ABSTRACT
There is an apparent benefit with extension of dual antiplatelet therapy (DAPT) beyond 1 year after implantation of drug‐eluting stents (DES). Assessment by a Double Randomization of a Conventional Antiplatelet Strategy vs a Monitoring‐Guided Strategy for Drug‐Eluting Stent Implantation, and of Treatment Interruption vs Continuation One Year After Stenting (ARCTIC)‐Generation assessed whether there is a difference of outcome between first‐ vs second‐generation DES and if there is an interaction with DAPT duration in the ARCTIC‐Interruption study. ARCTIC‐Interruption randomly allocated 1259 patients 1 year after stent implantation to a strategy of interruption of DAPT (n = 624), in which aspirin antiplatelet treatment only was maintained, or DAPT continuation (n = 635) for 6 to 18 additional months. The primary endpoint was the composite of death, myocardial infarction, stent thrombosis, stroke, or urgent revascularization. A total of 520 and 722 patients received a first‐ and a second‐generation DES, respectively. After a median follow‐up of 17 months (interquartile range, 15–18 months) after randomization, the primary endpoint occurred in 32 (6.2%) and 19 (2.6%) patients with first‐ and second‐generation DES, respectively (hazard ratio: 2.31, 95% confidence interval: 1.31‐4.07, P = 0.004). This was observed irrespective of the strategy of interruption or continuation of DAPT and timing of study recruitment. Major bleeding events occurred in 4 (0.8%) and 3 patients (0.4%) with first‐ and second‐generation DES, respectively (hazard ratio: 1.79, 95% confidence interval: 0.40‐8.02, P = 0.44). Results did not change after multiple adjustments for potential confounding variables. ARCTIC‐Generation showed worse clinical outcome with first‐ vs second‐generation DES, a difference that appeared to persist even with prolonged DAPT.
Introduction
Although more effective than bare‐metal stents in preventing restenosis and ischemia, first‐generation drug‐eluting stents (DES), such as paclitaxel‐eluting stents (PES) and sirolimus‐eluting stents (SES), demonstrate increased susceptibility to stent thrombosis (ST).1, 2, 3 Newer‐generation DES with thinner strut stent platforms, biocompatible durable or biodegradable polymers, and limus‐based antiproliferative agents have an approximately 50% lower risk of definite or probable ST compared with first‐generation DES, particularly during the late phase.4, 5, 6, 7 This lower risk of ST compared with bare‐metal stents,7, 8 together with improved survival compared with medical treatment in stable coronary artery disease (CAD), further support a broad use of this technology.9
Extended‐duration dual antiplatelet therapy (DAPT) prevents atherothrombotic events in patients with symptomatic vascular disease,10, 11 with a significant increased risk of major bleeding and a neutral effect on cardiovascular and noncardiovascular mortality.12 Duration of DAPT after coronary stenting remains a matter of debate. The susceptibility of first‐generation DES to ST, due to delayed vessel healing, polymer hypersensitivity, and other mechanisms, led to the recommendation of prolonged DAPT.13 Randomized interruption trials have selected patients according to their eligibility for DAPT, with a dominant proportion of coronary stenting for stable CAD patients but using various types of DES and durations of DAPT. Modest‐sized trials did not show any significant benefit but showed consistently increased bleeding events with prolonged DAPT14, 15 or no hazard.16, 17, 18, 19, 20, 21 In addition, some have suggested that DAPT duration may be device‐specific and that ST propensity is a time‐dependent phenomenon that can be modulated by adherence/duration of DAPT.22, 23 The only powered trial for hard endpoints, the Dual Antiplatelet Therapy Study (DAPT), demonstrated a significant benefit of extended DAPT beyond 1 year with increased major bleeding and no mortality benefit.1 Heterogeneity across stent types has been reported with significant interaction with treatment duration including lower event rates in zotarolimus‐eluting Endeavor Sprint stent (ZES‐S) patients undergoing short‐term when compared with long‐term DAPT therapy and higher rate of ST in the short‐term DAPT regimen.24
In the Assessment by a Double Randomization of a Conventional Antiplatelet Strategy vs a Monitoring‐Guided Strategy for Drug‐Eluting Stent Implantation and of Treatment Interruption vs Continuation One Year After Stenting (ARCTIC)‐Interruption study, there was no significant benefit with DAPT continuation beyond 1 year after coronary stenting but harm with more frequent major or minor bleeding.14 This study was started when both first‐ and second‐generation DES were available and was performed with DES only. The aim of ARCTIC‐Generation was to assess whether there is a difference of outcome between first‐ vs second‐generation DES and if there is an interaction with DAPT duration.
Methods
Study Design and Population
ARCTIC (http://www.clinicaltrials.gov: NCT00827411) was a multicenter, prospective, open‐label study with parallel arms and double randomization recruiting patients scheduled for planned DES implantation at 38 centers in France.25 Primary percutaneous coronary intervention (PCI) for ST‐segment elevation myocardial infarction (MI), planned use of glycoprotein IIb/IIIa inhibitors, chronic anticoagulation therapy, or bleeding diathesis were exclusion criteria. In the first phase (ARCTIC), 2440 patients were randomized a first time to a strategy of platelet function evaluation with adjustment of antiplatelet drugs and doses in patients with inadequate platelet inhibitory response (monitoring arm) or to a conventional strategy of treatment without platelet function assessment (conventional arm); no difference was observed between the 2 groups after 1 year of follow‐up.26, 27
In the second phase (ARCTIC‐Interruption), 1259 patients were randomized a second time at 1‐year follow‐up to interrupt or to continue thienopyridine according to the initial consent form, which engaged the patients also for the ARCTIC‐Interruption study.14 In the continuation arm, clopidogrel or prasugrel were maintained until the end of the study, with a minimum additional follow‐up of 6 months for the last randomized patient, whereas the others had an accrual follow‐up (last follow‐up in March 2012). In the continuation arm, DAPT was maintained at the same dose regimen as during the first year of follow‐up. Exclusion criteria for the second randomization were the occurrence of any ischemic event of the primary endpoint or any event of the primary safety endpoint during the first year of follow‐up after the first randomization, any new revascularization needing DAPT prolongation, or any contraindication to aspirin continuation, such as hemorrhagic gastrointestinal ulcer or aspirin resistance. (For the study flow chart, see Supporting Information, Figure, in the online version of this article.) ARCTIC‐Interruption showed no significant benefit of extended DAPT beyond 1 year but an excess of any bleeding as compared with interruption of DAPT.14
All patients underwent PCI with a DES according to the protocol. Drug‐eluting stents of first vs second generations were prespecified subgroups, and selection of stent type was left to physician discretion. First‐generation DES were identified as PES and SES, and second‐generation DES as ZES (Endeavor Sprint), everolimus‐eluting stents (EES), or any other recent stent types. When first‐ and second‐generation DES were used during the same procedure, patients were assigned to the first‐generation DES group.
