ABSTRACT
Background
The association between the development of heart failure (HF) and use of nonsteroidal anti‐inflammatory drugs (NSAIDs) is not well established.
Hypothesis
Use of NSAIDs may increase the risk of incident HF.
Methods
We conducted a systematic review and meta‐analysis of observational studies that reported odds ratio, relative risk, hazard ratio, or standardized incidence ratio comparing risk of incident HF in NSAID users vs nonusers. Pooled risk ratios (RR) and 95% confidence intervals (CI) for all NSAIDs and both subclasses (conventional NSAIDs and highly selective cyclooxygenase‐2 inhibitors [COXIBs]) were calculated using a random‐effect, generic inverse variance method.
Results
Seven studies with 7 543 805 participants were identified and included in our data analysis. Use of NSAIDs was associated with a significantly higher risk of developing HF, with a pooled RR of 1.17 (95% CI: 1.01‐1.36). Subgroup analysis showed a significantly elevated risk among users of conventional NSAIDs (RR: 1.35, 95% CI: 1.15‐1.57) but not users of COXIBs (RR: 1.03, 95% CI: 0.92‐1.16).
Conclusions
A significantly elevated risk of incident HF was observed among users of NSAIDs.
Introduction
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are a class of medications with analgesic and anti‐inflammatory properties. They are one of the most commonly used medications in the United States,1 despite their notorious adverse effects.2, 3 Inhibition of the cyclooxygenase (COX) enzyme, which has 2 isoforms, COX‐1 and COX‐2, is the fundamental pharmacological property of NSAIDs. COX‐1 is constitutively expressed under a normal physiologic condition, whereas COX‐2 is generally expressed under an inflammatory state. Conventional NSAIDs have been used in clinical practice as analgesic and anti‐inflammatory agents for decades. However, their utility is limited by the adverse effects associated with inhibition of the COX‐1 enzyme, particularly gastrointestinal (GI) ulcers and bleeding. The highly selective COX‐2 inhibitors (COXIBs) are a newer subgroup of NSAIDs that have been marketed as safer alternatives to conventional NSAIDs after several randomized controlled trials demonstrated a superior GI safety profile.4, 5 Nonetheless, over the past decade, attention has turned to the cardiovascular adverse effects of NSAIDs after rofecoxib, a COXIB, was withdrawn from the market after a randomized controlled trial demonstrated an increased incidence of myocardial infarction (MI) among the users.6 A subsequent meta‐analysis of observational studies confirmed this increased risk. In fact, a similar MI risk was also observed in some conventional NSAIDs, such as diclofenac and indomethacin.7
Use of NSAIDs also may be associated with an increased risk of heart failure (HF) as a result of salt and fluid retention secondary to the reduction of prostaglandin synthesis. In fact, use of these medications has been associated with the occurrence of HF in several reported cases.8, 9, 10 However, epidemiologic studies attempting to characterize this association yielded inconclusive results.11, 12, 13, 14, 15, 16 Therefore, we conducted a systematic review and meta‐analysis of epidemiologic studies that compared the incidence of HF in NSAID users vs nonusers to further investigate this possible adverse effect.
Methods
Search Strategy
Two investigators (P.U. and N.S.) independently searched published studies indexed in the MEDLINE and Embase databases from inception to April 2015. The search terms were compiled from terms for HF and NSAIDs, including names of individual drugs in conjunction with the terms for observational studies that were suggested by Furlan et al17 (for details, see Supporting Information, Search Strategy, in the online version of this article). A manual search of references of selected retrieved articles was also performed.
Inclusion Criteria
Studies were included into the data analysis if they met the following criteria: (1) studies had to be observational (case‐control or cohort studies); (2) the authors provided relative risk (RR), odds ratio (OR), hazard ratio (HR), or standardized incidence ratio with 95% confidence intervals (CI) of incident HF for conventional NSAIDs and/or COXIBs; and (3) NSAID nonusers were used as a reference group for cohort studies, and participants without HF were used as control for studies with a case‐control design.
