ABSTRACT
Background
Mineralocorticoid receptor antagonists (MRAs) reduce morbidity and mortality in heart failure with reduced ejection fraction (HFrEF). Their role in patients without heart failure, particularly in patients with coronary artery disease (CAD) and preserved EF, is still a matter of debate.
Hypothesis
The MRA eplerenone on top of standard medical therapy improves endothelial dysfunction and other markers of vascular health in CAD patients with preserved EF.
Methods
In this double‐blind, randomized, placebo‐controlled study, 42 patients (mean age: 63.5 ± 9.1 years; 37 males) were randomized to 4‐week treatment with eplerenone 25 mg daily or placebo. The primary endpoint was difference in endothelial function as assessed by flow‐mediated dilatation (FMD) of the brachial artery. Secondary endpoints included 24‐hour blood pressure (BP), endothelial progenitor cells, and platelet adhesion.
Results
No difference in the primary endpoint FMD was noted after 4 weeks of treatment with eplerenone compared with placebo (FMD: 4.7% ± 2.0% and 4.9% ± 2.1%, respectively; P = 0.77). There were no significant differences between eplerenone and placebo in 24‐hour BP (mean systolic BP: 126.9 ± 17.3 and 123.3 ± 9.7 mm Hg, P = 0.41; diastolic BP: 73.3 ± 12.9 and 72.0 ± 7.5 mm Hg, respectively, P = 0.69), number of endothelial progenitor cells, and platelet adhesion.
Conclusions
Adding low‐dose eplerenone to standard medical therapy did not improve important markers of vascular health in patients with CAD and preserved EF. Our results may help understand conflicting evidence from larger clinical trials on MRAs in patients with preserved EF.
Introduction
Mineralocorticoid receptor (MR) antagonism has become a main treatment paradigm in heart failure with reduced ejection fraction (HFrEF). Two MR antagonists (MRAs), spironolactone and eplerenone, have been shown to reduce all‐cause mortality and heart failure (HF) hospitalizations in HFrEF, with class IA recommendations supporting their use according to current guidelines.1, 2 Though the benefit of MR blockade in HFrEF is firmly established, its role in patients with coronary artery disease (CAD) with preserved ejection fraction (EF) still remains a matter of debate.3 Interestingly, plasma aldosterone levels predict long‐term clinical outcome not only in HF,4 but also in CAD.5 Indeed, aldosterone exerts multiple deleterious effects, particularly salt and water retention, ventricular hypertrophy, myocardial fibrosis, and endothelial dysfunction.6, 7 Endothelial dysfunction plays an important role in the pathogenesis of CAD and is a well‐established marker for cardiovascular risk and prognosis.8 Accordingly, MR blockade has been shown to improve endothelial dysfunction in patients with HFrEF.9 It was the aim of the present study to prospectively evaluate the effects of MR antagonism with eplerenone on vascular health in CAD patients with preserved EF.
Methods
This trial included 42 patients with a history of CAD, documented by coronary angiography, nuclear imaging, or positive stress testing. Other inclusion criteria were age >30 years and stable cardiovascular medication for ≥4 months. Female patients needed to be postmenopausal. Exclusion criteria were as follows: recent myocardial infarction, unstable angina, stroke, or any coronary interventions within 3 months prior to study entry; uncontrolled arterial hypertension (>160/90 mm Hg), any HF symptoms New York Heart Association class I or higher; left ventricular EF <50%; renal insufficiency (Cockcroft‐Gault estimated creatinine clearance <50 mL/min); insulin‐dependent diabetes mellitus; anemia (hemoglobin <10 g/dL); any conditions associated with chronic infection or inflammation; any malignancy; the presence of endocrine disorders (Cushing disease, Addison disease, thyroid dysfunction); and concomitant use of long‐acting nitrates, potassium supplements, potassium‐sparing diuretics (amiloride, eplerenone, spironolactone, or triamterene), strong cytochrome P450 3A4 inhibitors (eg, ketoconazole, itraconazole), and smoking.
Patients were recruited at the Cardiology Clinic at the University Hospital Zurich, Switzerland. Informed consent was obtained from and signed by all participants. The study was approved by the local ethics committee (Regulatory No. 1033) and was done in accordance with the Declaration of Helsinki. The trial was registered at http://www.ClinicalTrials.gov (NCT00427284).
