ABSTRACT
Fibrinolytic therapy is still used in patients with ST‐segment elevation myocardial infarction (STEMI) when the primary percutaneous coronary intervention cannot be provided in a timely fashion. Management strategies and outcomes in transferred fibrinolytic‐treated STEMI patients have not been well assessed in real‐world settings. Using the Nationwide Inpatient Sample from 2008 to 2012, we identified 18 814 patients with STEMI who received fibrinolytic therapy and were transferred to a different facility within 24 hours. The primary outcome was in‐hospital mortality. Secondary outcomes included gastrointestinal bleeding, bleeding requiring transfusion, intracranial hemorrhage (ICH), length of stay, and cost. The patients were divided into 3 groups: those who received medical therapy alone (n = 853; 4.5%), those who underwent coronary artery angiography without revascularization (n = 2573; 13.7%), and those who underwent coronary artery angiography with revascularization (n = 15 388; 81.8%). Rates of in‐hospital mortality among the groups were 20% vs 6.6% vs 2.1%, respectively (P < 0.001); ICH was 8.5% vs 1.1% vs 0.6%, respectively (P < 0.001); and gastrointestinal bleeding was 1.1% vs 0.4% vs 0.4%, respectively (P = 0.011). Multivariate analysis identified increasing age, higher Charlson Comorbidity Index score, cardiogenic shock, cardiac arrest, and ICH as the independent predictors of not performing coronary artery angiography and/or revascularization in patients with STEMI initially treated with fibrinolytic therapy. The majority of STEMI patients transferred after receiving fibrinolytic therapy undergo coronary angiography. However, notable numbers of patients do not receive revascularization, especially patients with cardiogenic shock and following a cardiac arrest.
Introduction
Acute coronary syndrome is one of the leading discharge diagnoses in the United States, accounting for approximately 700 000 patients discharged with this diagnosis each year.1 ST‐segment elevation myocardial infarction (STEMI) comprises 33% to 40% of acute coronary syndrome patients.2, 3 Previous studies have shown a significant decrease in the incidence of STEMI in the last decade, but the case fatality rate has remained unchanged.2, 4, 5 Successful early reperfusion of the culprit artery in STEMI patients is the most important intervention, which determines short‐term and long term outcomes regardless of which reperfusion strategy (fibrinolysis or percutaneous coronary intervention [PCI]) is used.6, 7 However, meta‐analysis comparing primary PCI with fibrinolytic therapy in STEMI patients showed reduction in mortality, reinfarction, and stroke with primary PCI.8 Early administration of fibrinolytic therapy, when primary PCI cannot be delivered in a timely fashion, followed by angiographic evaluation and intervention if indicated, may result in similar outcomes as with primary PCI.9, 10 A management strategy for patients who receive fibrinolytic therapy and are subsequently transferred to another hospital for further invasive evaluation, although evaluated in trials and recommended in guidelines, has not been well studied in real‐world settings in the United States. Therefore, we sought to evaluate the management and outcomes of STEMI patients transferred to a different facility for invasive evaluation after receiving fibrinolytic therapy, using patient cases from the Nationwide Inpatient Sample (NIS).
Methods
We used the NIS to determine the therapeutic strategies and outcomes of patients with STEMI transferred to a different facility after receiving fibrinolytic therapy. The NIS is part of the Agency for Healthcare Research and Quality's (AHRQ) Healthcare Cost and Utilization Project (HCUP) and contains information on all discharges from a 20% stratified sample of community hospitals in the United States. Each hospitalization is de‐identified and maintained in the NIS as a unique entry with 1 primary discharge diagnosis and <24 secondary diagnoses during that hospitalization. Each entry also carries information on demographic details, insurance status, comorbidities, primary and secondary procedures, hospitalization outcome, length of stay, and cost of care. The NIS contains the clinical and resource‐use information included in a typical discharge summary, with safeguards to protect the privacy of individual patients, physicians, and hospitals (as required by the data sources). Each record also includes a discharge weight to allow for national estimates.
Annual data quality assessments of the NIS are performed that assure the internal validity of the database. Furthermore, comparisons against the following data sources strengthen the external validity of the NIS: the American Hospital Association Annual Survey Database, the National Hospital Discharge Survey from the National Center for Health Statistics, and the MedPAR inpatient data from the Centers for Medicare & Medicaid Services.11, 12
Study Design and Cohorts
We queried HCUP's NIS between 2008 and 2012 using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnostic codes 410.0–410.6 and 410.8 for STEMI patients (n = 833 807) who had ICD‐9‐CM code of V45.88 (n = 19 062) to identify patients who received fibrinolytic therapy as the initial reperfusion strategy in a different facility within the previous 24 hours. We decided to use data from 2008, as the V45.88 code was introduced in October 2008. To restrict our evaluation to a typical adult population, only patients age ≥18 years were included.
Exclusion Criteria
To avoid the inclusion of the patients undergoing fibrinolytic therapy for other indications, we excluded patients having a secondary diagnosis of pulmonary embolism (ICD‐9 codes 415.11, 415.12, 416.2; n = 53) and stroke (ICD‐9 codes 434.00, 434.01, 434.10, 434.11; n = 185). We further excluded all the observations with missing data for sex (n = 5) and admission type for trauma (n = 5). The final study sample consisted of 18 814 patients (Figure 1). Because this study involved de‐identified data, it was exempt from institutional review board approval.
Figure 1.
