ABSTRACT
Background
In recent years, there has been growing evidence that vitamin D deficiency is associated with the development and progression of chronic heart failure (CHF).
Hypothesis
Additional supplementation of vitamin D may have protective effects in patients with CHF.
Methods
We searched PubMed, Embase, and Cochrane databases through June 2015 and included 7 randomized controlled trials that investigated the effects of vitamin D on cardiovascular outcomes in patients with CHF. Then, we performed a meta‐analysis of clinical trials to confirm whether vitamin D supplementation is beneficial in CHF patients. The weighted mean difference (WMD) and 95% confidence interval (CI) were calculated using fixed‐ or random‐effects models.
Results
Our pooled results indicated that additional supplementation of vitamin D was not superior to conventional treatment in terms of left ventricular ejection fraction, N‐terminal pro‐B‐type natriuretic peptide, and 6‐minute walk distance. Moreover, vitamin D supplementation was associated with significant decreases in the levels of tumor necrosis factor‐α (WMD: −2.42 pg/mL, 95% CI: −4.26 to −0.57, P < 0.05), C‐reactive protein (WMD: −0.72 mg/L, 95% CI: −1.42 to −0.02, P < 0.05), and parathyroid hormone (WMD: −13.44 pg/mL, 95% CI: −21.22 to −5.67, P < 0.05).
Conclusions
Vitamin D supplementation may decrease serum levels of parathyroid hormone and inflammatory mediators in CHF patients, whereas it has no beneficial effects on improvement of left ventricular function and exercise tolerance.
Introduction
Chronic heart failure (CHF) is a complex clinical syndrome that arises secondary to inherited or acquired abnormalities of cardiac structure and/or function that impair myocardial systolic and diastolic function. In the United States, CHF affects approximately 4.7 million people, with nearly 550 000 incident cases of CHF diagnosed annually.1 The medical treatment of CHF has made remarkable progress in the past few decades. Clinical applications of β‐receptor blockers, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, and aldosterone receptor antagonists have significantly reduced cardiovascular events and improved prognosis in patients with CHF.2 However, CHF remains a leading cause of morbidity and mortality throughout the world.
In recent years, epidemiological studies have demonstrated that vitamin D deficiency is associated with increased incidence of hypertension, myocardial infarction, and stroke, as well as chronic kidney disease and type 2 diabetes mellitus.3, 4, 5, 6, 7, 8 Low vitamin D levels have been found to activate the renin‐angiotensin‐aldosterone system, induce inflammatory response, and cause endothelial dysfunction.9, 10, 11 In patients with CHF, vitamin D deficiency is prevalent and is associated with a poor prognosis.12, 13, 14 Over the past few years, several randomized controlled trials (RCTs) have been carried out to evaluate the effects of vitamin D supplementation in CHF patients.15, 16, 17, 18, 19, 20, 21 These trials investigated clinical symptoms, cardiac function, quality of life, physical performance, cardiovascular events, and inflammation, comparing vitamin D with placebo. However, some results of clinical studies are inconsistent and the conclusions are contradictory. We therefore performed a meta‐analysis of RCTs to confirm whether vitamin D supplementation is beneficial in patients with CHF.
Methods
Search Strategy
We performed an electronic literature search of PubMed, Embase, and Cochrane databases through June 2015, using the terms vitamin D, cholecalciferol, 1,25‐dihydroxy vitamin D3, 25‐hydroxyvitamin D, calcitriol, heart failure, cardiac failure, cardiac dysfunction, cardiac insufficiency, cardiomyopathy, and ventricular dysfunction. Sensitive filters identified clinical trial or RCT in the PubMed database and the Embase database. The search was limited to human subjects, with no restriction for language.
Study Selection
Clinical trials reporting ≥1 of the outcomes were considered eligible. These outcomes included left ventricular ejection fraction (LVEF), New York Heart Association (NYHA) class, 6‐minute walk distance (6MWD), N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), tumor necrosis factor‐α (TNF‐α), C‐reactive protein (CRP), interleukin‐10 (IL‐10), parathyroid hormone (PTH), and renin.