After clinical examination, including measurement of blood pressure (BP) and body weight, endothelial function measurement was performed and blood samples were drawn and processed immediately. The participants were randomized in a double‐blind fashion to receive eplerenone 25 mg once daily or matched placebo for 4 weeks on top of current standard medical therapy. The low dose was chosen to allow for assessment of BP‐independent effects. Randomization and blinding of study drugs was provided by InterCorNet and the Cantonal Pharmacy (both in Zurich, Switzerland). All investigators were unaware of the allocation procedure at any time. Measurements were performed at baseline (time of randomization) and after 2 and 4 weeks of daily intake of the study drug or placebo, respectively.
All patients were instructed to fast for ≥12 hours and refrain from caffeine‐containing products for ≥24 hours prior to their appointments. The patients were advised not to take their usual drugs in the morning of the examination day. Measurement of flow mediated‐vasodilatation (FMD) and collection of blood samples was always done at rest, in the morning, and in a quiet, air‐conditioned room. Endothelial function was assessed by measuring FMD of the brachial artery, as described previously.10 In brief, the left brachial artery was visualized 2 to 10 cm above the elbow with a 10‐Mhz ultrasound system (Siemens X300; Siemens Switzerland AG, Zurich, Switzerland) using a stereotactic clamp and a video processing system (FMD Studio, Pisa, Italy).11 One minute after acquisition of the baseline diameter, a wrist cuff was inflated to 220 mm Hg for 5 minutes. Immediately after release, arterial diameter was continuously recorded. Endothelial‐independent vasodilatation was measured after sublingual application of 0.4 mg glyceryl trinitrate (Nitrolingual Spray; Pohl‐Boskamp, Hohenlockstedt, Germany).
For measurement of endothelial progenitor cells (EPCs), blood was collected and centrifuged at 1800 g for 30 minutes within 1 to 2 hours. The buffy layer was transferred to sterile centrifuge tubes. Mononuclear cells were washed twice with 15 mL phosphate‐buffered saline and seeded on fibronectin‐coated Lab‐Tek Chamber Slides (Lab‐Tek, Wiesbaden, Germany) at a density of 2 million per well in 20% fetal calf serum (FCS; Gibco, Invitrogen, Basel, Switzerland) endothelial cell growth medium (EGM)‐2 (Clonetics, Lonza, Verviers, Belgium). The medium was changed after 3 days. The cultures were analyzed at the fourth day of plating. Adherent cells were incubated with 1,1′‐dioctadecyl‐3,3,3′,3′‐tetramethylindocarbocyanine (DiI)‐labeled acetylated low‐density lipoprotein (acLDL; Molecular Probes, Eugene, Oregon, USA) and FITC‐labeled Ulex europaeus agglutinin (UEA)‐I (Sigma‐Aldrich, Steinheim, Germany) for 1 hour and nuclei were stained with DAPI (4′,6‐diamidino‐2‐phenylindole; Sigma‐Aldrich, Steinheim, Germany). Cells were visualized with an inverted fluorescent microscope in 3 different visual fields. The LDL/agglutinin double‐positive cells were counted and considered as EPCs.
Ambulatory BP measurements were obtained over 24 hours using a Tracker NIBP 2 device under the study medication (Del Mar Reynolds Medical, Hertford, United Kingdom). Shear‐stress‐dependent platelet adhesion was assessed using a cone and platelet analyzer as described previously.12 Results are expressed as the percentage of surface covered by platelets.
The difference in FMD was defined as the primary endpoint. A FMD Δ of 1.5% and SD of 1.5% for eplerenone vs placebo at 4 weeks was hypothesized. For a significance level of 5%, 20 patients per treatment group were assumed to be needed to reach a statistical power of ≥80%. The data were checked for normal distribution. Normally distributed data were expressed as mean ± SD. Non–normally distributed data were expressed as median and interquartile range (IQR). A P value <0.05 was considered statistically significant. ANOVA was used to assess intragroup as well as intergroup differences at any time points for FMD. The rest of the data were analyzed by paired and unpaired Student t test and the Wilcoxon test, as appropriate. All tests were 2‐sided and analysis was per protocol. Statistical analyses were performed using JMP version 9.0.1 (SAS Institute Inc., Cary, NC).