Study Objectives and Endpoints
ARCTIC‐Generation is a nonprespecified analysis of the randomized ARCTIC‐Interruption study. The aim of the present analysis was to assess whether there is a difference of outcome between first‐ vs second‐generation DES and if there is an interaction with DAPT duration. The primary endpoint was the composite of all‐cause death, MI, stroke or transient ischemic attack, urgent coronary revascularization, and ST, the same endpoint as for the first part of the study. All definitions have been described elsewhere.25, 27 The main secondary efficacy endpoint was the composite of ST (revascularized or not) and urgent revascularization. All the other prespecified endpoints of the study protocol were also analyzed. The main safety endpoint was defined as major bleeding using the PCI‐specific Safety and Efficacy of Enoxaparin in Percutaneous Coronary Intervention Patients, an International Randomized Evaluation (STEEPLE) definitions.28 All events were adjudicated by an independent Clinical Events Committee unaware of treatment assignments.
Statistical Analysis
The analysis was based on all events that occurred in the intention‐to‐treat population, defined as all randomized patients in ARCTIC‐Interruption who signed an informed‐consent form. In case of patients withdrawing consent during the study, only their data collected before the day of withdrawal were included in the database. The endpoints were analyzed using a Cox model for survival analysis. Adjustment for baseline characteristics and major determinants of ST were performed including sex, age, DM, smoking status, body mass index, prior coronary intervention, prior MI, number of stents, multivessel disease, prasugrel maintenance dose, and platelet reactivity status (P2Y12 reaction units [PRU] > 208). Interactions between stent generation (first or second) and duration of DAPT were evaluated for all prespecified endpoints. All patients were censored at the date of last available information. The 95% confidence interval (CI) of the hazard ratio (HR) is presented. Non‐Gaussian variables were summarized as median (quartiles) and compared by the Mann–Whitney U test. The χ2 test for frequency comparisons was used. All analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC), with a 2‐sided 5% significance level. To test the hypothesis of a time‐effect, interaction between stent generation and time categorized as first or second year of randomization was also tested.
The trial and the statistical analyses were performed by the nonprofit academic research organization ACTION (Allies in Cardiovascular Trials, Initiatives and Organized Networks), based at Pitié‐Salpêtrière Hospital, Paris (http://www.action‐coeur.org). Research grants were obtained from Fondation de France, Sanofi‐Aventis, Cordis, Medtronic, Boston Scientific, and Fondation SGAM, which had no involvement in the conduct of the study.
Results
Patients and Treatments
Of the 2440 patients who were originally randomized in the ARCTIC trial, 1259 were re‐randomized into the ARCTIC‐Interruption study: 624 were assigned to the strategy of DAPT interruption, leaving the patients on aspirin single antiplatelet therapy, and 635 to the strategy of thienopyridine continuation. Drug‐eluting stents of first and second generation were implanted into 520 and 722 patients, respectively.
Baseline characteristics of the ARCTIC‐Interruption study population according to DES generation were balanced (Table 1). Patients with first‐generation DES had more prior PCI than those with second‐generation DES. Platelet reactivity to adenosine diphosphate measured while on the maintenance dose of thienopyridine did not differ according to the study groups (Table 1).
Table 1.
Demographic, Clinical, and Procedural Characteristics of the Patients at Baseline
| Treatment Group | Second‐Generation DES, n = 722 | First‐Generation DES, n = 520 | P Value |
|---|---|---|---|
| Age, y | 64 (57–73) | 64 (57–73) | 0.65 |
| Age >75 y | 135 (18.7) | 81 (15.6) | 0.15 |
| Female sex | 141 (19.5) | 103 (19.8) | 0.90 |
| BMI, kg/m2 | 27 (25–30) | 27 (25–30) | 0.64 |
| PRU | 141 (75–208) | 142 (81–204) | 0.70 |
| DM | 245 (33.9) | 171 (32.9) | 0.70 |
| Dyslipidemia | 483 (66.9) | 364 (70) | 0.25 |
| Hypertension | 424 (58.7) | 333 (64) | 0.0583 |
| Current smoker | 171 (23.7) | 122 (23.5) | 0.93 |
| Prior stroke | 37 (5.1) | 28 (5.4) | 0.84 |
| Prior HF | 24 (3.3) | 19 (3.7) | 0.75 |
| Prior MI | 206 (28.5) | 170 (32.7) | 0.12 |
| Prior PCI | 270 (37.4) | 245 (47.1) | 0.0006 |
| Prior CABG | 50 (6.9) | 32 (6.2) | 0.59 |
| Medications | |||
| ACEI | 342 (47.4) | 304 (58.5) | 0.0001 |
| β‐Blocker | 418 (57.9) | 327 (62.9) | 0.0766 |
| Statin | 477 (66.1) | 357 (68.7) | 0.34 |
| PPI | 222 (30.7) | 163 (31.3) | 0.82 |
| CCB | 148 (20.5) | 115 (22.1) | 0.49 |
| Stented vessel | |||
| LM | 24 (3.3) | 17 (3.3) | 0.96 |
| LAD | 366 (50.7) | 298 (57.3) | 0.0211 |
| LCX | 227 (31.4) | 162 (31.2) | 0.91 |
| RCA, | 231 (32) | 177 (34) | 0.45 |
| CABG | 9 (1.2) | 4 (0.8) | 0.41 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; BMI, body mass index; CABG, coronary artery bypass grafting; CCB, calcium channel blocker; DES, drug‐eluting stent; DM, diabetes mellitus; HF, heart failure; IQR, interquartile range; LAD, left anterior descending artery; LCX, circumflex artery; LM, left main coronary artery; MI, myocardial infarction; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; PRU, P2Y12 reaction units; RCA, right coronary artery.