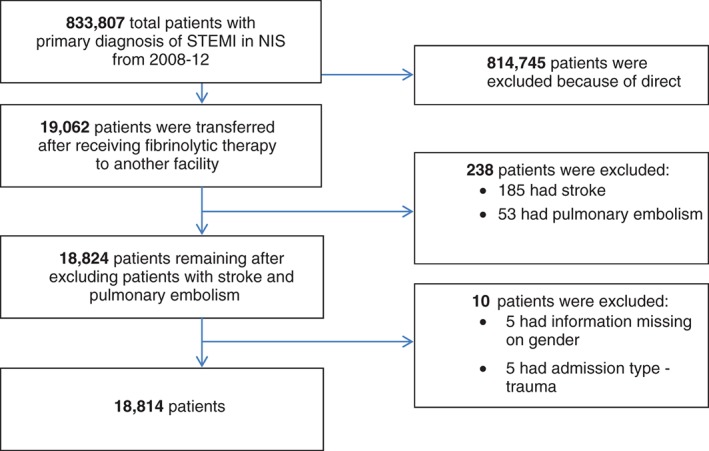
Flowchart of study population. Abbreviations: NIS, Nationwide Inpatient Sample; STEMI, ST‐segment elevation myocardial infarction.
Outcome Measures
To determine the patients who underwent coronary angiography, we used ICD‐9‐CM codes 88.55, 88.56, 88.57, 00.24, 00.59, and 37.22. The ICD‐9‐CM codes of 36.06 (insertion of non–drug‐eluting coronary artery stent) or 36.07 (insertion of drug‐eluting coronary artery stent) were used to identify patients who underwent PCI, and codes 36.10–36.17 and 36.19 were used to identify patients who underwent coronary artery bypass grafting (CABG). Patients with ICD‐9‐CM codes for coronary angiography without codes for PCI or CABG were placed in the group coronary angiography without revascularization, and patients with codes for coronary angiography plus codes for PCI or CABG were put in the group coronary angiography with revascularization. Patients without any of these codes were placed in the group medical therapy alone.
The primary outcome was in‐hospital mortality. The ICD codes for the secondary outcomes, which included gastrointestinal (GI) bleeding, bleeding requiring transfusion, and intracranial hemorrhage (ICH), are listed in Supporting Information, Table 1, in the online version of this article. Length of stay (LOS) and health care cost were also measured.
Definitions of Variables
We used NIS variables to identify patient age, sex, and race. We divided race into white, black, Hispanic, and other. We divided age into 5 subgroups: 18 to 34 years, 35 to 49 years, 50 to 64 years, 65 to 79 years, and ≥80 years. We defined the severity of comorbid conditions using the Deyo modification of the Charlson Comorbidity Index (CCI). This index contains 17 comorbid conditions with differential weights. The score ranges from 0 to 33, with higher scores corresponding to a greater burden of comorbid diseases (see Supporting Information, Table 2, in the online version of this article). Facilities were considered to be teaching hospitals if they had an American Medical Association–approved residency program, were a member of the Council of Teaching Hospitals, or had a full‐time equivalent interns and residents to patients ratio of ≥0.25. Hospital location (rural/urban) and number of beds were also recorded. The bed‐size cutoff points divided into small, medium, and large have been done so that approximately one‐third of the hospitals in a given region, location, and teaching‐status combination would fall within each bed‐size category.
Statistical Analysis
Stata IC version 11.0 (StataCorp, College Station, TX) and SAS version 9.4 (SAS Institute Inc., Cary, NC) were used for analysis, which accounted for the complex survey design and clustering. We stratified our study population into 3 groups: medical therapy alone, patients who underwent coronary artery angiography without revascularization, and patients who underwent coronary artery angiography and revascularization during the hospitalization. Differences in baseline characteristics were examined using 1‐way analysis of variance for continuous variables (reported as mean ± SD/SE) and χ2 test for categorical variables (reported as percentages). A P value <0.05 was considered significant.
Hierarchical Modeling
Hierarchical mixed‐effects models were generated to identify the independent multivariate predictors of in‐hospital mortality and also factors avoiding invasive strategy. Hierarchical modeling is designed to analyze data with nested observations and is more appropriate to simple regression modeling for an available dataset. The NIS dataset is inherently hierarchical, that is to say the data have the group (ie, hospital)‐specific attributes, and within each group there are patients who contribute specific patient attributes to the data. Hierarchical models take into consideration the effect of nesting. Two‐level hierarchical models (with patient‐level factors nested within hospital‐level factors) were created with the unique hospital identification number incorporated as random effects within the model (meaning that patients treated at the same hospital may experience similar outcomes as a result of other processes of care).
We excluded race from the multivariate models, as nearly 19.5% of the observations were missing. In all multivariate models, we included hospital‐level variables such as hospital region (Northeast, South, Midwest, with West as referent), hospital bed size, and teaching vs nonteaching status; and patient‐level variables such as age, sex, Deyo modification of CCI, admission over the weekend, median household income, and primary payer (with Medicare/Medicaid considered as referent). Model discrimination was assessed using the C‐index. Subgroup analyses were carried out in patients with cardiogenic shock and cardiac arrest.
Results
From 2008 to 2012, 18 814 patients with STEMI who received fibrinolytic therapy as the initial reperfusion strategy were transferred to a different facility within 24 hours for coronary artery angiography and revascularization, if indicated.
