronary Syndrome Registry; SBP, systolic blood pressure; STE‐ACS, ST‐segment elevation acute coronary syndrome; TC, total cholesterol; TG, triglycerides; TIA, transient ischemic attack.
Data are presented as % or median (IQR), unless otherwise noted.
As for the proportion of ACS presentations included in the Registry since 2008, we have only an approximate estimation because the public statistics contain only the data on MI, and not on ACS morbidity across Russia. There are about 185 000 MIs per year in Russia. The RusACSR contains data on about 100 000 MI patients.
Data Security
Some of the main features of database and Web security issues should be mentioned briefly. As mentioned above, all users are assigned a unique username/password combination that is used to log on to the RusACSR. In this way, all transactions are recorded automatically in the Web server's log.
All the data are pseudonymously entered into a Web‐based database protected by a password on a safe server of the Russian Cardiology Research and Production Complex using secure sockets layer (SSL) connections. Subject identification is possible only at the local study site, and participating centers are exclusively able to review and modify the patient data. The data on ACS patients added to the RusACSR can be changed but cannot be removed. The transmitted data are stored in the central database on the central server at the Russian Cardiology Research and Production Complex.
The purpose of all the above‐mentioned measures is to ensure the confidentiality of the data.
Ethical Aspects
The study protocol including patient information and consent forms has been reviewed and approved by the Ethics Commission of the Russian Cardiology Research and Production Complex.
All patients must give informed consent before inclusion of their personal and clinical data in the RusACSR. The standard informed‐consent form is available on the RusACSR Web site. Patients gave their informed consent after transfer from an intensive care unit to a cardiac/coronary care unit. If patients died in the intensive care unit, consent was given by their relatives.
The appropriate measures are used to guarantee maximal data confidentiality. All patient‐related clinical data are anonymized locally.
Data Analysis
Within the RusACSR, an analytical module was created for the assessment of health care quality for ACS patients in Russia. The main aim of this analytical module is to implement the system analysis of clinical cases for the development of health care quality indicators for ACS patients to achieve the clinical result (for example, decrease of mortality).
We present the structure of a statistical report on health care in ACS patients that could be calculated in the RusACSR for each clinical center, region, or the whole of Russia (see Supporting Information, Appendix C, in the online version of this article). This report is calculated for any selected date range.
In the RusACSR, the completeness of execution of clinical guidelines in real clinical practice is evaluated using the developed clinical indicators, which are calculated automatically by using the database query for a required cohort of patients (in clinical units, in clinics, in regions of Russia, or in all participating clinics). These indicators were developed according to the ACCF/AHA methodology for the development of quality measures for cardiovascular technology.27 (For details of clinical indicators, see Supporting Information, Appendix D, in the online version of this article.)
Based on the presented clinical indicators, the quality of care in ACS patients was compared among Russian hospitals. This approach allows us not only to evaluate the quality of care in a particular hospital based on clinical guidelines, but also to carry out a comparative evaluation with other hospitals in the city, region, or whole of Russia.
Discussion
Disease registries allow data to be collected from large patient populations. Currently, the RusACSR is used for different goals by Russian researchers. A number of public reports on the quality of health care in ACS patients have been published recently.17, 28 Some authors already use the data from the RusACSR to assess the mortality29, 30 and appropriateness of percutaneous coronary intervention (PCI) in ACS patients in Russia,31 to study the quality of health care in ACS patients,32, 33, 34, 35, 36, 37 and to analyze the clinical factors associated with PCI performance in Russia.30 The results of the RusACSR have caused debate among Russian cardiologists.38 Some authors reported their comments and suggestions with a view to improving this registry.
Because the RusACSR uses key data elements and definitions recommended by the ACCF/AHA, the results of our study have the potential to be used for cross‐country comparison with different registries in other European countries and the United States.
The ACC/AHA have proposed the performance measures for STEMI (ST‐segment elevation myocardial infarction) and NSTEMI (non–ST‐segment elevation myocardial infarction) patients.39 The clinical indicators of the RusACSR have a similar goal. But our indicators were proposed in accordance with the national features of health care in ACS patients.