Data are presented as n (%) or median (IQR).
The average number of stents per intervention was 1.65 and 1.53 for first‐ and second‐generation DES, respectively (P = 0.016). The types of vessels treated were also balanced between both groups; more than half were the left anterior descending artery, with a significant excess among first‐ vs second‐generation DES (57% vs 51%; P < 0.05). Of importance, both first‐ and second‐generation stents were available at the beginning of the study, and their use was left to the discretion of the physician.
At the time of re‐randomization, 1 out of 10 patients was on prasugrel or on a 150‐mg clopidogrel maintenance dose, reflecting prior treatment adjustment in patients initially randomized to the strategy of platelet function testing in the first phase of the study. Patients with a first‐generation DES were more frequently exposed to clopidogrel and less frequently to prasugrel as compared with patients implanted with a second‐generation DES at the time of re‐randomization (Table 2). During the follow‐up period, adherence to the assigned DAPT treatment progressively decreased, with 50% of patients on DAPT at the last follow‐up visit, without difference between the 2 groups (Table 2). We found a time‐effect of prasugrel use, with a significantly higher proportion of patients on prasugrel during the second year of randomization as compared with the first year at the time of re‐randomization (16.8% vs 2.9%; P < 0.0001). This was observed irrespective of first or second stent generation. Median follow‐up was 17 months (interquartile range, 15–18 months) without differences according to stent generation.
Table 2.
Use of Antiplatelet Medications During Trial
| Treatment Group | Second‐Generation DES, n = 722 | First‐Generation DES, n = 520 | P Value |
|---|---|---|---|
| Treatment at randomization for treatment interruption | |||
| Clopidogrel MD | 632 (87.5) | 488 (93.8) | 0.0002 |
| Clopidogrel MD 75 mg | 551 (87.2) | 428 (87.7) | 0.85 |
| Clopidogrel MD 150 mg | 77 (12.2) | 56 (11.5) | |
| Clopidogrel MD >150 mg | 4 (0.6) | 4 (0.8) | |
| Prasugrel MD 10 mg | 77 (10.7) | 27 (5.2) | 0.0006 |
| ASA MD | 717 (99.3) | 517 (99.4) | 1.00 |
| Treatment at last follow‐up visit | |||
| Clopidogrel MD | 299 (41.4) | 244 (46.9) | 0.0534 |
| ASA MD | 691 (95.7) | 496 (95.4) | 0.79 |
| Prasugrel MD 10 mg | 32 (4.4) | 18 (3.5) | 0.39 |
Abbreviations: ASA, aspirin; DES, drug‐eluting stent; MD, maintenance dose.
Data are presented as n (%).
Efficacy Endpoints
During the follow‐up period of 17 months, 16 patients died, 18 had an acute MI, 17 had an urgent revascularization, 3 had a definite or probable ST, and 10 had a stroke. At the end of follow‐up, the primary endpoint had occurred in 6.2% of patients in the first‐generation DES group and 2.6% of patients in the second‐generation DES group (P = 0.0039; Table 3 and Figure 1). The results were consistent for all the secondary endpoints (Table 3).
Table 3.
Study Endpoints During Follow‐up
| Variables | Second Generation, n = 722 | First Generation, n = 520 | HR (95% CI) | P Value |
|---|---|---|---|---|
| Any death, MI, ST, stroke or TIA, urgent revascularization (primary endpoint) | 19 (2.6) | 32 (6.2) | 2.31 (1.31‐4.07) | 0.0039 |
| ST (revascularized or not) or any urgent revascularization (main secondary) | 6 (0.8) | 12 (2.3) | 2.68 (1.01‐7.15) | 0.0486 |
| Any death, recurrent ACS, stroke or TIA | 16 (2.2) | 29 (5.6) | 2.48 (1.35‐4.57) | 0.0035 |
| Death or resuscitated cardiac arrest | 7 (1.0) | 9 (1.7) | 1.77 (0.66‐4.74) | 0.26 |
| Death or MI | 12 (1.7) | 19 (3.7) | 2.16 (1.05‐4.44) | 0.0371 |
| Any death, MI, ST, stroke or TIA, urgent revascularization, STEEPLE major bleed (net clinical benefit) | 22 (3.0) | 35 (6.7) | 2.18 (1.28‐3.72) | 0.0042 |
| Any death | 7 (1.0) | 9 (1.7) | 1.77 (0.66‐4.74) | 0.26 |
| MI | 7 (1.0) | 11 (2.1) | 2.12 (0.82‐5.47) | 0.12 |
| ST | 0 (0.0) | 3 (0.6) | ||
| ACS | 8 (1.1) | 16 (3.1) | 2.71 (1.16‐6.33) | 0.0213 |
| Stroke or TIA | 4 (0.6) | 6 (1.2) | 2.02 (0.57‐7.14) | 0.28 |
| Urgent revascularization | 6 (0.8) | 11 (2.1) | 2.45 (0.91‐6.62) | 0.08 |
| STEEPLE major bleed | 3 (0.4) | 4 (0.8) | 1.79 (0.40‐8.02) | 0.44 |
| STEEPLE minor bleed | 4 (0.6) | 3 (0.6) | 1.02 (0.23‐4.55) | 0.98 |
| STEEPLE major or minor bleed | 7 (1.0) | 7 (1.3) | 1.35 (0.47‐3.85) | 0.57 |
Abbreviations: ACS, acute coronary syndrome; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; ST, stent thrombosis; STEEPLE, Safety and Efficacy of Enoxaparin in Percutaneous Coronary Intervention Patients, an International Randomized Evaluation; TIA, transient ischemic attack.