Data Extraction and Quality Assessment
Two investigators independently reviewed all potentially eligible studies using predefined eligibility criteria and extracted data from the included RCTs. We collected details on study characteristics, patient characteristics, inclusion criteria, intervention strategies, duration of follow‐up, and clinical outcomes including LVEF, NYHA class, 6MWD, NT‐proBNP, TNF‐α, CRP, IL‐10, PTH, and renin. The quality of enrolled RCTs was evaluated by the Jadad scale, and a numerical score between 0 and 5 was assigned as a measure of study design.
Statistical Analysis
Continuous variables were analyzed using weighted mean differences (WMD) and 95% confidence intervals (CI). The heterogeneity of results across trials was assessed using the χ2‐based Q test. A P value >0.1 for the Q test indicated a lack of heterogeneity among the studies. Thus, the pooled effect was calculated using fixed‐effects model. Otherwise, a random‐effects model was used in case of significant heterogeneity across studies. A sensitivity analysis was performed to evaluate the influence of each individual study on overall estimates by sequential removal of individual studies. Potential publication bias was assessed by the Begg and Egger test. All statistical analyses were conducted using RevMan version 5.0 (the Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark) and Stata software version 10.0 (StataCorp LP, College Station, TX).
Results
Among the initial 144 studies, 18 trials were retrieved for detailed evaluation and 7 RCTs satisfying the inclusion criteria were finally included. The baseline characteristics of enrolled studies and patients are shown in Tables 1 and 2. Vitamin D dosage ranged from 1000 IU/d to 50 000 IU/wk and follow‐up periods from 6 weeks to 9 months in this meta‐analysis. (For the flow of study selection for this meta‐analysis and quality assessment of included RCTs by the Jadad scale, see Supporting Information, Figure 1 and Table 1, respectively, in the online version of this article).
Table 1.
Study Characteristics
| Study | No. of Patients (Vitamin D/ Control) | Vitamin D Dose | Follow‐up Duration | Inclusion Criteria | Endpoints |
|---|---|---|---|---|---|
| Dalbeni et al, 2014 | 36 (18/18) | 4000 IU/d | 6 mo | Age >40 y, chronic HF, LVEF <55%, NYHA class > II, 25(OH)D <30 ng/mL | LVEF, echocardiographic parameters, NYHA class, NT‐proBNP, PTH, renin, aldosterone |
| Boxer et al, 2014 | 64 (31/33) | 50 000 IU/wk | 6 mo | Age ≥50 y, NYHA class II–IV, 25(OH)D <37.5 ng/mL | LVEF, echocardiographic parameters, PTH, NT‐proBNP, hsCRP, renin, aldosterone |
| Schroten et al, 2013 | 101 (50/51) | 2000 IU/d | 6 wk | Age ≥18 y, chronic HF, LVEF <45% | Plasma renin activity and concentration, NT‐proBNP, fibrosis markers, PTH |
| Boxer et al, 2013 | 64 (31/33) | 50 000 IU/wk | 6 mo | Age ≥50 y, NYHA class II–IV, 25(OH)D <37.5 ng/mL | Peak VO2, exercise duration, 6MWD |
| Shedeed et al, 2012 | 80 (42/38) | 1000 IU/d | 12 wk | Infants with chronic HF, LVEF <40% | LVEF, echocardiographic parameters, TNF‐α, IL‐10, PTH |
| Witham et al, 2010 | 105 (53/52) | 100 000 IU at baseline and 10 wk | 20 wk | Age ≥70 y, chronic HF, LV systolic dysfunction, NYHA class II–III, 25(OH)D <20 ng/mL | 6MWD, renin, aldosterone, BNP, TNF‐α |
| Schleithoff et al, 2006 | 123 (61/62) | 2000 IU/d | 9 mo | Chronic HF, NYHA class II–IV | Survival rates, LVEF, NT‐proBNP, TNF‐α, CRP, IL‐10, PTH |
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; 6MWD, 6‐minute walk distance; BNP, B‐type natriuretic peptide; CRP, C‐reactive protein; HF, heart failure; IL‐10, interleukin‐10; IU, international units; LV, left ventricular; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PTH, parathyroid hormone; TNF‐α, tumor necrosis factor‐α; VO2, oxygen volume.