Study eligibility was independently determined by the 2 aforementioned investigators. Quality of the included studies was also independently appraised by the 2 investigators using the Newcastle‐Ottawa quality‐assessment scale, which evaluates each study in 3 main areas: (1) the selection of the study groups, (2) the comparability of the groups, and (3) the ascertainment of the exposure or outcome of interest for case‐control or cohort studies, respectively.18 The third investigator (C.T.) oversaw this literature‐review process and resolved any disagreements.
Data Extraction
A standardized data‐collection form was used to extract the following information: title of the article, first author's last name, country of origin, year when the study was conducted, year of publication, study design, study size, study population, names of evaluated NSAIDs, definition and diagnosis of HF, average duration of follow‐up (for cohort studies), potential confounders that were adjusted, and adjusted pooled‐effect estimates with 95% CI. To ensure the accuracy of the data extraction, this process was independently performed by all investigators. Any discrepancy was resolved by referring back to the original study.
Statistical Analysis
Data analysis was performed using Review Manager (RevMan) software, version 5.3 (the Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark). Adjusted point estimates were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird, which assigned weight of each study based on its standard error.19 We performed overall analysis and subgroup analysis for conventional NSAIDs and COXIBs. We reported the pooled‐effect estimate of HF risk using the combination of the data from case‐control and cohort studies to increase the power of our estimates. The OR of case‐control study was used as an estimate of the RR to pool this data with the RR or HR of cohort study. The use of OR as an estimate for RR was considered acceptable in this study, as the outcome of interest was relatively uncommon. We used a random‐effect model rather than a fixed‐effect model, given the high likelihood of between‐study variance due to the different study design, population, and outcome measurement. If the study provided data on risk of NSAIDs overall, that risk would be used to calculate the pooled‐effect estimate. If the study provided only risk of individual NSAIDs, the risk would be pooled together first, using the same random‐effect, generic inverse variance method. The pooled risk would be used as effect estimate for that study to combine with data from other studies.
The statistical heterogeneity of this study was assessed by the Cochran Q test. This test is complemented with the I2 statistic, which quantifies the proportion of the total variation across studies that is due to heterogeneity rather than chance. An I2 value of 0% to 25% indicates insignificant heterogeneity; 26% to 50% indicates low heterogeneity; 51% to 75% indicates moderate heterogeneity; and 76% to 100% indicates high heterogeneity.20
Publication bias was assessed by visualization of funnel plot and the Egger regression test. Comprehensive Meta‐analysis version 2.0 (Biostat, Englewood, NJ) was used to perform the regression analysis.
Results
Our search strategy yielded 8314 potentially relevant studies (3695 studies from MEDLINE and 4619 studies from Embase), including 3594 duplicates. Based on title and abstract review, 4646 studies were excluded that clearly did not meet our inclusion criteria (1504 basic science studies, 1132 non–case‐control/cohort observational studies, 1012 interventional studies, 512 review articles, 334 editorials/correspondences, and 152 miscellaneous), leaving 74 studies for full‐length article review. Fifty‐four studies were excluded because they were interventional studies, and another 11 studies were excluded as they were descriptive studies without a control group. Two studies reported the risk of first hospitalization for HF among NSAID users, but they included patients with prior history of HF; therefore, these studies were excluded, as their outcomes were not incident HF.21, 22 Seven studies (4 case‐control studies11, 13, 15, 23 and 3 cohort studies12, 14, 16) with 7 543 805 participants met our eligibility criteria and were included for data analyses. The detailed characteristics and Newcastle‐Ottawa quality‐assessment scale of the case‐control and cohort studies are described in tables 1 and 2, respectively.
Table 1.
