ABSTRACT
Background
Currently no research exists assessing lifestyle modifications and emotional state of acute aortic dissection (AAD) survivors. We sought to assess activity, mental health, and sexual function in AAD survivors.
Hypothesis
Physical and sexual activity will decrease in AAD survivors compared to pre‐dissection. Incidence of anxiety and depression will be significant after AAD.
Methods
A cross sectional survey was mailed to 197 subjects from a single academic medical center (part of larger IRAD database). Subjects were ≥18 years of age surviving a type A or B AAD between 1996 and 2011. 82 surveys were returned (overall response rate 42%).
Results
Mean age ± SD was 59.5 ± 13.7 years, with 54.9% type A and 43.9% type B patients. Walking remained the most prevalent form of physical activity (49 (60%) pre‐dissection and 47 (57%) post‐dissection). Physical inactivity increased from 14 (17%) before AAD to 20 (24%) after AAD; sexual activity decreased from 31 (38%) to 9 (11%) mostly due to fear. Most patients (66.7%) were not exerting themselves physically or emotionally at AAD onset. Systolic blood pressure (SBP) at 36 months post‐discharge for patients engaging in ≥2 sessions of aerobic activity/week was 126.67 ± 10.30 vs. 141.10 ± 11.87 (p‐value 0.012) in those who did not. Self‐reported new‐onset depression after AAD was 32% and also 32% for new‐onset anxiety.
Conclusions
Alterations in lifestyle and emotional state are frequent in AAD survivors. Clinicians should screen for unfounded fears or beliefs after dissection that may reduce function and/or quality of life for AAD survivors.
Introduction
Acute aortic dissection (AAD) is a life‐threatening emergency that involves a tear in the intimal wall. Studies have shown good long‐term survival after initial treatment.1 However, currently there is no research assessing the lifestyle modifications and emotional state of AAD survivors after discharge. Exercise recommendations are also lacking for this patient cohort. The goal of this study was to better understand the physical‐activity preferences of AAD survivors before and after AAD and the influence of functional status on physical activity and to offer recommendations for likely safe and beneficial aerobic activity. In addition, we sought to assess exercise goals; the association between physical and/or emotional exertion and AAD onset (unclear association at the moment)2, 3, 4; impact on occupation, lifestyle, and consequent activity restriction and hobbies; desire for specific activity recommendations; incidence of depression and anxiety and its association with consistent aerobic exercise; and the impact on sexual activity.
Methods
Study Design
This was a cross‐sectional survey of patients from a single center, coupled with retrospective data obtained from the center's aortic dissection registry.
Study Population
The sample of patients used for this study was derived from the single health system's aortic dissection registry, which is a site for the International Registry of Acute Aortic Dissection (IRAD), a multinational registry consisting of 34 referral centers in 12 countries. Comprehensive details regarding IRAD have been previously published.5, 6
Our subjects included only those from a single IRAD site. These patients experienced their AAD between 1996 and 2011. Patients were those with acute type A or type B aortic dissection. Type A AAD was defined as any nontraumatic dissection involving the ascending aorta and presenting within 14 days of symptom onset. Type B AAD was defined as any nontraumatic dissection involving the descending aorta and presenting within 14 days of symptom onset.
All subjects age ≥18 years who survived to discharge were considered for the study cohort. Subjects were excluded if their mailing address on record was incorrect or if they were deceased.
Data and Collection Procedure
Of the total patients registered in IRAD at this single academic center (326), 91 patients were deceased based on the Social Security Death Index as of June 2013 (n = 91; mean age, 71.40 ± 12.88 years). Of these patients, accurate mailing information was only available for 197 patients. Thus, the survey was sent to 197 patients, and 82 surveys were returned as completed (response rate of 42%).