Table 2.
Patient Characteristics
| Study | No. of Patients (Vitamin D/ Control) | Age, y | Male Sex, n | Ischemic Cause, n | HTN, n | LVEF, % | 25(OH)D, ng/mL | NYHA Class |
|---|---|---|---|---|---|---|---|---|
| Dalbeni et al, 2014 | 36 (18/18) | 74.2/74.3 | 12/10 | 16/17 | 17/18 | 42.3/44.3 | 17.2/17.6 | II–IV |
| Boxer et al, 2014 | 64 (31/33) | 65.8/66 | 15/18 | 8/10 | 26/28 | 39.2/36.1 | 19.1/17.8 | II–III |
| Schroten et al, 2013 | 101 (50/51) | 64/63.5 | 48/46 | 35/36 | NA | 35.7/33.6 | 27.6/28.8 | II–III |
| Boxer et al, 2013 | 64 (31/33) | 65.8/66 | 15/18 | 8/10 | 26/28 | 39.2/36.1 | 19.1/17.8 | II–III |
| Shedeed et al, 2012 | 80 (42/38) | 0.86/0.93 | 27/22 | NA | NA | 36.4/37.2 | NA | NA |
| Witham et al, 2010 | 105 (53/52) | 78.8/80.6 | 34/35 | 33/34 | 33/33 | 33/29 | 12.3/14.2 | II–III |
| Schleithoff et al, 2006 | 123 (61/62) | 57/54 | 52/50 | 47/40 | 38/32 | 31/33 | 14.4/15.3 | II–IV |
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; HTN, hypertension; LVEF, left ventricular ejection fraction; NA, not available; NYHA, New York Heart Association.
Data are expressed as vitamin D/control.
Our results indicated that additional supplementation of vitamin D was not superior to conventional treatment in terms of LVEF, NT‐proBNP, and 6MWD (WMD: 4.11%, 95% CI: −0.91 to 9.12, P = 0.11; WMD: −80.8 pg/mL, 95% CI: −305.3 to 143.7, P = 0.48; WMD: 8.90 m, 95% CI: −48.47 to 66.26, P = 0.76, respectively; Figure 1). Because a significant heterogeneity across studies was observed for LVEF, we conducted a sensitivity analysis to assess the effect of each study on the pooled estimate under the random‐effects model. The results showed that removal of any individual study could not significantly reduce the heterogeneity. In addition, there was no evidence of publication bias for LVEF among the included studies.
Figure 1.

Forest plots for (A) LVEF, (B) NT‐proBNP, and (C) 6MWD. Abbreviations: 6MWD, 6‐minute walk distance; CI, confidence interval; df, degrees of freedom; IV, inverse variance; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SD, standard deviation.
The pooled analysis also indicated that serum levels of TNF‐α and CRP were markedly decreased in the vitamin D group compared with those in the control group (WMD: −2.42 pg/mL, 95% CI: −4.26 to −0.57, P < 0.05; WMD: −0.72 mg/L, 95% CI: −1.42 to −0.02, P < 0.05, respectively; Figure 2A and 2B). However, there was no significant difference in serum IL‐10 level between patients treated with vitamin D and placebo (WMD: 0.94 pg/mL, 95% CI: −0.72 to 2.59, P = 0.27; Figure 2C).
Figure 2.