Characteristics of Case‐Control Studies in the Meta‐analysis
| Page et al11 | García‐Rodríguez et al13 | Huerta et al15 | Mangoni et al23 | |
|---|---|---|---|---|
| Country | Australia (New South Wales) | United Kingdom | United Kingdom | Australia |
| Year of publication | 2000 | 2003 | 2006 | 2010 |
| Study design | Case control | Case control | Case control | Case control |
| Cases | Patients who were admitted at the study hospitals with the primary diagnosis of HF from 1993 to 1995 were consecutively recruited. The study included patients both with and without prior diagnosis of heart diseases but also provided separate analysis for each category. | All patients age 40–84 y first diagnosed with HF between January 1, 1996, and December 31, 1996. Cases were identified from the United Kingdom General Practice Research Database, which covered approximately 3 million residents across the United Kingdom. Cases were verified by reviewing medical records. | All patients age 60–84 y first hospitalized with HF between January 1, 1997, and December 31, 2000. Cases were identified from the United Kingdom General Practice Research Database, which covered approximately 3 million residents across the United Kingdom. Cases were verified by reviewing medical records. The study included patients both with and without prior diagnosis of HF but also provided separate analysis for each category. | All patients age ≥65 y hospitalized with HF between January 1, 2002, and June 30, 2006. Cases were identified from the DVA database. DVA provided care with minimal copay to all Australian war veterans, spouses, and dependents. Cases with prior diagnosis of HF were excluded. |
| Controls | Sex‐ and age‐matched patients admitted at the same hospital. Controls must have no history of HF and must not have clinical or radiologic evidence of HF. | Sex‐ and age‐matched subjects without HF randomly selected from the same database | Sex‐ and age‐matched subjects without HF randomly selected from the same database | Sex‐, age‐, and state of residence–matched subjects without HF randomly selected from the same database |
| NSAIDs assessed in the study | Diclofenac, ibuprofen, ketoprofen, tiaprofenic acid, mefenamic acid, indomethacin, sulindac, diflunisal, naproxen, piroxicam, tenoxicam | Aceclofenac, acemetacin, diclofenac, etodolac, fenbufen, fenoprofen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, mefenamic acid, tiaprofenic acid, apazone, meloxicam, nabumetone, naproxen, piroxicam, sulindac, tenoxicam | Aceclofenac, acemetacin, azapropazone, diclofenac, diflunisal, etodolac, fenbufen, fenoprofen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, mefenamic acid, tiaprofenic acid, apazone, meloxicam, nabumetone, naproxen, piroxicam, sulindac, tenoxicam | Diclofenac, naproxen, ibuprofen, meloxicam, diflunisal, piroxicam, indomethacin, ketoprofen, mefenamic acid, tiaprofenic acid, celecoxib, lumiracoxib, rofecoxib |
| Definition of NSAID exposure | Use of any NSAID within 1 week prior to admission | Supply of the most recent prescription listed within 30 days prior to the index date | Supply of the most recent prescription listed within 30 days prior to the index date | Supply of the most recent prescription listed within 30 days prior to the index date |
| Verification of NSAID exposure | Structured interview within 1 week of admission | Prescription information from the database | Prescription information from the database | Prescription information from the database |
| Cases, n | 76 | 471 | 428 | 18 903 |
| Controls, n | 478 | 2807 | 2420 | 376 633 |
| Female sex, %, cases/ controls | 45.8/46.8 | 48.0/48.0 | 48.0/48.0 | 34.7/34.7 |
| Average age, y, cases/ controls | 76.6/75.1 | NA | NA | 65.3/65.3 |
| Confounders adjusted for | History of renal disease, respiratory disease, and PAD; use of β‐blockers, calcium channel antagonists, antidiabetic drugs, oral steroids | Age, sex, BMI, smoking, alcohol; use of steroids, acetaminophen, anticoagulants; DM, COPD, CAD, asthma, renal failure, valvular diseases, rhythm disorders, HTN | Age, sex, calendar year, BMI, smoking, alcohol; use of steroids, acetaminophen, anticoagulants; DM, CAD, COPD, asthma, valvular diseases, rhythm disorders, renal failure, HTN, hospitalizations in previous year | Age; public or private hospital admissions (for DM, obesity, dementia, HTN, CAD, respiratory disease, liver disease, RA, or renal disease) at any time prior to the index date; use of salicylic acid and derivatives or medications for obesity, DM, CAD, dementia, and COPD within 4 months prior to index date |
| Newcastle‐Ottawa scale | Selection, 3 stars; comparability, 1 star; exposure, 3 stars | Selection, 4 stars; comparability, 1 star; exposure, 3 stars | Selection, 4 stars; comparability, 1 star; exposure, 3 stars | Selection, 3 stars; comparability, 1 star; exposure, 3 stars |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; DVA, Department of Veterans' Affairs; HF, heart failure; HTN, hypertension; NA, not available; NSAIDs, nonsteroidal anti‐inflammatory drugs; PAD, peripheral arterial disease; RA, rheumatoid arthritis.