Data are presented as n (%).
Figure 1.
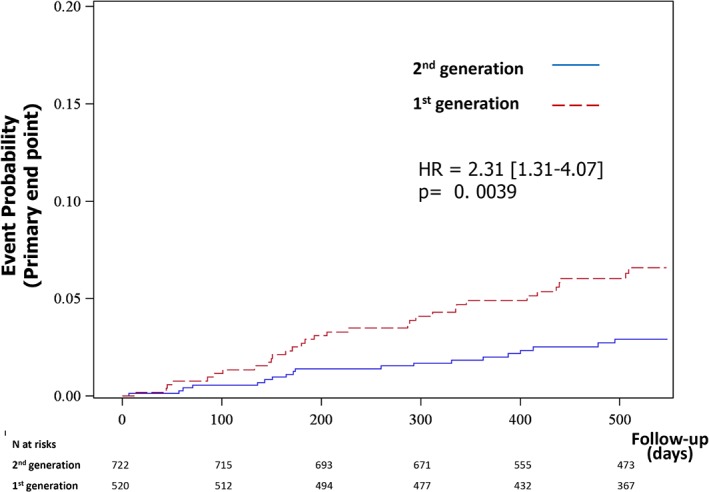
Kaplan‐Meier curve for the primary endpoint (death, MI, ST, stroke, or urgent revascularization). Abbreviations: HR, hazard ratio; MI, myocardial infarction; ST, stent thrombosis.
Results remained consistent after adjustment for established independent predictors of ST, including DM, active smoking status, prior MI, prior PCI, number of stents, body mass index, prasugrel maintenance dose at the time of randomization, and PRU > 208. The occurrence of the primary endpoint remained twice as frequent in the first‐generation DES group as in the second‐generation DES group, with consistent findings for all secondary endpoints (Table 4). Further adjustments for age, sex, and multivessel disease had no impact.
Table 4.
Study Endpoints After Adjustment for Major Determinants of ST (n = 1016)
| Stent Generation, N = 1016 | First Set of Adjustmentsa | Second Set of Adjustmentsb | Third Set of Adjustmentsc | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | Second, n = 589 | First, n = 427 | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Any death, MI, ST, stroke or TIA, urgent revascularization | 15 (2.5) | 30 (7) | 2.56 (1.37‐4.78) | 0.0033 | 2.58 (1.38‐4.83) | 0.0030 | 2.59 (1.38‐4.84) | 0.0030 |
| ST or any urgent revascularization | 5 (0.8) | 12 (2.8) | 3.03 (1.06‐8.68) | 0.0394 | 2.99 (1.04‐8.62) | 0.0426 | 3.02 (1.05‐8.74) | 0.0411 |
| Any death, recurrent ACS, stroke or TIA | 12 (2) | 27 (6.3) | 2.89 (1.46‐5.74) | 0.0024 | 2.91 (1.46‐5.79) | 0.0023 | 2.91 (1.46‐5.80) | 0.0023 |
| Death or resuscitated cardiac arrest | 4 (0.7) | 8 (1.9) | 2.98 (0.89‐10.01) | 0.0769 | 2.93 (0.88‐9.83) | 0.0812 | 2.76 (0.82‐9.33) | 0.1025 |
| Death or MI | 9 (1.5) | 17 (4) | 2.51 (1.11‐5.66) | 0.0274 | 2.50 (1.11‐5.66) | 0.0275 | 2.49 (1.10‐5.64) | 0.0284 |
| Any death, MI, ST, stroke or TIA, urgent revascularization, STEEPLE major bleed (net clinical benefit) | 18 (3.1) | 33 (7.7) | 2.35 (1.32‐4.20) | 0.0039 | 2.38 (1.33‐4.25) | 0.0035 | 2.38 (1.33‐4.25) | 0.0035 |
| Any death | 4 (0.7) | 8 (1.9) | 2.98 (0.89‐10.01) | 0.0769 | 2.93 (0.88‐9.83) | 0.0812 | 2.76 (0.82‐9.33) | 0.1025 |
| MI | 6 (1) | 10 (2.3) | 2.00 (0.72‐5.56) | 0.1815 | 2.02(0.73‐5.60) | 0.1771 | 2.02 (0.73‐5.60) | 0.1778 |
| ST | 0 (0) | 3 (0.7) | ||||||
| ACS | 6 (1) | 15 (3.5) | 3.20 (1.23‐8.32) | 0.0173 | 3.11 (1.19‐8.11) | 0.0204 | 3.12 (1.20‐8.15) | 0.0202 |
| Stroke or TIA | 4 (0.7) | 6 (1.4) | 1.66 (0.47‐5.94) | 0.4342 | 1.78 (0.49‐ 6.44) | 0.3805 | 1.78 (0.49‐6.44) | 0.3806 |
| Urgent revascularization | 5 (0.8) | 11 (2.6) | 2.74 (0.94‐7.99) | 0.0654 | 2.67 (0.91‐7.85) | 0.0745 | 2.69 (0.91‐7.95) | 0.0725 |
| STEEPLE major bleed | 3 (0.5) | 4 (0.9) | 1.77 (0.39‐8.04) | 0.4591 | 1.73 (0.38‐7.95) | 0.4835 | 1.61 (0.35‐7.48) | 0.5444 |
| STEEPLE minor bleed | 4 (0.7) | 3 (0.7) | 0.97 (0.21‐4.37) | 0.9659 | 1.02 (0.23‐4.65) | 0.9764 | 1.06 (0.23‐4.84) | 0.9410 |
| STEEPLE major or minor bleed | 7 (1.2) | 7 (1.6) | 1.31 (0.45‐3.77) | 0.6185 | 1.32 (0.46‐3.81) | 0.6081 | 1.31 (0.45‐3.77) | 0.6212 |
Abbreviations: ACS, acute coronary syndrome; BMI, body mass index; CI, confidence interval; HR, hazard ratio; MD, maintenance dose; MI, myocardial infarction; PCI, percutaneous coronary intervention; PRU, P2Y12 reaction units; ST, stent thrombosis; STEEPLE, Safety and Efficacy of Enoxaparin in Percutaneous Coronary Intervention Patients, an International Randomized Evaluation; TIA, transient ischemic attack.