Baseline Patient Characteristics
Of 18 814 consecutive patients with STEMI initially treated with fibrinolytic therapy and transferred to a different facility, 853 (4.5%) did not undergo angiographic evaluation and were considered to be treated with medical therapy alone, 2573 (13.7%) underwent coronary angiography without revascularization, and 15 388 (81.8%) underwent coronary angiography with revascularization, either PCI or CABG. Patients of older age, of female sex, with Medicare/Medicaid as primary insurance payer, and those with higher comorbidities (CCI ≥2), cardiogenic shock, and cardiac arrest were less likely to receive revascularization (Table 1).
Table 1.
Baseline Characteristics of STEMI Patients Transferred to a Different Facility After Receiving Fibrinolytic Therapy
| Overall | Medical Therapy Alone | CA Without Revascularization | CA With Revascularization | P Value | |
|---|---|---|---|---|---|
| No. of observations (%) | 18 814 (100.0) | 853 (4.5) | 2573 (13.7) | 15 388 (81.8) | |
| Patient age, y, % | <0.001 | ||||
| 18–34 | 1.1 | 0.6 | 3.9 | 0.7 | |
| 35–49 | 18.8 | 8.2 | 20.3 | 19.1 | |
| 50–64 | 44.5 | 30.5 | 40.5 | 45.9 | |
| 65–79 | 28.5 | 28.2 | 26.1 | 28.9 | |
| ≥80 | 7.2 | 32.5 | 9.1 | 5.4 | |
| Sex, % | <0.001 | ||||
| M | 73.5 | 61.3 | 66.2 | 75.4 | |
| F | 26.5 | 38.7 | 33.8 | 24.6 | |
| Race, % | <0.001 | ||||
| White | 65.9 | 67.5 | 69.0 | 65.3 | |
| Black | 3.2 | 4.7 | 4.4 | 2.9 | |
| Hispanic | 6.1 | 5.3 | 4.9 | 6.4 | |
| Other | 5.3 | 5.9 | 5.0 | 5.3 | |
| Missing | 19.5 | 16.6 | 16.7 | 20.1 | |
| Comorbidities, % | |||||
| Deyo modification of CCI scorea | <0.001 | ||||
| ≤ 1 | 53.1 | 35.2 | 49.7 | 54.6 | |
| 2 | 29.7 | 29.2 | 30.8 | 29.6 | |
| ≥ 3 | 17.2 | 35.6 | 19.4 | 15.8 | |
| Obesity | 14.7 | 9.2 | 17.3 | 14.6 | <0.001 |
| HTN | 60.0 | 63.5 | 61.0 | 59.7 | 0.045 |
| DM | 24.3 | 27.0 | 25.3 | 24.0 | 0.058 |
| CHF | 13.4 | 22.5 | 14.6 | 12.6 | <0.001 |
| COPD (Chronic Obstructive Pulmonary Disease) | 15.1 | 22.4 | 17.8 | 14.2 | <0.001 |
| PVD | 6.5 | 13.4 | 5.2 | 6.4 | <0.001 |
| Fluid‐electrolyte abnormalities and renal failure | 15.4 | 31.8 | 16.2 | 14.3 | <0.001 |
| Neurological disorder or paralysis | 3.3 | 12.6 | 4.2 | 2.6 | <0.001 |
| Anemia or coagulopathy | 9.5 | 16.7 | 8.9 | 9.2 | <0.001 |
| Solid tumors, metastatic cancers, or lymphoma | 1.1 | 1.2 | 0.8 | 1.1 | 0.220 |
| Depression, psychosis, or substance abuse | 7.4 | 7.6 | 10.8 | 6.8 | <0.001 |
| Median HHI category for patient Zip code, %b | 0.002 | ||||
| 0–25 | 42.4 | 43.7 | 42.4 | 42.3 | |
| 26–50 | 30.7 | 27.7 | 30.4 | 31.0 | |
| 51–75 | 14.3 | 17.2 | 15.8 | 13.8 | |
| 76–100 | 8.2 | 7.3 | 7.0 | 8.4 | |
| Primary payer, % | <0.001 | ||||
| Medicare/ Medicaid | 43.6 | 64.5 | 46.5 | 41.9 | |
| Private insurance (including HMOs) | 40.3 | 24.0 | 36.7 | 41.8 | |
| Other/self‐pay/no charge | 15.9 | 11.0 | 16.6 | 16.1 | |
| Hospital characteristics | |||||
| Bed size, % | 0.052 | ||||
| Small | 8.2 | 10.5 | 8.2 | 8.0 | |
| Medium | 21.6 | 20.2 | 22.6 | 21.5 | |
| Large | 69.4 | 69.3 | 67.7 | 69.7 | |
| Teaching status, % | <0.001 | ||||
| Nonteaching | 39.6 | 48.8 | 39.9 | 39.0 | |
| Teaching | 59.6 | 51.2 | 58.6 | 60.2 | |
| Region, % | <0.001 | ||||
| Northeast | 11.6 | 13.5 | 7.7 | 12.2 | |
| Midwest | 18.1 | 17.2 | 16.7 | 18.3 | |
| South | 36.0 | 32.3 | 39.7 | 35.6 | |
| West | 22.9 | 19.5 | 24.2 | 22.9 | |
| Admission day, % | 0.523 | ||||
| Weekday | 70.3 | 71.7 | 70.8 | 70.2 | |
| Weekend | 29.7 | 28.3 | 29.2 | 29.8 | |
| Disposition of survivors, % | <0.001 | ||||
| Home | 85.6 | 48.0 | 80.7 | 88.5 | |
| Transfer to short‐term hospital/other facilities/HHC | 10.3 | 29.2 | 11.52 | 9.03 | |
| Against medical advice (AMA) | 0.64 | 2.8 | 1.25 | 0.4 | |
| In‐hospital mortality, % | 3.51 | 20.0 | 6.6 | 2.1 | <0.001 |
| LOS, d, median (IQR) | 3.0 (2.0–4.0) | 3.0 (2.0–6.0) | 3.0(2.0–4.0) | 3.0 (2.0–4.0) | 0.323 |
| Adjusted cost, $, median (IQR) | 17 479 (12 912–24 619) | 7701 (3 936–14 001) | 10 892 (7868–15 461) | 18 773 (14 516–26 124) | <0.001 |