The evaluation of PCI in ACS patients has already become the target of some registries.40 These registries have provided useful insights regarding the practice of interventional cardiology in various countries. The RusACSR plays a similar role in Russia.
According to some authors, the employment of ACS registries in small territories (small countries, separate regions, or cities) can improve the quality of health care in ACS patients.41 The international experience shows that the most complete fulfillment of clinical guidelines in the care of STE‐ACS patients is achieved by using STEMI networks.42, 43 In Russia, several regions have already had a positive experience in implementing similar projects. Since 2008, the local STEMI networks based on the independent Samara STEMI Registry have been used successfully in the Samara region of Russia.44 The RusACSR can become a basis for future development of similar networks in the whole of Russia.
The use of the RusACSR in practical health care is associated with the need to reorganize the workload of staff in centers providing care for ACS patients. Untrained users may have some difficulties with the use of the RusACSR. To solve this problem, we developed a user manual.22 Another problem with the RusACSR is the formalization of medical information by users during the data transfer from a hospital chart to the Registry database. In our previous study, some problems were identified as the result of a test audit of 26 untrained users of the RusACSR.26 This study showed that the main errors are the incompleteness of data entry and the semantic content of entered data. The errors relating to incompleteness of data entry were most frequent for heart rate and blood pressure data (33%), results of instrumental examinations (55%), results of laboratory tests (55%), invasive interventions (38%), and advice at discharge on smoking, nutrition, and weight management (28%). The semantic errors were most frequent in data on past history (68%), main symptom at admission (51%), date and time of arrival of ambulance with the patient (49%), electrocardiogram parameters (48%), creatine phosphokinases and troponins (38%), coronary angiography (55%), and advice at discharge on smoking, nutrition, and weight management (50%). It was found that the error rate does not depend on the medical experience of users. After the training, these users entered about 80% of the data correctly. That is why obligatory annual training was organized for all Registry users. However, the problem of human error has still not been completely solved.
Another problem with the RusACSR is covering the Russian population of ACS patients. At first, only clinical centers from the All‐Russian Vascular Program participated in the RusACSR. Currently, different centers participate in the Registry. Nevertheless, covering the Russian population of ACS patients is insufficiently representative.38 The majority of centers that have participated in the RusACSR have quite good quality of health care, whereas most of the centers with low‐quality health care do not want to participate in the Registry. Widespread implementation of the RusACSR is the only solution to this problem.
Conclusion
The RusACSR is a perspective project for different epidemiologic studies in Russian ACS patients.
Supporting information
Appendix A. Key data elements of the database of the RusACSR
Appendix B. Addition data elements for coronary anatomy used in the RusACSR
Appendix C. Structure of statistical report on healthcare in ACS patients
Appendix D. Clinical indicators for assessment of quality of healthcare in ACS patients
Acknowledgments
The authors thank all participants of the RusACSR. This Registry is funded by the Russian Ministry of Health as a part of the Health National Project.