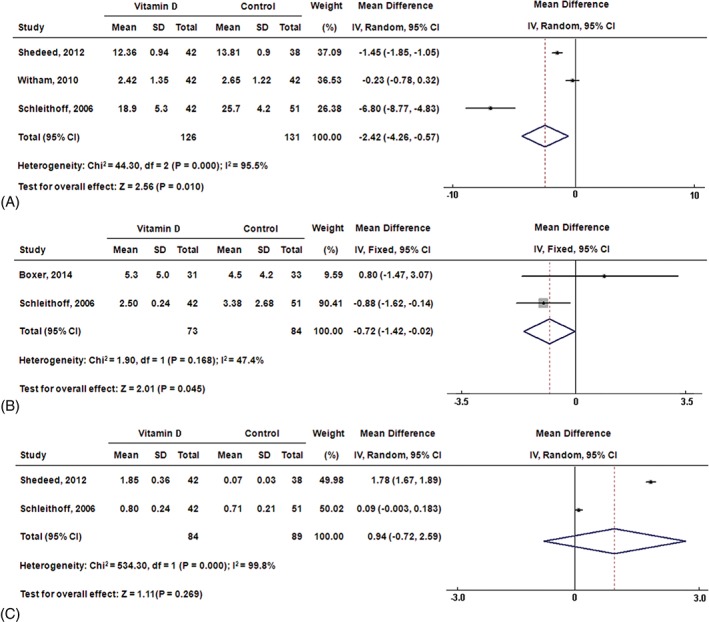
Forest plots for (A) TNF‐α, (B) CRP, and (C) IL‐10. Abbreviations: CI, confidence interval; CRP, C‐reactive protein; df, degrees of freedom; IL‐10, interleukin‐10; IV, inverse variance; SD, standard deviation; TNF‐α, tumor necrosis factor‐α.
Our results revealed that vitamin D supplementation was associated with a marked decline in PTH level in the vitamin D group compared with those in the control group (WMD: −13.44 pg/mL, 95% CI: −21.22 to −5.67, P < 0.01; Figure 3A). However, there was no significant difference in plasma renin concentration between CHF patients treated with vitamin D and placebo (WMD: −13.52 pg/mL, 95% CI: −43.66 to 16.63, P = 0.38; Figure 3B). In addition, we performed Begg and Egger tests and found that there was no publication bias for PTH among the included studies.
Figure 3.

Forest plots for (A) parathyroid hormone and (B) renin. Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; SD, standard deviation.
Discussion
We carried out a meta‐analysis of RCTs to evaluate the effects of vitamin D supplementation on prognosis of patients with CHF. Our results suggested that vitamin D therapy could decrease the serum levels of PTH, TNF‐α, and CRP in CHF patients, whereas it had no beneficial effects on improvement of left ventricular function and exercise tolerance.
In recent years, accumulating evidence has demonstrated that vitamin D deficiency is related to the increased occurrence of cardiovascular disease (CVD). Wang et al22 performed a meta‐analysis of prospective observational studies and showed a generally linear, inverse association between circulating 25(OH)‐vitamin D and risk of CVD. Comparing the lowest with the highest category of 25(OH)‐vitamin D concentration, the pooled relative risk for total CVD was 1.52 (95% CI: 1.30 to 1.77). Gotsman et al13 reported that vitamin D deficiency was highly prevalent in patients with CHF and was a significant predictor of reduced survival. Fall et al23 conducted a community‐based study among the elderly and found higher circulating vitamin D concentrations to be associated with better ventricular function after adjusting for several cardiovascular risk factors. Liu et al12 indicated that low 25(OH)‐vitamin D concentration was associated with a poor prognosis in CHF patients, and they found that activation of the renin‐angiotensin‐aldosterone system and inflammation may confer the adverse effects of low vitamin D levels.