Data are presented as n (%).
DM, smoking status, prior PCI, prior MI, number of stents, BMI, prasugrel MD, and PRU >208.
DM, smoking status, prior PCI, prior MI, number of stents, BMI, prasugrel MD, PRU >208, age, and sex.
DM, smoking status, prior PCI, prior MI, number of stents, BMI, prasugrel MD, PRU >208, age, sex, and multivessel disease.
There was no interaction between stent generation and interruption or continuation of thienopyridine for the primary study endpoint (P = 0.77) as for any other secondary ischemic endpoint (see Supporting Information, Table, in the online version of this article). Conversely, there was no interaction between stent generation and timing of randomization (first vs second year of randomization; P = 0.93).
Safety Endpoints
Overall, there were 14 bleeding events according to the STEEPLE definitions, of which 7 were major and 7 were minor. The rate of STEEPLE major bleeding events did not differ in the first‐generation DES group as compared with the second‐generation DES group (HR for second‐generation DES: 1.79, 95% CI: 0.4‐8.02, P = 0.44). Similarly, the endpoint combining major and minor bleeds did not differ significantly (HR for second‐generation DES: 1.35, 95% CI: 0.47‐3.85, P = 0.57; Table 3). There were no Thrombolysis in Myocardial Infarction (TIMI) major bleeds and no fatal bleeds. There was no significant interaction between DES generation and interruption or continuation of thienopyridine (see Supporting Information, Table, in the online version of this article). Finally, no interaction was observed between DES generation and timing of randomization.
Discussion
In this subanalysis of the ARCTIC‐Interruption study, the efficacy and safety of first‐ vs second‐generation DES were investigated in patients who were randomized to extension vs interruption of DAPT 1 year after stenting. The main results are the following:
The use of first‐generation DES was independently associated with the occurrence of the primary study endpoint, a composite of any death, MI, ST, stroke or transient ischemic attack, urgent revascularization, and of all main secondary ischemic endpoints.
This association persisted after adjustments for all measured potential confounders of ST.
This association was observed irrespective of DAPT duration, type of thienopyridine (clopidogrel or prasugrel), and date of entry into the study.
The use of first‐generation DES vs second‐generation DES was not associated with any safety endpoints.
The better safety profile of newer‐generation DES is now well established and is primarily driven by a reduction in MI and ST.4 This relies on experimental evidence of antithrombotic properties of EES29 but also on direct comparison demonstrating lower risk of MI as compared with PES,3, 30 and to a lesser degree with SES. By providing effective revascularization with significantly fewer stent‐related adverse effects, newer‐generation DES led to improved survival after coronary revascularization when compared with optimal medical treatment9 and has become the first choice for coronary stenting.31
The ARCTIC‐Interruption study demonstrated a nonsignificant trend for a reduction in ischemic events with extended DAPT beyond 1 year as compared with interruption but a significant increase in any bleeding irrespective of platelet reactivity.14 Second‐generation DES were used in more than half of PCIs, reflecting the market penetration of these devices. In the present ARCTIC‐Generation substudy, all types of thrombotic events are halved with second‐generation DES compared with first‐generation DES. These beneficial effects persisted after adjustments for ST‐associated risk factors,32 further supporting the initial findings.
In ARCTIC‐Interruption, the choice of thienopyridines was left to the discretion of the investigators but its interruption was randomized. Patients were censored if on‐treatment time exposure was <50% of follow‐up, avoiding poor antithrombotic adherence as a potential confounder leading to misinterpretation of our data, as has been observed in registries evaluating whether duration of DAPT should be device‐specific.23 Platelet reactivity on thienopyridine, a strong independent predictor of ST,33 did not differ between first‐ and second‐generation DES. Prasugrel was made available during the conduct of the trial and was utilized to overcome high on‐treatment platelet reactivity according to the protocol. Its use was less frequent in first‐ vs second‐generation DES at the time of randomization but was not present anymore at the end of follow‐up. In addition, our results were consistent after adjustment for prasugrel maintenance dose and platelet reactivity, further accounting for the lack of difference in safety events. These findings not only highlight that antiplatelet treatments were balanced between stent groups, but also that the observed differences in thrombotic events are primarily device‐related.
ARCTIC‐Generation demonstrates that first‐generation DES should be considered as an additional risk factor for recurrent ischemic events and may support an extended duration of DAPT,2 although our data did not show a significant interaction between the two. We found an unusual very high event rate in the first‐generation DES group, similar to that of the Taxus Liberté Post‐Approval Study, a surveillance study of DES performance following commercial release.2 In this randomized study, the Taxus Liberté–related safety concerns were overcome by an extended duration of prasugrel beyond 1 year. However, one major difference between ARCTIC‐Generation and the Taxus Liberté Post‐Approval Study is the type of thienopyridine for prolongation of DAPT (clopidogrel vs prasugrel). This suggests that we may need P2Y12 inhibitors stronger than clopidogrel to reduce the risk of first‐generation DES. Although prasugrel use was off‐label in the vast majority of patients in the Taxus Liberté Post‐Approval Study,34 it was rarely used in ARCTIC‐Interruption. We believe our findings are of clinical relevance, as first‐generation DES are still an option for coronary stenting in many countries. In addition, the vast majority of patients who were treated with a first‐generation DES are alive, and the decision of type and duration of DAPT remains a question.