Table 2.
Characteristics of Cohort Studies in the Meta‐analysis
| Feenstra et al12 | Mamdani et al14 | Bäck et al16 | |
|---|---|---|---|
| Country | The Netherlands | Canada | Sweden |
| Study design | Prospective cohort | Retrospective cohort | Retrospective cohort |
| Year | 2002 | 2004 | 2012 |
| Cases | All residents of Ommoord, a suburb city of Rotterdam, age ≥55 y, were invited to participate in this study. 78% of eligible residents agreed to participate. The first cross‐sectional study started in June 1990. Participants with prevalent HF at study entrance were excluded from the analyses. Cross‐sectional survey was conducted periodically by home interviews and participant visits to the research center. | All residents of Ontario, Canada, age ≥66 y and who were prescribed NSAIDs from April 17, 2000, to March 31, 2001, with a supply that lasted ≥30 days. Cases were identified from an administrative health care database that covered 1.3 million individuals ≥65 y. The study included patients with and without prior diagnosis of HF but provided separate analysis for each category. | All Swedish residents age ≥18 y. Medical information was retrieved from Swedish patient register, prescribed drug register, and cause of death register. Follow‐up started on July 1, 2005, and ended on December 31, 2008. Residents with history of MI, stroke, HF, and AF prior to the start date were excluded. |
| NSAIDs assessed in the study | Nonselective NSAIDs | Celecoxib, rofecoxib, and nonselective NSAIDs | Celecoxib, etoricoxib |
| Definition of NSAID exposure | Period of exposure was defined as duration of prescription plus a carryover period of 7 days | ≥2 prescriptions for NSAIDs from April 17, 2000, to March 31, 2001, with a supply that lasted ≥30 days | Period of exposure was defined as duration of prescription plus a carryover period of 30 days |
| Verification of NSAID exposure | Verified with the pharmacy database of the study, which comprehensively covered all prescriptions dispensed to participants | Verified with pharmacy records that were linked to the administrative health care database | Verified with the Swedish national prescribed drug register |
| Control | NSAID use was analyzed as a time‐dependent variable (ie, duration of nonexposure to NSAIDs served as control) | Residents age ≥66 y with no history of NSAID exposure, randomly selected from the same database | NSAID use was analyzed as a time‐dependent variable (ie, duration of nonexposure to NSAIDs served as control) |
| Diagnosis of HF | HF was of special interest in the study. The continuous follow‐up of all participants was aimed at identifying all events of interest, including HF. It was part of the routine follow‐up procedure that all available data on the events of interest, such as hospital discharge note and outpatient visit note, were analyzed. Two research physicians independently evaluated and verified the HF diagnosis. | The primary outcome was admission with primary diagnosis of HF, which was identified from the Canadian Institute for Health Information discharge abstract database. | Diagnostic code from the database. No further verification was performed. |
| Follow‐up | Until a diagnosis of incident HF, death, emigration out of system, or December 31, 1998 | Until occurrence of study endpoint, death, or March 31, 2001 | Until occurrence of study endpoint, death, or December 31, 2008 |
| Cases, n | 7277 | 44 258 (32 834 COXIBs and 11 424 nonselective NSAIDs) | 6 991 645 |
| Controls, n | 7277 | 98 409 | 6 991 645 |
| Average age, y, cases/controls | 70.0/70.0 | 75.4/76.3 | 50.0/50.0 |
| Female sex, %, cases/controls | 62.0/62.0 | 55.0/68.0 | 50.8/50.8 |
| Average follow‐up, y, cases/controls | 6.0/6.0 | 0.5/0.5 | NA/NA |
| Confounders adjusted for | Age, sex; sCr ≥1.1 mg/dL; HTN, history of MI, AF; concomitant cardiovascular and pulmonary medication | Age, sex; medications used; hospitalization; socioeconomic status | Age, sex; medications used; socioeconomic status, educational level; RA |
| Newcastle‐Ottawa scale | Selection, 4 stars; comparability, 2 stars; outcome, 3 stars | Selection, 4 stars; comparability, 1 star; outcome, 3 stars | Selection, 4 stars; comparability, 2 stars; outcome, 3 stars |
Abbreviations: AF, atrial fibrillation; COXIB, highly selective cyclooxygenase‐2 inhibitor; HF, heart failure; HTN, hypertension; MI, myocardial infarction; NA, not available; NSAID, nonsteroidal anti‐inflammatory drug; RA, rheumatoid arthritis; sCr, serum creatinine.