Although there is sufficient evidence that vitamin D deficiency is associated with increased incidence and poor prognosis of CHF, it remains unclear whether vitamin D supplementation can reduce cardiovascular events and improve clinical prognosis in CHF patients. In the past decade, several small RCTs have been carried out to evaluate the effects of vitamin D treatment in patients with CHF. Schleithoff et al21 reported that additional supplementation of vitamin D in CHF patients decreased proinflammatory cytokine levels, without changes in left ventricular function and NT‐proBNP levels. Witham et al20 showed that vitamin D supplementation did not improve functional capacity or quality of life in older patients with CHF. Conversely, Shedeed et al19 indicated that children with CHF achieved marked improvement in both cardiac function and inflammatory markers after 12 weeks of vitamin D supplementation. Collectively, these RCTs of vitamin D therapy in CHF patients have generated inconsistent results with regard to cardiovascular outcomes. We therefore performed a meta‐analysis of RCTs to confirm whether vitamin D supplementation is beneficial in patients with CHF. The pooled results showed that vitamin D therapy was not associated with improvement in LVEF, NT‐proBNP, and 6MWD, suggesting that additional supplementation of vitamin D in CHF patients has no significant effects on cardiovascular outcomes such as left ventricular function and exercise tolerance.
It has been well documented that inflammatory mediators are critically involved in the pathogenesis of ventricular remodeling and may serve as serum biomarkers reflecting the severity and prognosis of CHF.24, 25 Recently, several studies suggested that circulating concentration of PTH was correlated with the severity of CHF and it could serve as a useful biomarker of the disease.26, 27, 28 In the present meta‐analysis, our pooled results revealed that vitamin D supplementation was associated with a significant decrease in serum levels of TNF‐α, CRP, and PTH. We can therefore infer that vitamin D may exert protective effects in patients with CHF by reducing inflammatory factors and PTH levels.
Study Limitations
Although we included relatively high‐quality RCTs that investigated the effects of vitamin D supplementation on cardiovascular outcomes in CHF patients, there were also several limitations existing in this meta‐analysis. First, the methodological quality of enrolled studies was less than optimal, so we were not able to exclude the potential risk of bias in these trials. Second, the number of patients included in this meta‐analysis was relatively small, so further large‐sample‐size and well‐designed RCTs are needed. Third, substantial heterogeneity was observed across studies in this meta‐analysis, which might affect the outcomes, although a random‐effects model was used.
Conclusion
Our meta‐analysis demonstrates that vitamin D supplementation may decrease serum levels of PTH and inflammatory mediators such as TNF‐α and CRP in CHF patients, whereas it has no beneficial effects on improvement of left ventricular function and exercise tolerance. Our findings will provide valuable information for the clinicians in the treatment of CHF.
Supporting information
Supplementary Figure 1. Flow diagram of eligible studies included in the meta‐analysis.
Supplementary Table 1. Quality assessment of included studies
Wei‐Long Jiang, MD, and Hai‐Bo Gu, MD, contributed equally to this work.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Roger VL, Go AS, Lloyd‐Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association [published corrections appear in Circulation. 2011;123:e240 and Circulation. 2011;124:e426]. Circulation. 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krum H, Teerlink JR. Medical therapy for chronic heart failure. Lancet. 2011;378:713–721. [DOI] [PubMed] [Google Scholar]
- 3. Tamez H, Thadhani RI. Vitamin D and hypertension: an update and review. Curr Opin Nephrol Hypertens. 2012;21:492–499. [DOI] [PubMed] [Google Scholar]
- 4. Karakas M, Thorand B, Zierer A, et al. Low levels of serum 25‐hydroxyvitamin D are associated with increased risk of myocardial infarction, especially in women: results from the MONICA/KORA Augsburg case‐cohort study. J Clin Endocrinol Metab. 2013;98:272–280. [DOI] [PubMed] [Google Scholar]