The survey was designed to obtain data on physical‐activity preferences of patients before and after AAD, patient goals for exercise, effects of consistent low‐intensity aerobic exercise on resting blood pressure (BP), physical/emotional exertion during symptom onset, occupation/occupational changes, effects of AAD on lifestyle, patients' desire for specific activity recommendations, independence with activities of daily living, presence of depression and/or anxiety, and frequency of sexual activity before AAD (defined as in the last 8 weeks leading up to AAD) and after AAD (defined as in the last 8 weeks leading up to receiving this survey) and possible reasons for limitation. These items were developed by the researchers and are novel to our group (see Supporting Table, in the online version of this article). Figure 1 lists the relevant questions from various sections of the survey.
Figure 1.
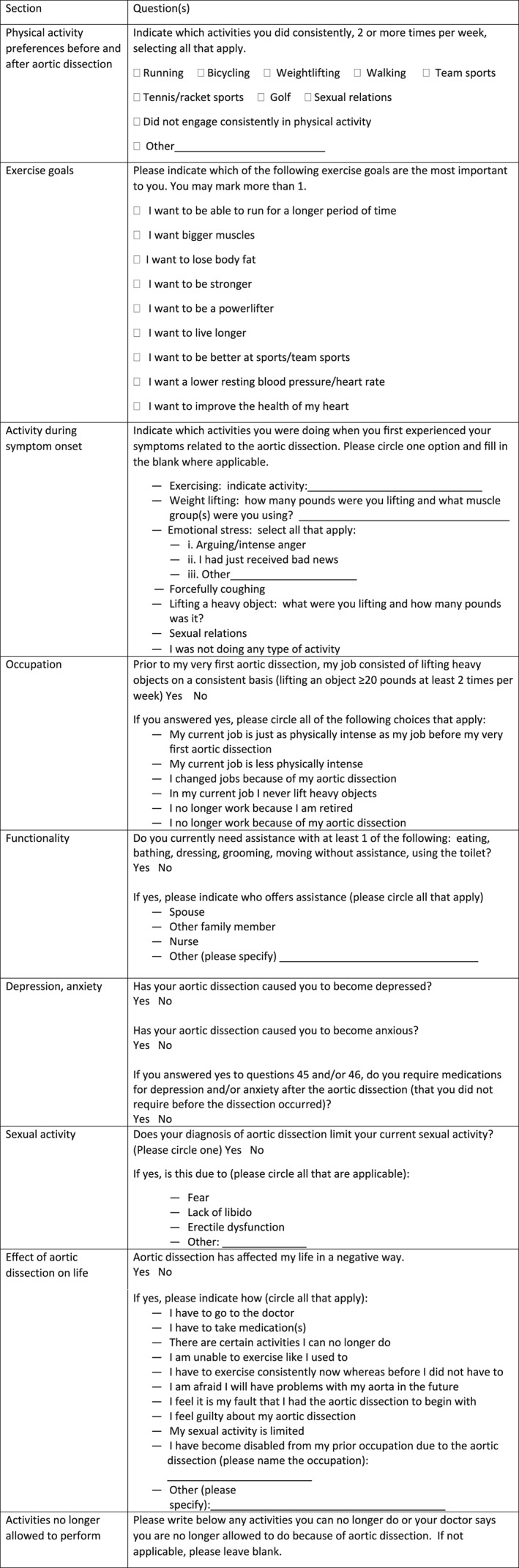
Relevant questions from the lifestyle survey.
Data obtained from this academic center's local IRAD registry included patient demographics, presenting symptoms and BP, conditions associated with AAD, dissection type and management, and aortic imaging information. Blood pressure for each patient was obtained from the retrospective chart review for clinic visits at 1, 3, 6, and 12 months after discharge, and annually until 5 years after discharge or the patient was lost to follow‐up. This was the BP value obtained at the clinic visit with either a cardiologist, cardiothoracic surgeon, or vascular surgeon. Aortic diameter was obtained from the computed tomography (CT) imaging report. In cases where multiple imaging modalities were used to assess the aorta, such as CT, magnetic resonance imaging, and transesophageal echocardiography, only the diameters recorded by CT were used.