| Cardiac arrest, % | 2.9 | 6.5 | 4.2 | 2.4 | <0.001 |
| Cardiogenic shock, % | 7.4 | 16.3 | 7.7 | 6.8 | <0.001 |
| Wall involved | <0.001 | ||||
| Anterolateral | 8.5 | 12.6 | 8.5 | 8.2 | |
| Anterior | 28.9 | 33.3 | 32.5 | 28.0 | |
| Inferolateral | 8.0 | 8.3 | 6.9 | 8.1 | |
| Inferoposterior | 5.6 | 5.7 | 5.4 | 5.7 | |
| Inferior | 45.6 | 36.5 | 41.0 | 47.0 | |
| Lateral | 2.5 | 1.8 | 4.5 | 2.2 | |
| Posterior | 0.4 | 1.3 | 0.2 | 0.4 | |
| Other specified | 0.5 | 0.6 | 1.0 | 0.4 | |
| Type of stent | |||||
| ≥1 DES | 49.9 | — | — | 61.1 | |
| Only BMS | 23.5 | — | — | 28.8 |
Abbreviations: AMA, against medical advice; BMS, bare‐metal stent; CA, coronary angiography; CCI, Charlson Comorbidity Index; CHF, congestive heart failure; DES, drug‐eluting stent; DM, diabetes mellitus; F, female; HHC, home health care; HHI, household income; HMO, health maintenance organization; HTN, hypertension; Hx, history; ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IQR, interquartile range; LOS, length of stay; M, male; PVD, peripheral vascular disease; STEMI, ST‐segment elevation myocardial infarction.
Charlson/Deyo Comorbidity Index was calculated as per Deyo classification. Comorbidities were identified by ICD‐9‐CM code mentioned in any of the diagnostic fields.
This represents a quartile classification of the estimated median HHI of residents in the patient's Zip code. These values are derived from Zip code demographic data obtained from Claritas. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually, the value ranges vary by year (http://www.hcup‐us.ahrq.gov/db/vars/zipinc_qrtl/nisnote.jsp).
Primary Outcome
There was a significant difference in mortality among the 3 groups (Table 2). Compared with the group who received medical therapy alone and did not undergo coronary artery angiography, the group who underwent coronary artery angiography but no revascularization and the group who underwent coronary artery angiography and revascularization had significantly lower in‐hospital mortality (20% vs 6.6% vs 2.1%, respectively; P < 0.001). Multivariate analysis identified age, female sex, cardiogenic shock, and cardiac arrest as significant predictors of death, and the adjusted odds ratio (OR) significantly favored coronary angiography and/or revascularization compared with medical therapy alone: the OR for coronary angiography and/or revascularization was 0.12 (95% confidence interval [CI]: 0.08‐0.18, P < 0.001).
Table 2.
Outcomes of Different Strategies in STEMI Patients Transferred to a Different Facility After Receiving Fibrinolytic Therapy
| Variable | Overall | Medical Therapy Alone | CA Without Revascularization | CA With Revascularization | P Value |
|---|---|---|---|---|---|
| Overall, n (%) | 18 814 (100.0) | 853 (4.5) | 2573 (13.7) | 15 388 (81.8) | |
| Primary outcome, % | |||||
| In‐hospital mortality | 3.5 | 20.0 | 6.6 | 2.1 | <0.001 |
| Secondary outcomes, % | |||||
| GI bleed | 0.4 | 1.1 | 0.4 | 0.4 | 0.011 |
| Bleeding requiring transfusion | 0.4 | 0.6 | 0.4 | 0.4 | 0.769 |
| ICH | 1 | 8.5 | 1.1 | 0.6 | <0.001 |
| LOS, d, median (IQR) | 3.0 (2.0–4.0) | 3.0 (2.0–6.0) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 0.323 |
| Adjusted cost, $, median (IQR) | 17 479 (12 912 –24 619) | 7701 (3936–14 001) | 10 892 (7868–15 461) | 18 773 (14 516–26 124) | <0.001 |
Abbreviations: CA, coronary angiography; GI, gastrointestinal; ICH, intracranial hemorrhage; IQR, interquartile range; LOS, length of stay; STEMI, ST‐segment elevation myocardial infarction.
Secondary Outcomes
The frequency of bleeding events was low across the entire study population. However, ICH and GI bleeds were significantly more frequent in the medical‐therapy‐only group compared with the coronary angiography and revascularization groups (ICH, 8.5% vs 1.1% vs 0.6%, respectively, P < 0.001; GI bleeds, 1.1% vs 0.4% vs 0.4%, respectively, P = 0.011; Table 2). There were no significant differences among the groups in the incidence of bleeding requiring transfusion.