- 5. Sun Q, Pan A, Hu FB, et al. 25‐Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta‐analysis. Stroke. 2012;43:1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nigwekar SU, Thadhani RI. Shining light on vitamin D trials in chronic kidney disease. Kidney Int. 2013;83:198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lim S, Kim MJ, Choi SH, et al. Association of vitamin D deficiency with incidence of type 2 diabetes in high‐risk Asian subjects. Am J Clin Nutr. 2013;97:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desai CK, Huang J, Lokhandwala A, et al. The role of vitamin supplementation in the prevention of cardiovascular disease events. Clin Cardiol. 2014;37:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li YC, Kong J, Wei M, et al. 1,25‐Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin‐angiotensin system. J Clin Invest. 2002;110:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase‐1. J Immunol. 2012;188:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caprio M, Mammi C, Rosano GM. Vitamin D: a novel player in endothelial function and dysfunction. Arch Med Sci. 2012;8:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu LC, Voors AA, van Veldhuisen DJ, et al. Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail. 2011;13:619–625. [DOI] [PubMed] [Google Scholar]
- 13. Gotsman I, Shauer A, Zwas DR, et al. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Eur J Heart Fail. 2012;14:357–366. [DOI] [PubMed] [Google Scholar]
- 14. Pourdjabbar A, Dwivedi G, Haddad H. The role of vitamin D in chronic heart failure. Curr Opin Cardiol. 2013;28:216–222. [DOI] [PubMed] [Google Scholar]
- 15. Dalbeni A, Scaturro G, Degan M, et al. Effects of six months of vitamin D supplementation in patients with heart failure: a randomized double‐blind controlled trial. Nutr Metab Cardiovasc Dis. 2014;24:861–868. [DOI] [PubMed] [Google Scholar]
- 16. Boxer RS, Hoit BD, Schmotzer BJ, et al. The effect of vitamin D on aldosterone and health status in patients with heart failure. J Card Fail. 2014;20:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schroten NF, Ruifrok WP, Kleijn L, et al. Short‐term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: an open‐label, blinded end point, randomized prospective trial (VitD‐CHF trial). Am Heart J. 2013;166:357.e2–364.e2. [DOI] [PubMed] [Google Scholar]
- 18. Boxer RS, Kenny AM, Schmotzer BJ, et al. A randomized controlled trial of high dose vitamin D3 in patients with heart failure. JACC Heart Fail. 2013;1:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shedeed SA. Vitamin D supplementation in infants with chronic congestive heart failure. Pediatr Cardiol. 2012;33:713–719. [DOI] [PubMed] [Google Scholar]
- 20. Witham MD, Crighton LJ, Gillespie ND, et al. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2010;3:195–201. [DOI] [PubMed] [Google Scholar]
- 21. Schleithoff SS, Zittermann A, Tenderich G, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double‐blind, randomized, placebo‐controlled trial. Am J Clin Nutr. 2006;83:754–759. [DOI] [PubMed] [Google Scholar]
- 22. Wang L, Song Y, Manson JE, et al. Circulating 25‐hydroxy‐vitamin D and risk of cardiovascular disease: a meta‐analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fall T, Shiue I, Bergeå af Geijerstam P, et al. Relations of circulating vitamin D concentrations with left ventricular geometry and function. Eur J Heart Fail. 2012;14:985–991. [DOI] [PubMed] [Google Scholar]
- 24. Gullestad L, Ueland T, Vinge LE, et al. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23–35. [DOI] [PubMed] [Google Scholar]
- 25. Hartupee J, Mann DL. Positioning of inflammatory biomarkers in the heart failure landscape. J Cardiovasc Transl Res. 2013;6:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gruson D, Lepoutre T, Ahn SA, et al. Increased circulating concentrations of bioactive PTH 1–84 in patients with heart failure. J Endocrinol Invest. 2012;35:987–991. [DOI] [PubMed] [Google Scholar]
- 27. Altay H, Zorlu A, Binici S, et al. Relation of serum parathyroid hormone level to severity of heart failure. Am J Cardiol. 2012;109:252–256. [DOI] [PubMed] [Google Scholar]
- 28. Altay H, Colkesen Y. Parathyroid hormone and heart failure: novel biomarker strategy. Endocr Metab Immune Disord Drug Targets. 2013;13:100–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Flow diagram of eligible studies included in the meta‐analysis.
Supplementary Table 1. Quality assessment of included studies