Frequencies with percentage or mean with standard deviation were determined for the respective variables. Pearson χ2 and Fisher exact tests were used to assess the association between consistent physical activity and resting BP, as well as consistent physical activity and new‐onset, self‐reported anxiety and/or depression.
Results
Study Population and Baseline Characteristics
Table 1 offers baseline characteristics of the study cohort for this analysis (n = 82). Twenty‐five (30.5%) patients were female. Forty‐five (55%) patients had type A AAD and 36 (43.9%) patients had type B AAD. Forty‐three (52.4%) patients were managed surgically. This survey was completed by patients at a median (Q1, Q3) of 7.1 years (5.6, 11.5) after discharge.
Table 1.
Patient Demographics, Medical History, Dissection Type, and Management in Patients Who Completed the Survey
| Characteristic | Overall (N = 82) |
|---|---|
| Patient age at presentation, y | 59.5 ± 13.7 |
| Patient age at completion of survey, y | 67.8 ± 13.7 |
| Male sex | 25 (30.5) |
| Caucasian ethnicity (vs other) | 51 (62.2) |
| Medical history | |
| Marfan syndrome | 6 (7.3) |
| HTN | 49 (59.8) |
| Dissection type | |
| Type A | 45 (54.9) |
| Male sex | 34 (75.6) |
| Caucasian ethnicity (vs other) | 34 (79.1) |
| Type B | 36 (43.9) |
| Male sex | 22 (61.1) |
| Caucasian ethnicity (vs other) | 30 (83.3) |
| Dissection management | |
| Surgery | 43 (52.4) |
| Type A | 39 (86.0) |
| Type B | 4 (9.0) |
| Endovascular repair | 9 (11.0) |
| Type B | 9 (100) |
| Medical management | 25 (30.5) |
| Type A | 2 (8.0) |
| Type B | 23 (92.0) |
| Hybrid | 3 (1.4) |
| Type A | 3 (100) |
Abbreviations: HTN, hypertension; SD, standard deviation.
Data are presented as mean ± SD or n (%).
Physical Activity Before and After Dissection
Figure 2 summarizes the study cohort in terms of various physical‐activity preferences before and after AAD. A majority of patients engaged in walking before and after AAD, 49 (60%) and 47 (57%), respectively. The number of patients who did not engage in any physical activity increased from 14 (17%) before AAD to 20 (24%) after AAD. A minimal number of patients took part in weightlifting prior to AAD, and this diminished after AAD (12 [14%] and 7 [8%], respectively). Table 2 describes patient goals for exercise.
Figure 2.
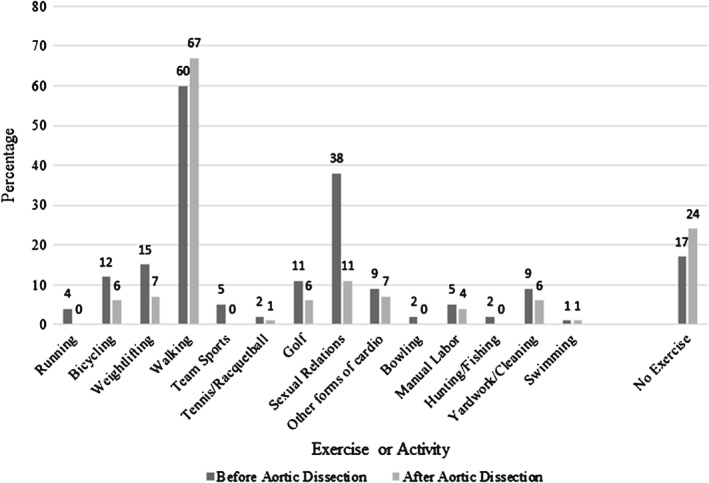
Percentage of patients engaging in various exercises and activities before and after AAD and P values. P values for before vs after: running (0.250), bicycling (0.125), weightlifting (0.180), walking (0.839), team sports (0.125), tennis/racket sports (1.00), golf (0.289), sexual relations (0.000), other forms of cardio (0.152), no exercise (0.263). Abbreviations: AAD, acute aortic dissection.