ARCTIC‐Generation directly raises the question of whether an optimal net clinical benefit can be reached by refining the potency and duration of DAPT. First‐generation PES and SES are a thick‐strut stainless‐steel platform, poorly deliverable or conformable, and embedded with durable inflammatory polymers that are nowadays rarely used or no longer manufactured in developed countries. However, whether DAPT should be device‐specific remains a matter of debate and is not answered by ARCTIC‐Generation, in contrast to the TAXUS Liberté Post‐Approval Study.2 So far, 5 trials collectively support the safety of 3 to 6 months of DAPT compared with a more prolonged course with E‐ZES, R‐ZES, and fluoropolymer‐based EES, whereas 3 trials of DAPT prolongation to 2 years have reported excess bleeding without reduced rates of ST, MI, or death.14, 15, 20 The only adequately powered trial to answer the question of the benefit of extending duration of DAPT beyond 1 year after DES demonstrated improved late outcomes by reducing stent‐related and/or non–stent‐related cardiovascular events with significantly more major bleedings.1 In this study, there was a significant interaction between DAPT duration and both the type of thienopyridine and stent generation for major adverse cardiac events, further supporting that type and duration of DAPT might be device‐specific.1
ARCTIC‐Generation is a subgroup analysis with its inherent limitations. It was not prespecified, and the sample size population is rather small. First randomization was not stratified by stent type, and some of the baseline characteristics and treatments were not adequately matched. Second, avoidance of all potential confounders is impossible and should be also seen as an important limitation when performing adjustments. Finally, the lack of interaction between DAPT duration and DES generation does not allow any robust conclusion.
Conclusion
ARCTIC‐Generation suggests that the use of first‐generation DES should be considered as an additional risk factor for recurrent ischemic events and that second‐generation DES should be the preferred strategy whenever possible. The lack of interaction between stent generation and duration of DAPT suggests that the type as well as the duration of DAPT may be decided on the basis of clinical characteristics but also on the type of device implanted.31
Supporting information
Table (n = 1242)
Study flow chart (ARCTIC and ARCTIC‐Interruption).
Acknowledgments
The authors would like to acknowledge Yannick Vacher, Abdel Ait Bachir, Andrea Bardon, Nabil Brouk, Julie Bussone, Hermione Folal, Vanessa Gallois, Sophie Gérard, Véronique Jouis, Azizath Karibou, Nathalie Kingue, Valérie Mazur, Hélène Mauro, Ghislain N'Gouala, Luminita Neculaita, Sissel Paulsrud, Virginie Rochaud, and Corinne Tchokothe.
Study Investigators
CHU Pitié‐Salpêtrière, Paris: Prs Montalescot/Collet; Hôpital Cardiologique du Haut Lévêque, Pessac: Pr Coste; Hôpital Pontchaillou, Rennes: Pr Le Breton; CH de Lagny, Marne‐la‐Vallée: Drs Elhadad/Cohen; Hôpital privé Beauregard, Marseille: Dr Wittenberg; Hôpital Arnaud de Villeneuve, Montpellier: Pr Leclercq; Hôpital Cochin, Paris: Pr Varenne; CHU Carémeau, Nîmes: Drs Ledermann/Cayla; Hôpital de la Timone, Marseille: Prs Bonnet/Cuisset; Hôpital Cardiologique Albert Calmette, Lille: Dr Van Belle; Hôpital Lariboisière, Paris: Pr Henry; CHU Jean Minjoz, Besançon: Pr Bassand; Hôpital Cardio‐Vasculaire Louis Pradel, Lyon: Pr Finet; Hôpital Nord Marseille: Dr Paganelli; Hôpital de Rangueil, Toulouse: Dr Carrié; Clinique de l'Orangerie, Strasbourg: Dr Aleil; CH de la Région Annecienne, Annecy: Dr Belle; Clinique Nantaise, Nantes: Dr Brunel; Clinique Nantaise, Nantes: Dr Brunel; CH de Chartres: Dr Rangé; CH d'Avignon: Drs Pansieri/Barney; GH du Centre Alsace: Drs Lhoest/Levai; CH Marie Lannelongue, Le Plessis‐Robinson: Dr Caussin; CH de Cannes: Drs Tibi/Zemour; Hôpital François Mitterrand, Pau: Dr Delarche; Hôpital Saint‐Joseph, Marseille: Dr D'Houdain; CHU de Poitiers: Dr Christiaens; Clinique Sainte Clothilde, La Réunion:, Dr Pouillot; Polyclinique de Bordeaux Caudéran, Bordeaux: Dr Casteigt; Hôpital Pasteur, Nice: Pr Ferrari; CH Dijon: Pr Cottin; CHR Strasbourg: Pr Ohlmann; CH Lens: Dr Pecheux; CH de Compiègne: Dr Sayah; CH Clermont‐Ferrand: Dr Motreff; Clinique du Tonkin, Villeurbanne: Dr Champagnac; Clinique de l'Europe, Amiens: Dr Py; Clinique du Parc, Castelnau‐le‐Lez: Dr Shadfar; CH de Bastia: Dr Boueri.