Results
A total of 42 patients (21 per group; mean age, 63.5 ± 9.1 years; 37 male; mean body mass index 27.1 ± 3.5 kg/m2) were included in the study. Their clinical characteristics and medications are presented in Table 1. Both study groups were well matched with no significant differences in baseline characteristics.
Table 1.
Baseline Characteristics of Participants
| Parameters | Eplerenone, n = 21 | Placebo, n = 21 | P Value |
|---|---|---|---|
| Clinical characteristics | |||
| LVEF, % | 61.1 ± 6.8 | 61.8 ± 7.0 | 0.75 |
| BMI, kg/m2 | 27.6 ± 3.2 | 26.6 ± 3.7 | 0.35 |
| Office SBP, mm Hg | 129.9 ± 9.6 | 133.0 ± 13.5 | 0.39 |
| Office DBP, mm Hg | 79.5 ± 8.5 | 79.0 ± 8.7 | 0.83 |
| Heart rate, bpm | 58.8 ± 9.3 | 59.9 ± 8.2 | 0.69 |
| Comorbidities | |||
| History of smoking | 11 (52) | 11 (55) | 0.87 |
| Dyslipidemia | 19 (90) | 18 (86) | 0.63 |
| HTN | 14 (67) | 13 (62) | 0.75 |
| DM | 0 (0) | 0 (0) | — |
| History of MI | 16 (76) | 15 (71) | 0.40 |
| Family history for CAD | 15 (79) | 12 (67) | 0.40 |
| CV risk factors | 3.5 ± 1.0 | 3.0 ± 1.0 | 0.11 |
| Concomitant drug therapy | |||
| ASA | 19 (90) | 21 (100) | 0.09 |
| Clopidogrel | 10 (48) | 4 (19) | 0.05 |
| ACEI and/or ARB | 12 (57) | 14 (67) | 0.53 |
| β‐Blocker | 14 (67) | 15 (71) | 0.74 |
| CCB | 5 (24) | 4 (19) | 0.71 |
| Diuretic | 3 (14) | 5 (24) | 0.43 |
| Statin | 19 (90) | 21 (100) | 0.09 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, aspirin; BMI, body mass index; CAD, coronary artery disease; CCB, calcium channel blocker; CV, cardiovascular; DBP, diastolic blood pressure; DM, diabetes mellitus; HR, heart rate; HTN, hypertension; LVEF, left ventricular ejection fraction; MI, myocardial infarction; SBP, systolic blood pressure; SD, standard deviation.
Values are presented as n (%) or mean ± SD.
No adverse effects were noted during the duration of the study. Baseline levels and changes of the primary and secondary endpoints are shown in Table 2. The primary endpoint FMD showed no significant difference between eplerenone and placebo after 4 weeks of treatment (FMD: 4.7% ± 2.0% and 4.9% ± 2.1%, respectively; P = 0.77; Table 2 and Figure 1, part A). Consistency of the measurement was verified using endothelium‐independent vasodilatation after glyceryl trinitrate, which showed no significant differences. In regard to laboratory and hemodynamic parameters, no significant differences in pro‐brain natriuretic peptide, potassium levels, high‐sensitivity C‐reactive protein, as well as ambulatory and office BP and heart rate were seen between both groups (Table 2). There was no significant difference in the number of circulating EPCs after treatment with eplerenone compared with placebo (1.3 [IQR, 1–4.5] and 1.7 [IQR, 0.8–2.4], respectively; P = 0.62). Likewise, no significant differences in platelet adhesion were seen between eplerenone and placebo (adhering platelets: 3.9% ± 1.6% and 4.1% ± 2.3%, respectively; P = 0.83).
Table 2.