Drs. Gridnev, Kiselev, Posnenkova, and Prokhorov took part in the manuscript preparation. Drs. Gridnev, Kiselev, Posnenkova and Dmitriev designed the RusACSR. Drs. Dovgalevsky and Oschepkova contributed to the establishment of the federal RusACSR. Drs. Gridnev, Dovgalevsky, and Oschepkova contributed to the establishment of the local RusACSR. All authors reviewed and approved the final manuscript. The RusACSR is funded by the Russian Ministry of Health as a part of the Health National Project. The Russian Ministry of Health was not involved in the collection, analysis, and interpretation of data; in the manuscript preparation; or in making the decision to submit the article for publication. The authors have not received any financial support for the preparation of this article.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Fox KA, Eagle KA, Gore JM, et al; GRACE and GRACE2 Investigators . The Global Registry of Acute Coronary Events, 1999 to 2009—GRACE. Heart. 2010;96:1095–1101. [DOI] [PubMed] [Google Scholar]
- 2. Herrett E, Smeeth L, Walker L, et al; MINAP Academic Group . The Myocardial Ischaemia National Audit Project (MINAP). Heart. 2010;96:1264–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashrafi R, Hussain H, Brisk R, et al. Clinical disease registries in acute myocardial infarction. World J Cardiol. 2014;6:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leurent G, Garlantézec R, Auffret V, et al. Gender differences in presentation, management and in‐hospital outcome in patients with ST‐segment elevation myocardial infarction: data from 5000 patients included in the ORBI prospective French regional registry. Arch Cardiovasc Dis. 2014;107:291–298. [DOI] [PubMed] [Google Scholar]
- 5. Lin TH, Hsin HT, Wang CL, et al; Taiwan ACS Full Spectrum Registry Investigators. Impact of impaired glomerular filtration rate and revascularization strategy on one‐year cardiovascular events in acute coronary syndrome: data from Taiwan Acute Coronary Syndrome Full Spectrum Registry. BMC Nephrol. 2014;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw C, Nitsch D, Steenkamp R, et al. Inpatient coronary angiography and revascularisation following non–ST‐elevation acute coronary syndrome in patients with renal impairment: A cohort study using the Myocardial Ischaemia National Audit Project. PLoS One. 2014;9:e99925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trzeciak P, Gierlotka M, Gąsior M, et al. In‐hospital and 12‐month outcomes after acute coronary syndrome treatment in patients aged <40 years of age (from the Polish Registry of Acute Coronary Syndromes). Am J Cardiol. 2014;114:175–180. [DOI] [PubMed] [Google Scholar]
- 8. Gutierrez JA, Karwatowska‐Prokopczuk E, Murphy SA, et al. Effects of ranolazine in patients with chronic angina in patients with and without percutaneous coronary intervention for acute coronary syndrome: observations from the MERLIN‐TIMI 36 trial. Clin Cardiol. 2015;38:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huo Y, Lee SW, Sawhney JP, et al. Rationale, design, and baseline characteristics of the EPICOR Asia study (Long‐term Follow‐up of Antithrombotic Management Patterns in Acute Coronary Syndrome Patients in Asia). Clin Cardiol. 2015;38:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reibis R, Völler H, Gitt A, et al. Management of patients with ST‐segment elevation or non–ST‐segment elevation acute coronary syndromes in cardiac rehabilitation centers. Clin Cardiol. 2014;37:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thukkani AK, Fonarow GC, Cannon CP, et al; Get With the Guidelines Steering Committee and Investigators. Quality of care for patients with acute coronary syndromes as a function of hospital revascularization capability: insights from Get With the Guidelines‐CAD. Clin Cardiol. 2014;37:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fox KA. Registries and surveys in acute coronary syndrome. Eur Heart J. 2006;27:2260–2262. [DOI] [PubMed] [Google Scholar]
- 13. Gitt AK, Bueno H, Danchin N, et al. The role of cardiac registries in evidence‐based medicine. Eur Heart J. 2010;31:525–529. [DOI] [PubMed] [Google Scholar]
- 14. Bradley EH, Nallamothu BK, Herrin J, et al. National efforts to improve door‐to‐balloon time: results from the Door‐to‐Balloon Alliance. J Am Coll Cardiol. 2009;54:2423–2429. [DOI] [PubMed] [Google Scholar]