Table 2.
Patient Exercise Goals
| Exercise Goal, n (%) | |
|---|---|
| Live longer | 57 (70) |
| Improved heart health | 51 (62) |
| Be stronger | 34 (41) |
| Lower resting heart rate and blood pressure | 32 (39) |
| Increase stamina and run for a longer period of time | 3 (4) |
| Bigger muscles | 3 (4) |
| Lose fat | 41 (50) |
Eight (10%) patients required functional assistance, with 5 (6%) receiving assistance from their spouse and/or family member, 1 (1%) residing in an assisted living facility, and 1 (1%) requiring a home nurse.
Aerobic Exercise and Resting Blood Pressure
The systolic blood pressure (in mm Hg) at 36 months after discharge for patients who engaged in ≥2 sessions of aerobic activity per week (defined as “consistent aerobic activity” per author consensus) was 126.67 ± 10.30, compared with 141.10 ± 11.87 for patients who did not engage in consistent aerobic activity (P = 0.012). The difference in diastolic blood pressure at 36 months between the 2 groups was not statistically significant. There was no significant difference in resting BP for patients who engaged in consistent low‐intensity aerobic activity compared with those who did not at any of the other follow‐up time points. The overall exercise goals of our patient population are presented in Table 2. The most common goals included desire to live longer and improved cardiovascular health.
Activity During Dissection Onset
Table 3 summarizes self‐reported physical and/or emotional stressors at symptom onset. Two patients reported weightlifting, 7 patients reported lifting heavy objects, 6 patients engaged in strenuous aerobic exertion, and 8 patients described an intense emotional state or emotional trigger. Thus, a total of 23 (28%) patients reported physical exertion or intense emotions at symptom onset. Not every patient filled out this section of the survey.
Table 3.
Activity and Aortic Size for Patients Who Reported Physical and/or Emotional Exertion at Symptom Onset
| Activity | Age at Dissection Onset | Sex | History of HTN | History of Marfan Syndrome | Family History of Aortic Disease | Type of Dissection | Treatment | Aortic Size, cm |
|---|---|---|---|---|---|---|---|---|
| Lifting chalkboard (100 lb) | 64 | M | Yes | No | No | B | Medical | |
| Arguing | 43 | M | Yes | No | No | B | Endovascular | |
| Forcefully coughing | 73 | M | Yes | No | No | A | Surgical | 5.7 |
| Lifting 50–100 lb | 60 | F | Yes | Yes | No | B | Medical | 4 |
| Weightlifting, 60 lb pectoral | 72 | M | No | No | No | A | Surgical | |
| Golfing | 82 | M | No | No | No | B | Medical | 4.2 |
| Running softball bases | 41 | M | No | No | No | A | Surgical | |
| Lifting wall frames | 43 | M | Yes | No | No | A | Surgical | 5.2 |
| Lifting a heavy object | 53 | F | Yes | No | No | B | Medical | 3.7 |
| Riding carnival ride | 62 | F | No | No | No | B | Medical | 3.3 |
| Bad news | 46 | M | No | Yes | No | B | Endovascular | 3.7 |
| Holiday stress | 71 | F | No | No | No | A | Hybrid | |
| Deadlifting 310 lb | 81 | M | Yes | No | No | B | Endovascular | 4 |
| Arguing | 59 | F | Yes | No | No | A | Medical | 4.4 |
| Holiday stress | 59 | F | No | No | No | A | Surgical | 6 |
| Exercise stationary bike | 44 | F | No | No | Yes | A | Surgical | 6 |
| Exercise climbing hill | 49 | F | No | Yes | No | B | Medical | 2.8 |
| Forcefully coughing | 65 | F | No | No | No | A | Surgical | 4.3 |
| Walking | 62 | M | Yes | No | No | B | Endovascular | 4.1 |