Main Outcome Measures of the Trial
| Parameters | Eplerenone Baseline | Eplerenone 4 Weeks | Placebo Baseline | Placebo 4 Weeks | P Valuea |
|---|---|---|---|---|---|
| Endothelial function | |||||
| FMD, % | 4.6 ± 2.4 | 4.7 ± 2.0 | 5.1 ± 2.2 | 4.9 ± 2.1 | 0.77 |
| GTN, % | 12.4 ± 4.9 | 12.6 ± 5.7 | 13.8 ± 5.7 | 12.8 ± 12.8 | 0.90 |
| Hemodynamic measures | |||||
| 24‐hour SBP, mm Hg | 128.1 ± 14.0 | 126.9 ± 17.3 | 124.1 ± 11.5 | 123.3 ± 9.7 | 0.41 |
| 24‐hour DBP, mm Hg | 75.3 ± 9.6 | 73.3 ± 12.9 | 72.5 ± 7.6 | 72.0 ± 7.5 | 0.69 |
| 24‐hour heart rate, bpm | 65.5 ± 9.7 | 66.8 ± 9.8 | 65.2 ± 7.6 | 66.3 ± 8.4 | 0.86 |
| Office SBP, mm Hg | 129.9 ± 9.6 | 126.2 ± 11.8 | 133.0 ± 13.5 | 127.5 ± 11.7 | 0.72 |
| Office DBP, mm Hg | 79.5 ± 8.5 | 75.6 ± 9.5 | 79.0 ± 8.7 | 77.1 ± 6.2 | 0.54 |
| Heart rate, bpm | 58.8 ± 9.3 | 58.3 ± 10.2 | 59.9 ± 8.2 | 60.4 ± 7.9 | 0.46 |
| Laboratory parameters | |||||
| Na, mmol/L | 139.8 ± 2.4 | 139.4 ± 1.8 | 140.0 ± 1.7 | 140.0 ± 1.6 | 0.22 |
| K, mmol/L | 4.0 ± 0.3 | 4.0 ± 0.2 | 3.9 ± 0.3 | 3.9 ± 0.2 | 0.05 |
| Cr, µmol/L | 83.9 ± 11.0 | 92.1 ± 18.7 | 83.9 ± 11.0 | 84.5 ± 12.3 | 0.13 |
| Glucose, mmol/L | 5.5 ± 0.7 | 5.5 ± 0.8 | 5.3 ± 0.5 | 5.3 ± 0.9 | 0.53 |
| proBNP, ng/L | 142 (65.3–254.8) | 119 (56–249.8) | 129 (84.5–217.5) | 110 (52.5–202.5) | 0.41 |
| hsCRP, mg/L | 1.0 (0.4–3.3) | 0.85 (0.43–2.4) | 1.0 (0.6–1.2) | 0.7 (0.6–1.5) | 0.95 |
| EPC, % | 0.7 (0.5–1.7) | 1.3 (1–4.5) | 0.7 (0.4–1.3) | 1.7 (0.8–2.4) | 0.62 |
| Platelet adhesion, % | 3.9 ± 1.9 | 3.9 ± 1.6 | 3.9 ± 1.2 | 4.1 ± 2.3 | 0.83 |
Abbreviations: Cr, creatinine; DBP, diastolic blood pressure; EPC, endothelial progenitor cells; FMD, flow‐mediated vasodilatation; GTN, glycerol trinitrate–mediated vasodilatation; hsCRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; K, potassium; Na, sodium; proBNP, pro‐brain natriuretic peptide; SBP, systolic blood pressure; SD, standard deviation.
Values are presented as mean ± SD or median (IQR).
Difference between intervention and control at 4 weeks.
Figure 1.
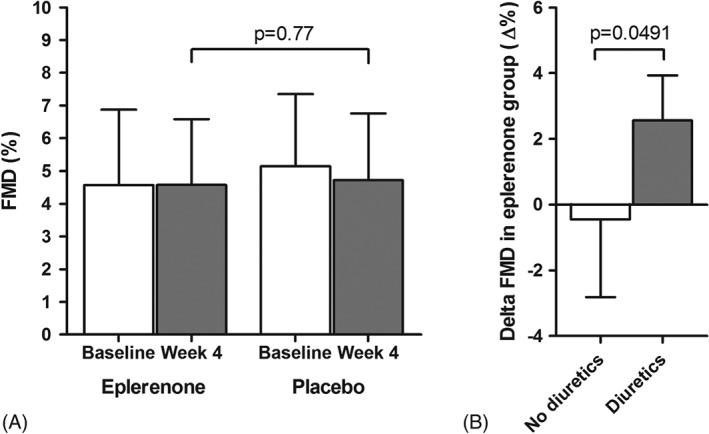
Effect of eplerenone on the primary endpoint FMD after 4 weeks of treatment compared with placebo (A). Subgroup analysis of change in FMD from baseline to 4 weeks in the eplerenone group (Δ FMD), separated by concurrent use of diuretics (B). Abbreviations: FMD, flow‐mediated vasodilatation.