Length of Stay and Cost
There was no difference in the LOS among the 3 groups (Table 2). However, adjusted cost of hospitalization was significantly higher in the revascularization group (medical therapy alone, $7701 [interquartile range {IQR}, $3936–$14 001] vs coronary angiography without revascularization, $10 892 [IQR, $7868–$15 461] vs coronary angiography with revascularization,$18 773 [IQR, $14 516–$26 124]; P < 0.001).
Predictors of Avoiding Coronary Angiography and/or Revascularization in ST‐Segment Elevation Myocardial Infarction Patients Initially Treated With Fibrinolytic Therapy
As the predictors of avoiding coronary angiography and/or revascularization in STEMI patients initially treated with fibrinolytic therapy, multivariate analysis identified age (OR: 1.97, 95% CI: 1.77‐2.20, P < 0.001), higher CCI score (OR: 2.03, 95% CI: 1.56‐2.63, P < 0.001), cardiogenic shock (OR: 1.66, 95% CI: 1.21‐2.27, P = 0.002), cardiac arrest (OR: 3.42, 95% CI: 2.16‐5.42, P < 0.001), and ICH (OR: 13.96, 95% CI: 8.28‐23.54, P < 0.001; Table 3).
Table 3.
Predictors of Avoiding Invasive Strategy in STEMI Patients Transferred to Different Facility After Receiving Fibrinolytic Therapy
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Cardiogenic shock | 1.66 | 1.21‐2.27 | 0.002 |
| Cardiac arrest | 3.42 | 2.16‐5.42 | <0.001 |
| ICH | 13.96 | 8.28‐23.54 | <0.001 |
| Age (per 10‐unit change) | 1.97 | 1.77‐2.20 | <0.001 |
| Sex | |||
| M | Ref | ||
| F | 1.07 | 0.86‐1.33 | 0.571 |
| Deyo modification of CCI scorea | |||
| 0–1 | Ref | ||
| 2 | 1.46 | 1.14‐1.88 | 0.003 |
| ≥3 | 2.03 | 1.56‐2.63 | <0.001 |
| Hospital bed size | |||
| Small | Ref | ||
| Medium | 0.85 | 0.26‐2.78 | 0.783 |
| Large | 0.89 | 0.31‐2.52 | 0.820 |
| Hospital teaching status | |||
| Nonteaching | Ref | ||
| Teaching | 0.81 | 0.43‐1.53 | 0.513 |
| Hospital region | |||
| Northeast | Ref | ||
| Midwest | 0.82 | 0.29‐2.28 | 0.697 |
| South | 0.93 | 0.37‐2.37 | 0.882 |
| West | 0.70 | 0.25‐1.93 | 0.488 |
| Median HHI category for patient Zip code, %b | |||
| 0–25 | Ref | ||
| 26–50 | 1.11 | 0.85‐1.45 | 0.444 |
| 51–75 | 0.77 | 0.53‐1.12 | 0.174 |
| 76–100 | 0.70 | 0.44‐1.12 | 0.136 |
| Primary payer | |||
| Medicare/Medicaid | Ref | ||
| Private insurance (including HMOs) | 0.93 | 0.70‐1.24 | 0.628 |
| Other/self‐pay/no charge | 1.13 | 0.76‐1.67 | 0.551 |
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; F, female; HHI, household income; HMO, health maintenance organization; ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICH, intracranial hemorrhage; M, male; OR, odds ratio; Ref, referent; STEMI, ST‐segment elevation myocardial infarction.
Charlson/Deyo Comorbidity Index was calculated as per Deyo classification. Comorbidities were identified by ICD‐9‐CM code mentioned in any of the diagnostic fields.
This represents a quartile classification of the estimated median HHI of residents in the patient's Zip code. These values are derived from Zip code demographic data obtained from Claritas. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually, the value ranges vary by year (http://www.hcup‐us.ahrq.gov/db/vars/zipinc_qrtl/nisnote.jsp).
Subgroup Analysis of Patients With Cardiogenic Shock
Of 1382 patients with cardiogenic shock, 139 (10.1%) received medical therapy alone, 197 (14.3%) received coronary angiography without revascularization, and 1046 (75.7%) received coronary angiography with revascularization. There was a very significant difference in mortality among the 3 groups, favoring the revascularization group (56.6% vs 44.3% vs 20%, respectively; P < 0.001). There was no difference in GI bleeding or bleeding requiring transfusion. However, incidence of ICH was higher in the medical‐therapy‐only group (3.6% vs 0% vs 1.3%; P = 0.02). Regarding LOS and adjusted hospital cost (see Supporting Information, Table 3, in the online version of this article), there was no significant difference in LOS, and adjusted hospital cost was significantly higher in the revascularization group ($13 735 [IQR, $5565–$33 345] vs $21 182 [IQR, $11 607–$35 091] vs $32 511 [IQR, $21 433–$49 899], respectively, P = 0.004).