| Walking while shopping | 60 | F | Yes | No | No | A | Surgical | 5.3 |
| Lifting a car tire | 65 | M | Yes | No | No | B | Medical | 5 |
| Acute anxiety | 49 | M | No | No | No | A | Surgical | 3.3 |
| Lifting another man (weight 120 lb) | 26 | F | Yes | Yes | Yes (Marfan) | A | Surgical | 3.5 |
| Sexual relations | 70 | M | Yes | No | No | B | Surgical | 3.5 |
| Emotional stress | 74 | F | No | No | No | A | Surgical and endovascular | 5.1 |
| Emotional stress | 54 | M | Yes | No | No | A | Surgical | |
| Playing volleyball | 49 | M | Yes | No | No | A | Surgical | |
| Walking up the stairs | 74 | F | Yes | No | No | B | Medical | 4.5 |
| Pushing lawn mower | 56 | M | Yes | No | No | A | Surgical | 4.1 |
| Forcefully coughing, running on treadmill, and later lifting 20 lb with arms, chest | 52 | F | Yes | No | No | A | Medical | 5.0 |
| Bicycling | 53 | M | Yes | No | No | A | Surgical | 6.2 |
Abbreviations: F, female; HTN, hypertension; M, male.
The median (Q1, Q3) aortic diameter for type A AAD at onset with physical or emotional stress was 4.9 cm (4.0, 5.8), and it was 5.1 cm (4.2, 5.9) at onset without physical or emotional stress (P = 0.942). The median (Q1, Q3) aortic diameter for type B AAD at onset with physical or emotional stress was 4.3 cm (3.7, 5.0), compared with 4.0 cm (3.5, 4.2) at onset without physical or emotional stress (P = 0.140).
Occupation
A total of 31 (38%) patients reported that their occupation prior to AAD involved consistently lifting objects weighing >20 lb. After AAD, of these 31 patients, only 1 (3%) patient still performed the same job requiring the same physical intensity. Eleven (35%) patients changed to jobs consisting of less lifting or no lifting at all; 12 (39%) patients are disabled due to their AAD and thus no longer work. Seven (23%) patients have retired as a result of their AAD.
Other Effects of Dissection on Lifestyle
Sixty‐two (76%) patients believe that AAD has negatively affected their life. Reasons include having to visit the doctor consistently, taking medications daily, no longer being able to do certain activities and exercise in the same way, fear of future aortic complications, limitations in sexual activity, and change of occupation or disability due to AAD. Furthermore, when patients were asked which activities they were no longer allowed to perform after AAD, as recommended by their aortic clinician, many patients reported not taking part in strenuous activity, snow shoveling, pushing/pulling/lifting >50 lb, traveling and driving long distances, coughing strenuously, team sports, prior occupation, going on roller coasters, hunting and fishing, hang gliding and parachuting, and long‐distance walking.
A total of 23 (28%) patients believe that surviving the AAD has motivated them to exercise more, and 53 (65%) patients felt it motivated them to eat healthier. A majority, 51 (62%) patients, indicated a desire to take fewer medications, and 62 (76%) patients limit the amount of weight they lift, push, or pull since their dissection.
Communication With the Health Care Provider
A majority, 65 (79%) patients, indicated that their physician had a discussion regarding exercise and physical activity with them, whereas 57 (70%) of the total patients felt that their doctor was clear in what exercises they should and should not perform. However, 58 (71%) patients still wished that there were specific recommendations about which activities are likely safe and which may not be safe in post‐AAD patients.