The overall risk of incident HF among NSAID users was 1.17 (95% CI: 1.01‐1.36). Elevated risk was consistently observed in most studies, although without reaching statistical significance. Our meta‐analysis, taking advantage of combining all existing data, was able to prove a statistical significance. The I2 of this study was 53%, which indicated a moderately significant statistical heterogeneity. Subgroup analysis according to study design revealed an increased risk in both subgroups, although without reaching statistical significance. Figure 1 demonstrates a forest plot of all NSAIDs.
Figure 1.
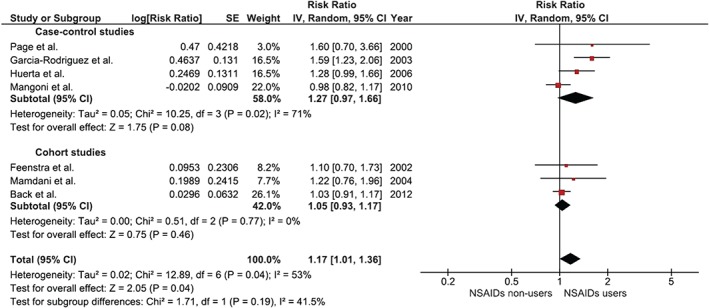
Forest plot of all NSAIDs. Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; NSAID, nonsteroidal anti‐inflammatory drug; SE, standard error.
Five studies11, 12, 13, 14, 15 reported risk of incident HF in users of conventional NSAIDs compared with nonusers. Elevated risk was, again, consistently observed in all studies, even though it was not statistically significant in most studies. The pooled RR was 1.35, and it was statistically significant (95% CI: 1.15‐1.57). The I2 of this analysis was 0%, which indicated a nonsignificant statistical heterogeneity. Figure 2 demonstrates a forest plot of these 5 studies.
Figure 2.
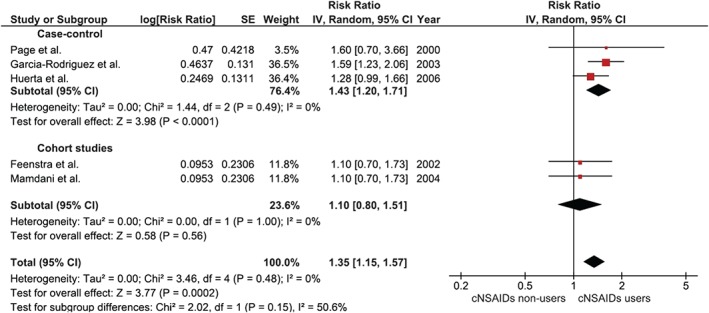
Forest plot of conventional NSAIDs. Abbreviations: CI, confidence interval; cNSAIDs, conventional nonsteroidal anti‐inflammatory drugs; df, degrees of freedom; IV, inverse variance; SE, standard error.
On the other hand, risk of incident HF among COXIB users was reported in only 2 studies.14, 16 The pooled RR was modestly elevated, at 1.03, without reaching statistical significance (95% CI: 0.92‐1.16). The statistical heterogeneity was insignificant, with an I2 of 0%. Figure 3 demonstrates a forest plot of the COXIB studies.
Figure 3.
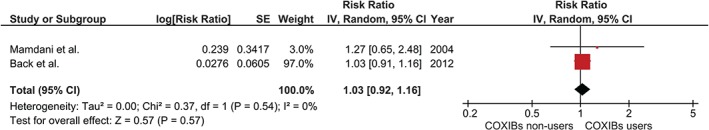
Forest plot of COXIBs. Abbreviations: CI, confidence interval; COXIB, highly selective cyclooxygenase‐2 inhibitor; df, degrees of freedom; IV, inverse variance; SE, standard error.