J.‐P. Collet reports receiving research grants from Bristol‐Myers Squibb, Sanofi‐Aventis, Eli Lilly, Medtronic, Boston Scientific, Cordis, Stago, Fondation de France, INSERM, Nanospheres, Fédération Française de Cardiologie, and Société Française de Cardiologie; consulting fees from Sanofi‐Aventis, Eli Lilly, and Bristol‐Myers Squibb; and lecture fees from Bristol‐Myers Squibb, Sanofi‐Aventis, Eli Lilly, and AstraZeneca. J. Silvain reports receiving research grants to the Institution from AstraZeneca, Brahms, Daiichi‐Sankyo, Eli Lilly, Institute of Cardiometabolism (ICAN), INSERM, Fédération Française de Cardiologie, Fondation de France, Société Française de Cardiologie and Sanofi‐Aventis; consulting fees from Actelion, AstraZeneca, Daiichi‐Sankyo, Eli Lilly, and Sanofi‐Aventis; lecture fees from Algorythm, AstraZeneca, and Bristol‐Myers Squibb; and travel fees from AstraZeneca, Braun, Bristol‐Myers Squibb, and Pfizer. M. Kerneis reports receiving a grant from Fédération Française de Cardiologie. G. Cayla reports receiving a research grant from la Fédération Française de Cardiologie, la Fondation Coeur et Recherche; consultant fees from Abbott Vascular, AstraZeneca, CLS Behring, Daiichi‐Sankyo, and Eli Lilly; and lecture fees from Abbott Vascular, AstraZeneca, Biotronik, Boston, Bristol‐Myers Squibb, CLS Behring, Daiichi‐Sankyo, Eli Lilly, Iroko Cardio, and Pfizer. E. Van Belle reports receiving research grants from Sanofi‐Aventis, Medtronic, Cordis, Boston Scientific, Fondation SGAM, Fondation de France, ACTION, and APHP; consulting fees from Pfizer, Eli Lilly, Sanofi, LFB, Abbott, and Fresenius; a grant from Boehringer Ingelheim; and lecture fees from Merck and Pfizer. O. Barthélémy reports receiving lecture fees from Sanofi. P. Sabouret reports receiving honoraria for advisory board/lectures from AstraZeneca, Bayer, Bristol‐Myers Squibb, Daiichi‐Sankyo, Eli Lilly, Johnson & Johnson, Merck Sharpe and Dohme, and Pfizer. E. Vicaut reports receiving honoraria for advisory board/lectures from AstraZeneca, Bayer, Bristol‐Myers Squibb, Daiichi‐Sankyo, Eli Lilly, Johnson & Johnson, Merck Sharpe and Dohme, and Pfizer. G. Montalescot reports receiving research grants to the institution or consulting/lecture fees from Acuitude, ADIR, Amgen, AstraZeneca, Bayer, Berlin Chimie AG, Boehringer Ingelheim, Bristol‐Myers Squibb, Brigham and Women's Hospital, Cardiovascular Research Foundation, Celladon, CME Resources, Daiichi‐Sankyo, Eli Lilly, Europa, Fédération Française de Cardiologie, Gilead, Hopitaux Universitaires Genève, ICAN, Janssen‐Cilag, Lead‐Up, Medcon International, Menarini, Medtronic, MSD, Pfizer, Recor, Sanofi‐Aventis, Stentys, The Medicines Company, TIMI Study Group, Universität Basel, WebMD, and Zoll Medical.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators . Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med. 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garratt KN, Weaver WD, Jenkins RG, et al. Prasugrel plus aspirin beyond 12 months is associated with improved outcomes after TAXUS Liberté paclitaxel‐eluting coronary stent placement. Circulation. 2015;131:62–73. [DOI] [PubMed] [Google Scholar]
- 3. Stone GW, Rizvi A, Newman W, et al; SPIRIT IV Investigators . Everolimus‐eluting versus paclitaxel‐eluting stents in coronary artery disease. N Engl J Med. 2010;362:1663–1674. [DOI] [PubMed] [Google Scholar]
- 4. Stefanini GG, Baber U, Windecker S, et al. Safety and efficacy of drug‐eluting stents in women: a patient‐level pooled analysis of randomised trials [published correction appears in Lancet. 2013;382:1878]. Lancet. 2013;382:1879–1888. [DOI] [PubMed] [Google Scholar]
- 5. Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug‐eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR‐TEST 3, ISAR‐TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33:1214–1222. [DOI] [PubMed] [Google Scholar]
- 6. Stefanini GG, Kalesan B, Serruys PW, et al. Long‐term clinical outcomes of biodegradable polymer biolimus‐eluting stents versus durable polymer sirolimus‐eluting stents in patients with coronary artery disease (LEADERS): 4‐year follow‐up of a randomised non‐inferiority trial. Lancet. 2011;378:1940–1948. [DOI] [PubMed] [Google Scholar]
- 7. Palmerini T, Biondi‐Zoccai G, Della Riva D, et al. Stent thrombosis with drug‐eluting and bare‐metal stents: evidence from a comprehensive network meta‐analysis. Lancet. 2012;379:1393–1402. [DOI] [PubMed] [Google Scholar]
- 8. Bangalore S, Kumar S, Fusaro M, et al. Short‐ and long‐term outcomes with drug‐eluting and bare‐metal coronary stents: a mixed‐treatment comparison analysis of 117 762 patient‐years of follow‐up from randomized trials. Circulation. 2012;125:2873–2891. [DOI] [PubMed] [Google Scholar]
- 9. Windecker S, Stortecky S, Stefanini GG, et al. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta‐analysis. BMJ. 2014;348:g3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morrow DA, Braunwald E, Bonaca MP, et al; TRA 2P–TIMI 50 Steering Committee and Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–1413. [DOI] [PubMed] [Google Scholar]
- 11. Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators . Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–1988. [DOI] [PubMed] [Google Scholar]
- 12. Elmariah S, Mauri L, Doros G, et al. Extended duration dual antiplatelet therapy and mortality: a systematic review and meta‐analysis. Lancet. 2015;385:792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wijns W, Kolh P, Danchin N, et al; Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI). Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555. [DOI] [PubMed] [Google Scholar]