Depression, Anxiety, and Aerobic Exercise
Twenty‐six (32%) patients self‐reported depression due to their AAD. Twenty‐six (32%) patients also self‐reported anxiety due to their AAD. Of these 52 patients, 19 patients self‐reported both new‐onset depression and anxiety after dissection. Of the 26 (32%) patients who did not consistently engage in exercise (<2 sessions per week), 13 (50%) reported new‐onset depression (P = 0.020). Of the 54 (66%) patients who performed exercise consistently, 13 (24%) reported new‐onset depression. No association was found between aerobic exercise and anxiety. Nine out of 24 patients managed medically self‐reported new‐onset depression after treatment, and 11 out of 31 patients managed surgically self‐reported new‐onset depression after treatment (P = 0.336).
Sexual Activity
Prior to AAD, 31 (38%) patients engaged in sexual activity. After AAD, 9 (11%) patients reported sexual activity (Figure 2). Twenty‐seven (33%) patients feel their diagnosis limits their current sexual activity. Eleven (13%) patients express fear of adverse aortic events resulting from sexual activity; 12 (15%) patients feel a lack of libido; and 16 (20%) patients suffer from erectile dysfunction.
Discussion
There is no information available about activity levels, lifestyle changes, quality of life, and mood disorders in survivors of AAD. Our survey of AAD survivors illustrated an increase in physical inactivity after dissection. Although we did not specifically ask why this may be the case, our clinical experience suggests it is likely in part due to fear. It is less likely due to impaired functionality, as most of our patients indicated adequate functional status, with only 1% of patients requiring a home nurse after AAD. Nevertheless, this increase in physical inactivity is most likely detrimental to overall health, resting BP, and mental well‐being. Mild aerobic activity has been shown to promote mental and physical health and lower resting BP.7 Our subjects who engaged in consistent mild aerobic activity had a lower resting BP. Thus, clinicians should encourage AAD survivors to consistently engage in mild to moderate aerobic exercise (3–5 metabolic equivalents) such as walking or other forms of aerobic activity (eg, golf, low‐level bicycling, and other forms of mild aerobic cardiovascular activity).
The majority of our patients described their goals for exercise as living healthier and longer. It is plausible that strenuous exertion may be detrimental to the health of AAD survivors, as the acute increase in BP in the context of a weakened aortic wall may promote further aortic complications, which is suggested by Laplace's law. However, to achieve these patient‐stated goals, strenuous exertion is not necessary. Studies have shown that consistent low‐intensity aerobic exercise is beneficial in lowering resting BP, which has been shown to lower mortality and improve outcomes in AAD survivors. Our patients who reported consistent aerobic exercise also had a lower resting BP. As a result, AAD survivors should be asked about their exercise goals when formulating an exercise prescription and should be encouraged to engage in consistent, low‐ to moderate‐intensity aerobic activity (3–5 metabolic equivalents).
Previously, Hatzaras et al identified 31 patients with a history of aortic aneurysm who suffered an AAD in the context of intense physical exertion, predominantly weightlifting or lifting heavy objects.2 Furthermore, in another study by Hatzaras et al, 60 out of 90 patients (66%) with prior aortic aneurysm reported strenuous physical exertion and/or intense emotions at AAD symptom onset.4 However, our results suggest that aortic size at type A and type B AAD onset is nearly equal among patients enduring physical and/or intense emotions compared with those not enduring physical and/or emotional stress at AAD onset, and only 28% of our patients reported intense emotions or physical exertion at dissection onset. Thus, physical and emotional stress may not play as large a role in triggering aortic dissection in patients with no prior history of aortic aneurysm compared with those with a prior history of aortic aneurysm.
More than one‐third of patients reported consistently lifting >20 lb as a part of their occupation prior to AAD. A similar frequency of patients also reported disability for work after AAD. This, along with the likely prevalence of new‐onset anxiety and/or depression after AAD, suggests that cardiac rehabilitation after discharge may be beneficial. A study by Corone et al showed that cardiac rehabilitation after discharge, for patients experiencing a type A AAD, improved functionality, allowed patients to return to work earlier, increased muscular strength, and improved mental well‐being.8 In addition, patients expressed a desire for specific activity recommendations and felt that consequent activity restriction reduced their quality of life, suggesting a need for better patient education and further research to develop specific activity guidelines for this patient cohort.