Sensitivity Analysis
We conducted a jackknife sensitivity analysis by excluding 1 study at a time from the pooled analysis. The pooled RRs for all NSAIDs from this sensitivity analysis ranged from 1.06 to 1.24.
Evaluation for Publication Bias
For a funnel plot evaluating publication bias for all NSAID studies, see Supporting Information, Figure, in the online version of this article. Visual inspection of this funnel plot does not reveal any obvious asymmetry, and, therefore, does not provide suggestive evidence for publication bias. Furthermore, there is no evidence of publication bias detected by the Egger regression test (P = 0.57).
Discussion
To the best of our knowledge, this is the first meta‐analysis of observational studies that summarizes the risk of incident HF among users of NSAIDs. We were able to assess the risk for NSAIDs as a single group, as well as performing subgroup analysis for both conventional NSAIDs and COXIBs.
An increased risk of incident HF among NSAID users has been consistently observed in several epidemiological studies.11, 12, 13, 14, 15, 16 However, because this outcome of interest was relatively uncommon, most of these studies failed to show a statistical significance. Our meta‐analysis, by combining all available data, was able to demonstrate a statistically significant elevated risk of incident HF among NSAID users, with 17% excess risk. Subgroup analysis showed a significantly elevated risk among users of conventional NSAIDs and a modestly increased risk among users of COXIBs, without a statistical significance. The statistical heterogeneity was moderate in the overall analysis but was insignificant in each subgroup analysis, suggesting that the difference between subgroups was responsible for this heterogeneity.
There are a few possible pathophysiologic explanations for this increased risk. The most widely accepted one is related to elevated blood pressure and fluid retention. Endothelium‐derived prostaglandins, such as prostaglandin I2 (PGI2), have a vasodilatory effect on the peripheral vasculature. Thus, inhibition of the COX enzyme could directly lead to vasoconstriction and hypertension.24, 25
Moreover, renal prostaglandins, particularly prostaglandin E2 (PGE2) and PGI2, play a major role in maintaining renal blood flow and glomerular filtration rate, especially in a volume‐depleted state. They also have an inhibitory effect on the sodium reabsorption in the loop of Henle and cortical collecting tubule, and on the water reabsorption in the collecting tubules, driven by the antidiuretic hormone.26, 27, 28 These prostaglandins are locally derived from the COX‐2 enzyme in the renal cortex and juxtaglomerular cells. Thus, inhibition of the COX‐2 enzyme by either conventional NSAIDs or COXIBs could lead to blood‐volume expansion, resulting in clinically evident HF, particularly in those who have an underlying ventricular dysfunction.
In fact, the vasoconstrictive and volume‐expansion effects of NSAIDs are even more pronounced in patients with established HF, as the included studies have also demonstrated that use of NSAIDs was associated with a higher incidence of HF exacerbation, with RRs ranging from 8.6 to 26.3.11, 12, 15
Based on this pathogenesis, theoretically, increased risk of incident HF should be seen with use of both conventional NSAIDs and COXIBs. Our meta‐analysis, however, could not demonstrate a statistically significant increased risk among COXIB users. This nonsignificant result could be secondary to a smaller sample size, as only 2 studies reported the risk of incident HF among COXIB users. More studies are required to clarify this risk.
Another possible explanation is related to the fact that use of some NSAIDs (such as rofecoxib and diclofenac) is linked to an increased risk of MI,7 which is a major risk factor for HF.
Study Limitations
Even though the primary studies included in this meta‐analysis are of high quality, there are some limitations, and, thus, our results should be interpreted with caution.