- 15. Widimsky P, Wijns W, Fajadet J, et al; European Association for Percutaneous Cardiovascular Interventions . Reperfusion therapy for ST‐elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J. 2010;31:943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Érlikh AD, Kharchenko MS, Barbarash OL, et al. Adherence to guidelines on management of acute coronary syndrome in Russian hospitals and outcomes of hospitalization (data from the RECORD‐2 Registry) [article in Russian]. Kardiologiia. 2013;53:14–22. [PubMed] [Google Scholar]
- 17. Posnenkova OM, Korotin AS, Kiselev AR, et al. Performance of recommended treatment measures in patients with acute coronary syndrome in 2014: a report on the data from Federal Registry [article in Russian]. Cardio‐IT. 2015;2:e0101. [Google Scholar]
- 18. National guidelines for treatment of acute coronary syndromes without persistent ST elevation on ECG. Cardiovasc Ther Prevent. 2006;5(8 suppl 1):1–32. [Google Scholar]
- 19. National guidelines for diagnosis and treatment of patients with acute myocardial infarction with ST‐segment elevation on ECG. Cardiovasc Ther Prevent. 2007;6(8 suppl 1):415–500. [Google Scholar]
- 20. Bassand JP, Hamm CW, Ardissino D, et al; Task Force for Diagnosis and Treatment of Non–ST‐segment Elevation Acute Coronary Syndromes of European Society of Cardiology. Guidelines for the diagnosis and treatment of non–ST‐segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. [DOI] [PubMed] [Google Scholar]
- 21. Van de Werf F, Bax J, Betriu A, et al; ESC Committee for Practice Guidelines (CPG) . Management of acute myocardial infarction in patients presenting with persistent ST‐segment elevation: the Task Force on the Management of ST‐Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. [DOI] [PubMed] [Google Scholar]
- 22. Oshchepkova EV, PYa Dovgalevsky, Gridnev VI, et al. Federal Registry of Acute Coronary Syndrome user guide [article in Russian]. Cardio‐IT. 2014;1:0203. [Google Scholar]
- 23. Cannon CP, Battler A, Brindis RG, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patient with acute coronary syndromes: a report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol. 2001;38:2114–2130. [DOI] [PubMed] [Google Scholar]
- 24. Weintraub WS, Karlsberg RP, Tcheng JE, et al. ACCF/AHA 2011 key data elements and definitions of a base cardiovascular vocabulary for electronic health records: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards [published correction appears in Circulation. 2011;124:e174]. Circulation. 2011;124:103–123. [DOI] [PubMed] [Google Scholar]
- 25. Cannon CP, Brindis RG, Chaitman BR, et al. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Acute Coronary Syndromes and Coronary Artery Disease Clinical Data Standards). Circulation. 2013;127:1052–1089. [DOI] [PubMed] [Google Scholar]
- 26. Posnenkova OM, Kiselev AR, Gridnev VI. Experience of untrained users' work audit conducted during the testing of federal registry of patients with acute coronary syndrome [article in Russian]. Cardio‐IT. 2014;1:0301. [Google Scholar]
- 27. Bonow RO, Douglas PS, Buxton AE, et al. ACCF/AHA methodology for the development of quality measures for cardiovascular technology: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures. Circulation. 2011;124:1483–1502. [DOI] [PubMed] [Google Scholar]
- 28. Oschepkova EV, Dmitriev VA, Gridnev VI, et al. The three‐year experience of the Russian Acute Coronary Syndrome Registry in the some acute care hospitals [article in Russian]. Kardiol Vest. 2012;7:5–9. [Google Scholar]
- 29. Oshchepkova EV, Efremova IuE, Karpov IuA. Myocardial infarction morbidity and mortality in the Russian Federation in 2000–2011 [article in Russian]. Ter Arkh. 2013;85:4–10. [PubMed] [Google Scholar]
- 30. Posnenkova OM, Kiselev AR, Popova YV, et al. Impact of patient‐related and treatment‐related factors on in‐hospital mortality of patients with ST‐elevation myocardial infarction: data of Russian Acute Coronary Syndrome Registry. Cor et Vasa. 2014;56:e217–e227. [Google Scholar]
- 31. Kiselev AR, Popova YV, Posnenkova OM, et al. Implementation of percutaneous coronary interventions in patients with acute coronary syndrome in Russia and clinical factors influencing decision making. Cor et Vasa. 2014;56:e1–e10. [Google Scholar]