- 14. Collet JP, Silvain J, Barthélémy O, et al. Dual‐antiplatelet treatment beyond 1 year after drug‐eluting stent implantation (ARCTIC‐Interruption): a randomised trial. Lancet. 2014;384:1577–1585. [DOI] [PubMed] [Google Scholar]
- 15. Valgimigli M, Campo G, Monti M, et al; Prolonging Dual Antiplatelet Treatment After Grading Stent‐Induced Intimal Hyperplasia Study (PRODIGY) Investigators. Short‐ versus long‐term duration of dual‐antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–2026. [DOI] [PubMed] [Google Scholar]
- 16. Colombo A, Chieffo A, Frasheri A, et al. Second‐generation drug‐eluting stent implantation followed by 6‐ versus 12‐month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol. 2014;64:2086–2097. [DOI] [PubMed] [Google Scholar]
- 17. Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators . Three vs twelve months of dual antiplatelet therapy after zotarolimus‐eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310:2510–2522. [DOI] [PubMed] [Google Scholar]
- 18. Gwon HC, Hahn JY, Park KW, et al. Six‐month versus 12‐month dual antiplatelet therapy after implantation of drug‐eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125:505–513. [DOI] [PubMed] [Google Scholar]
- 19. Kim BK, Hong MK, Shin DH, et al; RESET Investigators . A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3‐month dual antiplatelet Therapy following Endeavor zotarolimus‐eluting stent implantation). J Am Coll Cardiol. 2012;60:1340–1348. [DOI] [PubMed] [Google Scholar]
- 20. Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug‐eluting stent implantation: a randomized, controlled trial. Circulation. 2014;129:304–312. [DOI] [PubMed] [Google Scholar]
- 21. Gilard M, Barragan P, Noryani AAL, et al. 6‐ versus 24‐month dual antiplatelet therapy after implantation of drug‐eluting stents in patients nonresistant to aspirin: the randomized, multicenter ITALIC trial. J Am Coll Cardiol. 2015;65:777–786. [DOI] [PubMed] [Google Scholar]
- 22. Camenzind E, Boersma E, Wijns W, et al; PROTECT Steering Committee and Investigators . Modifying effect of dual antiplatelet therapy on incidence of stent thrombosis according to implanted drug‐eluting stent type. Eur Heart J. 2014;35:1932–1948. [DOI] [PubMed] [Google Scholar]
- 23. Silber S, Kirtane AJ, Belardi JA, et al. Lack of association between dual antiplatelet therapy use and stent thrombosis between 1 and 12 months following Resolute zotarolimus‐eluting stent implantation. Eur Heart J. 2014;35:1949–1956. [DOI] [PubMed] [Google Scholar]
- 24. Valgimigli M, Borghesi M, Tebaldi M, et al; Prolonging Dual Antiplatelet Treatment After Grading Stent‐Induced Intimal Hyperplasia Study (PRODIGY) Investigators . Should duration of dual antiplatelet therapy depend on the type and/or potency of implanted stent? A pre‐specified analysis from the Prolonging Dual Antiplatelet Treatment After Grading Stent‐Induced Intimal Hyperplasia Study (PRODIGY). Eur Heart J. 2013;34:909–919. [DOI] [PubMed] [Google Scholar]
- 25. Collet JP, Cayla G, Cuisset T, et al. Randomized comparison of platelet function monitoring to adjust antiplatelet therapy versus standard of care: rationale and design of the assessment by a double randomization of (1) a fixed dose versus a monitoring‐guided dose of aspirin and clopidogrel after DES implantation, and (2) treatment interruption versus continuation, 1 year after stenting (ARCTIC) study. Am Heart J. 2011;161:5.e5–12.e5. [DOI] [PubMed] [Google Scholar]
- 26. Montalescot G, Rangé G, Silvain J, et al; ARCTIC Investigators . High on‐treatment platelet reactivity as a risk factor for secondary prevention after coronary stent revascularization: a landmark analysis of the ARCTIC study. Circulation. 2014;129:2136–2143. [DOI] [PubMed] [Google Scholar]
- 27. Collet JP, Cuisset T, Rangé G, et al; ARCTIC Investigators . Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367:2100–2109. [DOI] [PubMed] [Google Scholar]
- 28. Montalescot G, White HD, Gallo R, et al; STEEPLE Investigators . Enoxaparin versus unfractionated heparin in elective percutaneous coronary intervention. N Engl J Med. 2006;355:1006–1017. [DOI] [PubMed] [Google Scholar]
- 29. Kolandaivelu K, Swaminathan R, Gibson WJ, et al. Stent thrombogenicity early in high‐risk interventional settings is driven by stent design and deployment and protected by polymer‐drug coatings. Circulation. 2011;123:1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kedhi E, Joesoef KS, McFadden E, et al. Second‐generation everolimus‐eluting and paclitaxel‐eluting stents in real‐life practice (COMPARE): a randomised trial. Lancet. 2010;375:201–209. [DOI] [PubMed] [Google Scholar]
- 31. Windecker S, Alfonso F, Collet JP, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS), developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 32. Cayla G, Hulot JS, O'Connor SA, et al. Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. JAMA. 2011;306:1765–1774. [DOI] [PubMed] [Google Scholar]
- 33. Stone GW, Witzenbichler B, Weisz G, et al; ADAPT‐DES Investigators . Platelet reactivity and clinical outcomes after coronary artery implantation of drug‐eluting stents (ADAPT‐DES): a prospective multicentre registry study [published correction appears in Lancet. 2014;383:1128]. Lancet. 2013;382:614–623. [DOI] [PubMed] [Google Scholar]
- 34. Collet JP, Silvain J, Montalescot G. Long‐term secondary prevention after high‐risk stenting: a good drug for a bad stent. Circulation. 2015;131:13–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table (n = 1242)
Study flow chart (ARCTIC and ARCTIC‐Interruption).