First, most of the included studies were conducted using coding‐based medical registry, raising a concern of coding inaccuracy and incompleteness. Most of the included studies also relied on prescription information from their database, which did not guarantee consumption of the medications and would not capture over‐the‐counter NSAID use. The only exception is the study by Page et al,11 which used structured interviews to identify NSAID exposure. However, this method had its own problem, as cases (participants with the event of interest) tended to recall exposure better than controls (recall bias).29 Second, most of the included studies were conducted in older populations. In fact, the mean ages of participants in 3 cohort studies were 70.0, 75.4, and 50.0 years. Therefore, our results are not generalizable to the general population, especially to younger patients with lower baseline cardiovascular risk. Third, most of the primary studies included in this meta‐analysis identified incident cases of HF from an admission database. Therefore, cases of milder HF that did not require hospitalization would not be identified. We also did not have data on the subtypes of HF. Fourth, the definition of NSAID exposure was not entirely consistent between studies, with time of exposure ranging from 7 to 30 days prior to the index date. It would also raise the question of how much HF could be attributed to NSAID use for the short period of time. Fifth, we did not search for unpublished data; therefore, publication bias might have been present. It should be noted that evaluation for publication bias was relatively unreliable in this study, as the number of included studies was small. Last, this is a meta‐analysis of observational studies, which, by study design, are at risk for several types of bias.30 For example, participants who took NSAIDs might undergo more medical examination and laboratory surveillance, resulting in detection bias. Physicians might also preferentially avoid prescribing NSAIDs to patients with increased baseline risk for cardiac dysfunction, leading to selection bias of healthier participants to the exposed group.
Conclusion
A statistically significant elevated risk of incident HF was observed among NSAID users in this meta‐analysis. Subgroup analysis showed a significantly elevated risk among users of conventional NSAIDs. Although we could not demonstrate a statistically significant elevated risk among COXIB users, we acknowledge that this could be related to the smaller sample size. Physicians must weigh the potential risks of HF and other adverse events related to NSAIDs, such as renal failure, MI, and GI bleeding, against their clinical benefit when prescribing these medications.
Supporting information
AppendixS1. Supplementary data
FigureS1
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Curhan GC, Bullock AJ, Hankinson SE, et al. Frequency of use of acetaminophen, nonsteroidal anti‐inflammatory drugs, and aspirin in US women. Pharmacoepidemiol Drug Saf. 2002;11:687–693. [DOI] [PubMed] [Google Scholar]
- 2. Ungprasert P, Cheungpasitporn W, Crowson CS, et al. Individual non‐steroidal anti‐inflammatory drugs and risk of acute kidney injury: a systematic review and meta‐analysis of observational studies. Eur J Intern Med. 2015;26:285–291. [DOI] [PubMed] [Google Scholar]
- 3. Ungprasert P, Srivali N, Wijarnpreecha K, et al. Non‐steroidal anti‐inflammatory drugs and risk of venous thromboembolism: a systematic review and meta‐analysis. Rheumatology (Oxford).> 2015;54:736–742. [DOI] [PubMed] [Google Scholar]
- 4. Vonkeman HE, van de Laar MA. Nonsteroidal anti‐inflammatory drugs: adverse effects and their prevention. Semin Arthritis Rheum. 2010;39:294–312. [DOI] [PubMed] [Google Scholar]
- 5. Bensen WG, Zhao SZ, Burke TA, et al. Upper‐gastrointestinal tolerability of celecoxib, a COX‐2‐specific inhibitor, compared to naproxen and placebo. J Rheumatol. 2000;27:1876–1883. [PubMed] [Google Scholar]
- 6. Bresalier RS, Sandler RS, Quan H, et al; Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial Investigators . Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial [published correction appears in N Engl J Med. 2006;355:221]. N Engl J Med.> 2005;352:1092–1102. [DOI] [PubMed] [Google Scholar]