A subgroup analysis showed that in patients with concurrent use of diuretic drugs (n = 3, all thiazide diuretics), eplerenone significantly improved FMD, whereas it showed no significant benefit in patients not taking diuretics (mean FMD increase 2.57% ± 1.37% in patients taking diuretics; mean change −0.45% ± 2.36% in patients not taking diuretics; P = 0.049; Figure 1, part B). Potassium serum levels and BP were not different in patients taking diuretics compared with patients without diuretics, and no differences between diuretic users and nonusers were seen in the placebo group.
Discussion
In this randomized, placebo‐controlled trial, a 4‐week treatment with 25 mg eplerenone daily did not improve endothelial function as assessed by FMD or affect 24‐hour BP, EPC numbers, and platelet adhesion in patients with stable CAD and preserved EF.
There are several potential explanations for our findings. First, renin‐angiotensin‐aldosterone system (RAAS) activation may not be pronounced enough in our specific subset of CAD patients with preserved EF to benefit from MR blockade. Our study excluded patients with significant renal insufficiency, HF, and uncontrolled hypertension, all conditions known to result in increased RAAS activation. Our patients were also well treated in regard to BP, and most were concomitantly treated with an angiotensin‐converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), or a statin, all drugs that can improve endothelial dysfunction.13, 14 With these prerequisites, eplerenone may not have been able to further improve vascular function. Interestingly, in the small subgroup of patients treated with thiazide diuretics, eplerenone significantly improved FMD, whereas no effect was seen in patients without concomitant diuretic treatment. These results suggest that although MR antagonists may not be helpful in all patients with CAD and preserved EF, they may be useful in specific subgroups. In the subgroup of thiazide diuretic users, MR antagonists may counteract the potassium‐wasting and RAAS‐activating effects of thiazide diuretics, resulting in a restoration of body potassium levels and thereby beneficial vascular and metabolic effects.15 This is in line with previous clinical findings indicating that MR blockade is particularly effective when used in combination with diuretics.16 Similar results were seen with potassium‐sparing diuretics, which prevented thiazide diuretic–induced glucose intolerance while improving BP control in a recent trial.17
Second, MR antagonism may be beneficial in CAD, but the effect is not mediated by an improvement in peripheral endothelial dysfunction, but rather by local cardiomyocyte‐specific effects. The activation of MR on cardiomyocytes can trigger coronary endothelial dysfunction by paracrine factors, oxidative stress in particular.18 Indeed, specific MR deletion in cardiomyocytes of mice led to infarct healing and prevented cardiovascular remodeling,19 an effect not seen by deletion of MR receptors on other cells.20 Direct assessment of the coronary vasculature and myocardial microcirculation may yield more positive results, and new compounds, such as more selective MR antagonists, may be helpful in delineating the differential effects of MR antagonists on the heart and the vasculature.
A third explanation for our findings is that MR blockade is not beneficial in CAD patients with preserved EF. Indeed, recent large randomized controlled trials have yielded conflicting results with regard to MR blockade in patients with a preserved EF. In the Impact of Eplerenone on Cardiovascular Outcomes in Patients Post–Myocardial Infarction (REMINDER) trial, myocardial infarction patients without HF symptoms and with a preserved EF received eplerenone or placebo for >1 year.21 Although the trial met its primary composite endpoint, this observation was largely mediated by a reduction in N‐terminal pro‐brain natriuretic peptide levels, a component of the primary endpoint, but not significantly by other more relevant components such as HF hospitalizations and cardiovascular mortality. In the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial, spironolactone was tested in patients with heart failure with preserved ejection fraction (HFpEF).3 Here, spironolactone failed to reduce the composite primary endpoint of cardiovascular death, aborted cardiac arrest, and HF hospitalizations. Both studies stand in contrast to the unequivocally positive results of MR antagonists in HFrEF.1, 2 Interestingly, MR blockers have been shown to also improve endothelial function in HFrEF, paralleling the benefit on hard endpoints.9, 22 The reasons for these diverging results remain elusive. One possibility may be more pronounced RAAS activation in patients with reduced compared with preserved EF, although clear data on this is lacking. Regional or other phenotypic differences may also play a decisive role. In a post‐hoc analysis of the TOPCAT trial, spironolactone significantly reduced the primary endpoint in HFpEF patients from the Americas, whereas it had no effect in HFpEF patients from Eastern Europe.23 In light of these conflicting results, a beneficial effect of MR antagonism in CAD or HF patients with a preserved EF currently cannot be excluded.