- 32. Boitsov SA, PYa Dovgalevsky, Gridnev VI, et al. Comparative analysis of the data of Russian and foreign acute coronary syndrome registries [article in Russian]. Kardiol Vest. 2010;5:82–86. [Google Scholar]
- 33. Oshchepkova EV, Dmitriev VA, Gridnev VI, et al. Assessment of the quality of medical assistance for patients with acute ST‐elevation coronary syndrome for 2009–2010 in regions of the Russian Federation participating in the “vascular program” (by the data of the Russian ACS Registry) [article in Russian]. Ter Arkh. 2012;84:23–29. [PubMed] [Google Scholar]
- 34. Oshchepkova EV, Dmitriev VA, Gridnev VI, et al. Organization of medical care for patients with non–ST‐segment elevation acute coronary syndrome in regional vascular centers and primary vascular units in 2009–2012 (according to the data of the ACS register) [article in Russian]. Ter Arkh. 2013;85:4–8. [PubMed] [Google Scholar]
- 35. Posnenkova OM, Kiselev AR, Gridnev VI, et al. Assessment of myocardial reperfusion quality in patients with acute coronary syndrome and ST‐segment elevation, based on the criteria by the American College of Cardiology/American Heart Association. Cardiovasc Ther Prevent. 2013;12:40–44. [Google Scholar]
- 36. Popova YV, Posnenkova OM, Kiselev AR, et al. Implementation of evidence‐based clinical‐and‐morphological appropriate use criteria for coronary revascularization in patients with acute coronary syndrome in Russia [article in Russian]. Cardiovasc Ther Prevent. 2014;13:24–28. [Google Scholar]
- 37. Posnenkova OM, Kiselev AR, Popova YuV, et al. Novel approach to evaluation of medical care quality delivered to patients with ST‐segment elevation acute coronary syndrome: course to clinical result. Saratov J Med Sci Res. 2014;10:408–413. [Google Scholar]
- 38. Ganyukov VI, Shilov AA. Come back to an issue of performance of recommended treatment measures in patients with acute coronary syndrome in hospitals of the Russian Federation [article in Russian]. Cardio‐IT. 2015;2:e0201. [Google Scholar]
- 39. Krumholz HM, Anderson JL, Bachelder BL, et al. ACC/AHA 2008 performance measures for adults with ST‐elevation and non–ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to develop performance measures for ST‐elevation and non–ST‐elevation myocardial infarction): developed in collaboration with the American Academy of Family Physicians and the American College of Emergency Physicians: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. Circulation. 2008;118:2596–2648. [DOI] [PubMed] [Google Scholar]
- 40. Papaioannou GI, Chatzis DG, Kotsanis A, et al; Working Group of Hemodynamics and Interventional Cardiology, Hellenic Society of Cardiology. Organization, structure and data of the Hellenic Heart Registry on Percutaneous Coronary Interventions: a step forward towards outcomes research. Hellenic J Cardiol. 2014;55:227–234. [PubMed] [Google Scholar]
- 41. Pipilis AG, Paschidi MD, Andrikopoulos GK, et al; Working Group on Clinical Epidemiology, Prevention and Metabolic Syndrome of the Hellenic Cardiological Society. Seven plus one reasons for surveys of acute myocardial infarction in Greece. Hellenic J Cardiol. 2006;47:194–197. [PubMed] [Google Scholar]
- 42. PhG Steg, James SK, Atar D, et al; Task Force on the management of ST‐segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 43. García‐García C, Merono O, Recasens LL, et al. In‐hospital prognosis of STEMI before and after a primary PCI reperfusion network: eleven years' experience. Eur Heart J. 2014;3(suppl 2):41. [Google Scholar]
- 44. Posnenkova OM, Kiselev AR, Duplyakov DV, et al. Using the Russian system of indicators for healthcare quality assessment in patients with acute coronary syndrome with ST‐segment elevation: a step towards the practice of public reporting on quality [article in Russian]. Cardio‐IT. 2014;1:0401. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Key data elements of the database of the RusACSR
Appendix B. Addition data elements for coronary anatomy used in the RusACSR
Appendix C. Structure of statistical report on healthcare in ACS patients
Appendix D. Clinical indicators for assessment of quality of healthcare in ACS patients