The prevalence of mood disorders in the United States ranges from 5% to 10% in elderly patients.9, 10 The prevalence of depression and anxiety in survivors of myocardial infarction is 17% to 37% and 24% to 31%, respectively.11 Depression is prevalent in >20% of heart failure patients.12 Our results suggest that post‐AAD self‐reported depression and anxiety is common, and it may be more prevalent than in survivors of other heart diseases. Thus, aortic specialists should screen patients for and treat anxiety and depression. Although currently no association has been established between mood disorders and outcomes in AAD survivors, anxiety and depression have been associated with increased morbidity and mortality in survivors of myocardial infarction and heart failure.13, 14 Furthermore, >50% of our patients who did not consistently perform physical activity reported new‐onset depression after AAD, suggesting a possible association between depression and decreased aerobic exercise. Because exercise may play an important role in the treatment of depression,15 it may be especially important for aortic specialists to encourage mild aerobic exercise for AAD survivors who suffer from depression.
Avoidance of sexual activity was also common postdissection and is unnecessary,8 with the most common reason reported being fear. Health care providers should evaluate sexual health and allay this unfounded fear. Although it is certainly possible that this could be due to social factors (eg, impaired health of significant other, divorce, single status), our patients did not provide this as a reason for impaired sexual activity. Decreased sexual activity may contribute to decreased quality of life and consequent impaired mental health.
Study Limitations
We acknowledge the presence of recall bias. It is certainly plausible that nonresponders were doing so well as to not respond, are extremely debilitated and not able to respond, or were deceased since the time of mailing the survey. It is unclear which of these 2 groups the nonresponders belong to. It is also possible that only healthy subjects responded to our survey. The response rate may have also been higher had this survey been given to patients during clinic follow‐up visits rather than through mail. However, several of these patients received initial management at our academic medical institution but received follow‐up care with their local cardiologist and did not return to our institution. In addition, we acknowledge that it is difficult for patients to remember as far back as 15 years and describe their lifestyle habits before AAD, further contributing to recall bias. In addition, these data are also self‐reported and unable to be independently verified, reducing external validity.
We are unable to account for the influence of cardiac rehabilitation on these outcomes, as some patients may have received cardiac rehabilitation and others may not have. Given that cardiac rehabilitation has been shown to positively affect lifestyle, it is a topic for further study in AAD survivors. Currently no data exist on the effects of cardiac rehabilitation on outcomes in AAD survivors.
No additional instructions were given to patients for filling out this survey besides those listed in the instructions in the survey (Figure 1). However, we did our best to make the survey as comprehensible as possible and also used large font, understanding that many of our patients are older and may have impaired vision. In returned surveys, our patients wrote how grateful they were that we had sent them this survey and wanted to study this topic further.
The survey utilized in this study is one developed solely by the researchers and has not been validated. The questions were developed based on the clinical experience of the researchers. It was felt that a better understanding of these topics would further improve patient care. In addition, we are unable to examine the influence of social support (married vs single) on these outcomes of interest.
Ideas for future research include impact of social support on these outcomes, cognitive testing and IQ assessment for survivors treated medically vs surgically, and further screening for incidence of depression, suicidal rate/intension, anxiety, and quality of life.