Subgroup Analysis of Patients With Cardiac Arrest
Of 536 patients with cardiac arrest, 55 (10.3%) received medical therapy alone, 109 (20.2%) received coronary angiography without revascularization, and 372 (69.5%) received coronary angiography with revascularization. Similar to the cardiogenic shock subgroup, there was a very significant difference in mortality favoring the revascularization group (79.5% vs 45.4% vs 15.6%, respectively; P < 0.001). There was no significant difference in bleeding complications. The LOS was higher in the revascularization group (0 days [IQR, 0–4 days] vs 5 days [IQR, 2–9 days] vs 5 days [IQR, 3–9 days], P < 0.001). Similarly, cost of hospitalization was higher in the revascularization group ($4482 [IQR, $1569–$17 363] vs $17 411 [IQR, $12 773–$30 053] vs $27 475 [IQR, $18 966–$43 359], respectively, P < 0.001; see Supporting Information, Table 3, in the online version of this article).
Trends and Regional Variation
Only a small number of STEMI patients did not undergo coronary angiography after initial treatment with fibrinolytic therapy and transfer to the different facility. However, we observed an increased trend of not performing coronary angiography in these patient populations in all regions except the South from 2009 to 2012. The proportion of patients who did not receive coronary angiography in the Northeast was 1.9% in 2009, which increased to 7.1% in 2012 (P for trend < 0.001); over the same time period, the increases were from 3.6% to 6.0% in the Midwest (P for trend < 0.008), from 4.1% to 5.0% in the South (P for trend = 0.083), and from 3.1% to 6.0% in the West (P for trend < 0.001; see Supporting Information, Figure 1, in the online version of this article).
Discussion
Our study investigated the contemporary therapeutic strategies and in‐hospital outcomes in STEMI patients initially treated with fibrinolytic therapy and who were subsequently transferred to a different facility for coronary angiography and possible revascularization in the real‐world setting. Compared with medical therapy alone and coronary angiography without revascularization, the invasive strategy of coronary angiography with revascularization was associated with a marked decrease in mortality, especially in patients with cardiogenic shock and cardiac arrest. Although only a small number of patients did not receive the invasive strategy, the trend increased from 2009 to 2012 in all regions except South.
Current evidence suggests that fibrinolytic therapy alone could result in suboptimal outcomes in STEMI patients. Wijeysundera et al concluded in their meta‐analysis that routine early invasive strategy was superior to ischemia‐guided treatment after fibrinolytic therapy.13 Subsequently, D'Souza et al demonstrated in their meta‐analysis the efficacy and safety of routine early PCI within the first 24 hours of fibrinolytic therapy, with significant reduction in the primary composite endpoint of mortality, reinfarction, and ischemia compared with conservative management.14 Moreover, real‐world data from the French Registry on Acute ST‐elevation Myocardial Infarction (FAST‐MI), the Global Registry of Acute Coronary Events (GRACE), and the Canadian Registry of Acute Coronary Events (CANRACE) suggested better outcomes with routine angiography and revascularization after fibrinolytic therapy.15, 16 The American College of Cardiology Foundation/American Heart Association STEMI Guidelines also recommend angiography within 3 to 24 hours for patients who have received fibrinolytic therapy as an initial revascularization strategy.1
Our data are also consistent with previously reported findings suggesting benefit with routine angiography and revascularization if indicated after fibrinolytic therapy.15, 16 A recent study from the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) registry demonstrated that 97.1% of fibrinolytic‐treated STEMI patients received coronary angiography after transfer.17 In our study, 95.5% of patients underwent coronary angiography after being transferred. Only 4.5% of patients did not receive coronary angiography, but the trend of not receiving angiography increased significantly in all regions except South from 2009 to 2012. This patient population who did not undergo coronary angiography had a high prevalence of ICH, and 32% were age >80 years and had a higher prevalence of comorbidities. Moreover, ICH and major bleeding after fibrinolytic therapy could be the potential reasons for not performing coronary angiography. These findings suggest the cautious use of coronary angiography and revascularization, with avoidance in patient populations more susceptible to harm.
Our study also noted that 13.7% of patients did not receive revascularization after angiography and had less favorable outcomes. Additionally, our findings demonstrate real‐world therapeutic and outcomes disparities in transferred fibrinolytic‐treated STEMI patients. An aggressive invasive strategy with revascularization is already established to have a significant survival benefit.18, 19, 20 Despite this fact, 24.3% of patients with cardiogenic shock and 30.5% of patients with cardiac arrest did not undergo revascularization. Furthermore, cardiogenic shock and cardiac arrest were independent predictors of avoiding the invasive therapeutic strategy, and those patients had a marked increase in mortality without revascularization. Moreover, higher CCI score was also a predictor for avoiding the invasive strategy. Possible reasons include fear of bleeding complications and unfavorable outcomes. This hesitation may be explained in Northeast region, where New York, Massachusetts, Pennsylvania, and New Jersey implement public reporting of PCI outcomes, which has been well established in previous studies.21, 22, 23 However, results are consistent in other regions where public reporting is not mandated.
Interestingly, 73.1% of the study population had a median household income at <50th percentile of the median household income in the patient's residential zip code. This suggests that the majority of the fibrinolytic‐treated STEMI patients are from the area with lower socioeconomic status (SES). It is already established that patients from the area with lower SES have decreased timely reperfusion following STEMI, compared with patients from higher SES.24 Our findings may propose a lack of resources, such as PCI‐capable hospitals, in areas with lower SES. The number of PCI‐capable hospitals has been increasing consistently since 2001.25, 26 Previous reports have also demonstrated that access to PCI in the population held steady, even with the increasing number of PCI‐capable hospitals.26 This may suggest a tendency to establish new PCI programs in neighborhoods with high SES, where PCI programs already exist, and not where they are needed.