- 7. McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–1644. [DOI] [PubMed] [Google Scholar]
- 8. Campbell RJ, Sneed KB. Acute congestive heart failure induced by rofecoxib. J Am Board Fam Pract. 2004;17:131–135. [DOI] [PubMed] [Google Scholar]
- 9. Nevins M, Berque S, Corwin N, et al. Phenylbutazone and pulmonary oedema. Lancet. 1969;2:1358. [DOI] [PubMed] [Google Scholar]
- 10. Van den Ouweland FA, Gribnau FW, Meyboom RH. Congestive heart failure due to nonsteroidal anti‐inflammatory drugs in the elderly. Age Ageing. 1988;17:8–16. [DOI] [PubMed] [Google Scholar]
- 11. Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an unrecognized public health problem. Arch Intern Med. 2000;160:777–784. [DOI] [PubMed] [Google Scholar]
- 12. Feenstra J, Heerdink ER, Grobbee DE, et al. Association of nonsteroidal anti‐inflammatory drugs with first occurrence of heart failure and with relapsing heart failure: the Rotterdam study. Arch Intern Med. 2002;162:265–270. [DOI] [PubMed] [Google Scholar]
- 13. García‐Rodríguez LA, Hernández‐Díaz S. Nonsteroidal anti‐inflammatory drugs as a trigger of clinical heart failure. Epidemiology. 2003;14:240–246. [DOI] [PubMed] [Google Scholar]
- 14. Mamdani M, Juurlink DN, Lee DS, et al. Cyclo‐oxygenase‐2 inhibitors versus non‐selective non‐steroidal anti‐inflammatory drugs and congestive heart failure outcomes in elderly patients: a population‐based cohort study. Lancet. 2004;363:1751–1756. [DOI] [PubMed] [Google Scholar]
- 15. Huerta C, Varas‐Lorenzo C, Castellsague J, et al. Non‐steroidal anti‐inflammatory drugs and risk of first hospital admission for heart failure in the general population. Heart. 2006;92:1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bäck M, Yin L, Ingelsson E. Cyclooxygenase‐2 inhibitors and cardiovascular risk in a nationwide cohort study after the withdrawal of rofecoxib. Eur Heart J. 2012;33:1928–1933. [DOI] [PubMed] [Google Scholar]
- 17. Furlan AD, Irvin E, Bombardier C. Limited search strategies were effective in finding relevant nonrandomized studies. J Clin Epidemiol. 2006;59:1303–1311. [DOI] [PubMed] [Google Scholar]
- 18. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trial. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG, Deeks JJ, et al.> Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGettigan P, Han P, Jones L, et al. Selective COX‐2 inhibitors, NSAIDs and congestive heart failure: differences between new and recurrent cases. Br J Clin Pharmacol. 2008;65:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kohli P, Steg PG, Cannon CP, et al; REACH Registry Investigators . NSAID use and association with cardiovascular outcomes in outpatients with stable atherothrombotic disease. Am J Med.> 2014;127:53.e1–60.e1. [DOI] [PubMed] [Google Scholar]
- 23. Mangoni AA, Woodman RJ, Gaganis P, et al. Use of non‐steroidal anti‐inflammatory drugs and risk of incident myocardial infarction and heart failure, and all‐cause mortality in the Australian veteran community. Br J Clin Pharmacol. 2010;69:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pope JE, Anderson JJ, Felson DT. A meta‐analysis of the effects of nonsteroidal anti‐inflammatory drugs on blood pressure. Arch Intern Med. 1993;153:477–484. [PubMed] [Google Scholar]
- 25. Ungprasert P, Kittanamongolchai W, Price C, et al. What is the “safest” non‐steroidal anti‐inflammatory drugs? Am Med J. 2012;3:115–123. [Google Scholar]
- 26. Aneja A, Farkouh ME. Adverse cardiovascular effects of NSAIDs: driven by blood pressure, or edema? Ther Adv Cardiovasc Dis. 2008;2:53–66. [DOI] [PubMed] [Google Scholar]
- 27. Stichtenoth DO, Marhauer V, Tsikas D, et al. Effects of specific COX‐2 inhibition on renin release and renal and systemic prostanoid synthesis in healthy volunteers. Kidney Int. 2005;68:2197–2207. [DOI] [PubMed] [Google Scholar]
- 28. White WB. Cardiovascular risk, hypertension, and NSAIDs.> Curr Rheumatol Rep. 2007;9:36–43. [DOI] [PubMed] [Google Scholar]
- 29. Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol. 1990;43:87–91. [DOI] [PubMed] [Google Scholar]
- 30. Signorello LB, McLaughlin JK, Lipworth L, et al. Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther. 2002;9:199–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS1. Supplementary data
FigureS1