Conclusion
Alterations in lifestyle and emotional state are frequent in survivors of AAD. Following AAD, physical inactivity increased most likely due to fear; functional status was mostly intact. This is unhealthy, as consistent low‐intensity aerobic exercise promotes mental and physical well‐being. Our subjects who engaged in consistent low‐intensity aerobic activity had a lower resting BP. Thus, walking or other forms of mild aerobic activity should be encouraged, as the most common exercise goal for patients was improved cardiovascular health. In addition, physical and emotional stress may not play as large a role in triggering AAD in patients with no prior history of aortic aneurysm compared with those with a prior history of aortic aneurysm. Many patients postdissection were unable to return to their prior occupation. The majority of patients wished there were specific recommendations regarding which activities are likely safe and which may not be safe after dissection, pointing toward a better need for postprocedure patient education and further research developing specific activity guidelines for this patient cohort. Post‐AAD depression and anxiety are common and should be screened for and treated. These findings suggest that cardiac rehabilitation would likely be beneficial in this patient cohort. Patients also avoided sexual activity after AAD. Health care providers should screen for and allay this unfounded fear. In general, clinicians should screen for unfounded fears or beliefs after AAD that may reduce function and/or quality of life for AAD survivors.
Supporting information
Supporting Information
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Tsai TT, Fattori R, Trimarchi S, et al; International Registry of Acute Aortic Dissection. Long‐term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation. 2006;114:2226–2231. [DOI] [PubMed] [Google Scholar]
- 2. Hatzaras I, Tranquilli M, Coady M, et al. Weight lifting and aortic dissection: more evidence for a connection. Cardiology. 2007;107:103–106. [DOI] [PubMed] [Google Scholar]
- 3. de Virgilio C, Nelson RJ, Milliken J, et al. Ascending aortic dissection in weight lifters with cystic medial degeneration. Ann Thorac Surg. 1990;49:638–642. [DOI] [PubMed] [Google Scholar]
- 4. Hatzaras IS, Bible JE, Koullias GJ, et al. Role of exertion or emotion as inciting events for acute aortic dissection. Am J Cardiol. 2007;100:1470–1472. [DOI] [PubMed] [Google Scholar]
- 5. Mehta RH, O'Gara PT, Bossone E, et al; International Registry of Acute Aortic Dissection Investigators. Acute type A aortic dissection in the elderly: clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol. 2002;40:685–692. [DOI] [PubMed] [Google Scholar]
- 6. Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. [DOI] [PubMed] [Google Scholar]
- 7. Brook RD, Appel LJ, Rubenfire M, et al; American Heart Association Professional Education Committee of the Council for High Blood Pressure Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension. 2013;61:1360–1383. [DOI] [PubMed] [Google Scholar]
- 8. Corone S, Iliou MC, Pierre B, et al; Cardiac Rehabilitation Working Group of the French Society of Cardiology. French registry of cases of type I acute aortic dissection admitted to a cardiac rehabilitation center after surgery. Eur J Cardiovasc Prev Rehabil. 2009;16:91–95. [DOI] [PubMed] [Google Scholar]
- 9. Weissman MM, Myers JK. Affective disorders in a US urban community: the use of research diagnostic criteria in an epidemiological survey. Arch Gen Psychiatry. 1978;35:1304–1311. [DOI] [PubMed] [Google Scholar]
- 10. Borson S, Barnes RA, Kukull WA, et al. Symptomatic depression in elderly medical outpatients. I. Prevalence, demography, and health service utilization. J Am Geriatr Soc. 1986;34:341–347. [DOI] [PubMed] [Google Scholar]
- 11. Lane D, Carroll D, Lip GY. Anxiety, depression, and prognosis after myocardial infarction: is there a causal association? J Am Coll Cardiol. 2003;42:1808–1810. [DOI] [PubMed] [Google Scholar]
- 12. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure: a meta‐analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. [DOI] [PubMed] [Google Scholar]
- 13. Carney RM, Blumenthal JA, Stein PK, et al. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. [DOI] [PubMed] [Google Scholar]
- 14. Mayou RA, Gill D, Thompson DR, et al. Depression and anxiety as predictors of outcome after myocardial infarction. Psychosom Med. 2000;62:212–219. [DOI] [PubMed] [Google Scholar]
- 15. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62:633–638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