Fibrinolytic‐treated patients who underwent coronary angiography with or without revascularization after transfer to the different facility had no difference in LOS but a higher total hospital cost of coronary angiography with revascularization. It is been well established that patients treated with fibrinolytic therapy alone have a high risk of death, reinfarction, and stroke.27 Our study does not have long‐term outcomes, which precludes cost‐effective analysis. However, previous studies have demonstrated the cost‐effectiveness of an early invasive strategy with revascularization in patients treated with fibrinolytic therapy by reducing mortality, reinfarction, and stroke.28
Study Limitations
Several limitations of our study merit consideration. First, the timing of fibrinolytic therapy from first medical contact, the fibrinolytic agent used (alteplase, reteplase, or tenecteplase) and dose (full dose or half‐dose with glycoprotein IIb/IIIa receptor antagonist), the failure or success of fibrinolytic therapy, and transfer times are not captured and would not be represented in the findings of our study. Second, timing of revascularization after transfer is not known. Third, reasons for not performing angiography and/or revascularization in individual patients would be difficult to ascertain. Fourth, information about the use of anticoagulation agents and antiplatelet agents is not available. Firth, residual measured and unmeasured confounding may influence these findings. Also, the NIS is primarily an administrative dataset based on hospital claims and not on medical‐chart review. We addressed this limitation by including only those patients who had a principal discharge diagnosis of STEMI, which has a high positive predictive value (95%) compared with medical chart reviews.29 Coding errors are always a limitation with the NIS database. Our analysis only pertains to inpatient outcomes; long‐term outcomes are not known. Another potential limitation is that the NIS does not provide clinical data for a more detailed explanation of outcomes.
Conclusion
In this large observational study, we found that the majority of transferred fibrinolytic‐treated STEMI patients undergo coronary angiography. However, notable numbers of patients do not receive revascularization, especially patients with cardiogenic shock and following a cardiac arrest.
Supporting information
Supplementary table 1 Procedural complications by ICD 9 code.
Supplementary Table 2: Deyo's modification of Charlson's co‐morbidity index (CCI).
Supplementary Table 3: Subgroup analysis of patients' with cardiogenic shock and cardiac arrest
Figure S1: Trend of not performing coronary angiography in STEMI patient transferred to different facility after receiving fibrinolytic therapy from 2009 to 2012 in different region
Dr. Gregg C. Fonarow discloses the following relationships – Honoraria: Amgen, Boston Scientific, Johnson & Johnson, Medicines Company, Medtronic, Novartis, and Takeda; Research Funding: Gambro, Medtronic, National Heart, Lung, and Blood Institute, National Institute of Health/National Institute of Allergy and Infectious Diseases. Dr. Mauricio G. Cohen discloses the following relationships – Honoraria: Abiomed, Accumed, Merit Medical, Terumo Medical, and Medtronic. Research Funding: Astra Zeneca.
References
- 1. O'Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2013;128:e481]. Circulation. 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 2. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. [DOI] [PubMed] [Google Scholar]
- 3. Wiviott SD, Morrow DA, Frederick PD, et al. Performance of the thrombolysis in myocardial infarction risk index in the National Registry of Myocardial Infarction‐3 and ‐4: a simple index that predicts mortality in ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2004;44:783–789. [DOI] [PubMed] [Google Scholar]
- 4. Gjesing A, Gislason GH, Køber L, et al. Nationwide trends in development of heart failure and mortality after first‐time myocardial infarction 1997–2010: a Danish cohort study. Eur J Intern Med. 2014;25:731–738. [DOI] [PubMed] [Google Scholar]
- 5. Arciero TJ, Jacobsen SJ, Reeder GS, et al. Temporal trends in the incidence of coronary disease. Am J Med. 2004;117:228–233. [DOI] [PubMed] [Google Scholar]
- 6. Boersma E, Mercado N, Poldermans D, et al. Acute myocardial infarction. Lancet. 2003;361:847–858. [DOI] [PubMed] [Google Scholar]
- 7. De Luca G, Suryapranata H, Ottervanger JP, et al. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–1225. [DOI] [PubMed] [Google Scholar]
- 8. Huynh T, Perron S, O'Loughlin J, et al. Comparison of primary percutaneous coronary intervention and fibrinolytic therapy in ST‐segment‐elevation myocardial infarction: Bayesian hierarchical meta‐analyses of randomized controlled trials and observational studies. Circulation. 2009;119:3101–3109. [DOI] [PubMed] [Google Scholar]
- 9. Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER‐AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705–2718. [DOI] [PubMed] [Google Scholar]
- 10. Denktas AE, Athar H, Henry TD, et al. Reduced‐dose fibrinolytic acceleration of ST‐segment elevation myocardial infarction treatment coupled with urgent percutaneous coronary intervention compared to primary percutaneous coronary intervention alone: results of the AMICO (Alliance for Myocardial Infarction Care Optimization) Registry. JACC Cardiovasc Interv. 2008;1:504–510. [DOI] [PubMed] [Google Scholar]
- 11. Healthcare Cost and Utilization Project (HCUP) . Overview of the Nationwide Inpatient Sample (NIS). http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed February 15, 2015. Updated November 9, 2015. [Google Scholar]
- 12. Barrett M, Wilson E, Whalen D. 2007 HCUP Nationwide Inpatient Sample (NIS) Comparison Report. HCUP Methods Series report no. 2010‐03. http://www.hcup‐us.ahrq.gov/reports/methods/2010_03.pdf. Published September 9, 2010. Accessed February 15, 2015. [Google Scholar]
- 13. Wijeysundera HC, You JJ, Nallamothu BK, et al. An early invasive strategy versus ischemia‐guided management after fibrinolytic therapy for ST‐segment elevation myocardial infarction: a meta‐analysis of contemporary randomized controlled trials. Am Heart J. 2008;156:564–572. [DOI] [PubMed] [Google Scholar]
- 14. D'Souza SP, Mamas MA, Fraser DG, et al. Routine early coronary angioplasty versus ischaemia‐guided angioplasty after thrombolysis in acute ST‐elevation myocardial infarction: a meta‐analysis. Eur Heart J. 2011;32:972–982. [DOI] [PubMed] [Google Scholar]
- 15. Danchin N, Coste P, Ferrières J, et al; FAST‐MI Investigators . Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST‐segment‐elevation acute myocardial infarction: data from the French Registry on Acute ST‐elevation Myocardial Infarction (FAST‐MI). Circulation. 2008;118:268–276. [DOI] [PubMed] [Google Scholar]
- 16. Czarnecki A, Welsh RC, Yan RT, et al; Global Registry of Acute Coronary Events (GRACE/GRACE2); Canadian Registry of Coronary Events (CANRACE) Investigators . Reperfusion strategies and outcomes of ST‐segment elevation myocardial infarction patients in Canada: observations from the Global Registry of Acute Coronary Events (GRACE) and the Canadian Registry of Acute Coronary Events (CANRACE). Can J Cardiol. 2012;28:40–47. [DOI] [PubMed] [Google Scholar]
- 17. Vora AN, Holmes DN, Rokos I, et al. Fibrinolysis use among patients requiring interhospital transfer for ST‐segment elevation myocardial infarction care: a report from the US National Cardiovascular Data Registry. JAMA Intern Med. 2015;175:207–215. [DOI] [PubMed] [Google Scholar]
- 18. Hochman JS, Sleeper LA, Webb JG, et al; SHOCK Investigators . Early revascularization in acute myocardial infarction complicated by cardiogenic shock. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625–634. [DOI] [PubMed] [Google Scholar]
- 19. Berger PB, Holmes DR Jr, Stebbins AL, et al. Impact of an aggressive invasive catheterization and revascularization strategy on mortality in patients with cardiogenic shock in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO‐I) trial: an observational study. Circulation. 1997;96:122–127. [DOI] [PubMed] [Google Scholar]
- 20. Lettieri C, Savonitto S, De Servi S, et al. Emergency percutaneous coronary intervention in patients with ST‐elevation myocardial infarction complicated by out‐of‐hospital cardiac arrest: early and medium‐term outcome. Am Heart J. 2009;157:569.e1–575.e1. [DOI] [PubMed] [Google Scholar]
- 21. Moscucci M, Eagle KA, Share D, et al. Public reporting and case selection for percutaneous coronary interventions: an analysis from two large multicenter percutaneous coronary intervention databases. J Am Coll Cardiol. 2005;45:1759–1765. [DOI] [PubMed] [Google Scholar]
- 22. Joynt KE, Blumenthal DM, Orav EJ, et al. Association of public reporting for percutaneous coronary intervention with utilization and outcomes among Medicare beneficiaries with acute myocardial infarction. JAMA. 2012;308:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCabe JM, Joynt KE, Welt FG, et al. Impact of public reporting and outlier status identification on percutaneous coronary intervention case selection in Massachusetts. JACC Cardiovasc Interv. 2013;6:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agarwal S, Garg A, Parashar A, et al. Outcomes and resource utilization in ST‐elevation myocardial infarction in the United States: evidence for socioeconomic disparities. J Am Heart Assoc. 2014;3:e001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Culler SD, Kugelmass AD, Brown PP, et al. Trends in coronary revascularization procedures among Medicare beneficiaries between 2008 and 2012. Circulation. 2015;131:362–370. [DOI] [PubMed] [Google Scholar]
- 26. Concannon TW, Nelson J, Goetz J, et al. A percutaneous coronary intervention lab in every hospital? Circ Cardiovasc Qual Outcomes. 2012;5:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armstrong PW, Collen D, Antman E. Fibrinolysis for acute myocardial infarction: the future is here and now. Circulation. 2003;107:2533–2537. [DOI] [PubMed] [Google Scholar]
- 28. Coleman CI, McKay RG, Boden WE, et al. Effectiveness and cost‐effectiveness of facilitated percutaneous coronary intervention compared with primary percutaneous coronary intervention in patients with ST‐segment elevation myocardial infarction transferred from community hospitals. Clin Ther. 2006;28:1054–1062. [DOI] [PubMed] [Google Scholar]
- 29. Varas‐Lorenzo C, Castellsague J, Stang MR, et al. Positive predictive value of ICD‐9 codes 410 and 411 in the identification of cases of acute coronary syndromes in the Saskatchewan Hospital automated database. Pharmacoepidemiol Drug Saf. 2008;17:842–852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1 Procedural complications by ICD 9 code.
Supplementary Table 2: Deyo's modification of Charlson's co‐morbidity index (CCI).
Supplementary Table 3: Subgroup analysis of patients' with cardiogenic shock and cardiac arrest
Figure S1: Trend of not performing coronary angiography in STEMI patient transferred to different facility after receiving fibrinolytic therapy from 2009 to 2012 in different region