Study Limitations
The relatively low dose of eplerenone was chosen to allow for an evaluation of BP‐independent effects of the drug, as lowering of BP itself is known to alter endothelial function. Although similar doses of MR antagonists were used in the large outcome trials, dose and duration of treatment may at least in part account for the observed neutral effects under the conditions of the present study. In patients with untreated hypertension and no background therapy with ACEIs or ARBs, and thus potentially higher RAAS activation than in the present study, 50 mg eplerenone daily did not improve endothelial function after 4 weeks but after 12 and 48 weeks of treatment.24 It is possible that longer treatment duration or a higher dose of MR antagonists is necessary to see beneficial vascular effects in CAD patients with preserved EF. Given the different receptor specificities of currently available MR antagonists and the lack of comparative studies, we also cannot rule out that spironolactone has a different effect on endothelial function compared with eplerenone.25 Finally, aldosterone levels were not assessed in this study due to the concomitant therapy with ACEIs, ARBs, and β‐blockers. Correlation between changes in aldosterone levels as a response marker for MR antagonism and endothelial dysfunction may yield additional valuable information in future studies.
Conclusion
In summary, the MR antagonist eplerenone on top of standard medical therapy failed to improve endothelial function and other surrogate markers of cardiovascular health in patients with CAD and preserved EF. Further studies that focus on specific CAD subgroups such as diuretic users, patients with significant hypertension, or patients with chronic kidney disease may be helpful in further elucidating whether MR blockade is helpful in this patient population.
Isabella Sudano, MD, Matthias Naegele, MD, Frank Ruschitzka, MD, and Andreas J. Flammer, MD, contributed equally.
The study was supported by an unrestricted research grant from Pfizer Inc., New York, New York; by a grant of the Swiss Heart Foundation, Bern, Switzerland; and the Zurich Center of Integrative Human Physiology, Zurich, Switzerland. Trial registration at http://www.ClinicalTrials.gov: NCT00427284.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Pitt B, Zannad F, Remme WJ, et al; Randomized Aldactone Evaluation Study Investigators . The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 2. Pitt B, Remme W, Zannad F, et al; Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction [published correction appears in N Engl J Med. 2003;348:2271]. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 3. Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 4. Güder G, Bauersachs J, Frantz S, et al. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115:1754–1761. [DOI] [PubMed] [Google Scholar]
- 5. Ivanes F, Susen S, Mouquet F, et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33:191–202. [DOI] [PubMed] [Google Scholar]
- 6. Pitt B. Heart failure: the role for mineralocorticoid receptor antagonists. Swiss Med Wkly. 2014;144:w13959. [DOI] [PubMed] [Google Scholar]
- 7. Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone‐induced vasculopathy. Clin Sci (Lond). 2002;103:425–431. [DOI] [PubMed] [Google Scholar]
- 8. Gutiérrez E, Flammer AJ, Lerman LO, et al. Endothelial dysfunction over the course of coronary artery disease. Eur Heart J. 2013;34:3175–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–597. [DOI] [PubMed] [Google Scholar]
- 10. Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gemignani V, Bianchini E, Faita F, et al. Ultrasound measurement of the brachial artery flow‐mediated dilation without ECG gating. Ultrasound Med Biol. 2008;34:385–391. [DOI] [PubMed] [Google Scholar]
- 12. Spieker LE, Flammer AJ, Amacker N, et al. C‐reactive protein influences shear stress‐dependent platelet adhesion in patients with familiar hypercholesterolemia and coronary artery disease undergoing LDL apheresis. Thromb Haemost. 2006;96:540–542. [PubMed] [Google Scholar]
- 13. Ferrari R, Guardigli G, Ceconi C. Secondary prevention of CAD with ACE inhibitors: a struggle between life and death of the endothelium. Cardiovasc Drugs Ther. 2010;24:331–339. [DOI] [PubMed] [Google Scholar]
- 14. Frick M, Alber HF, Hügel H, et al. Short‐ and long‐term changes of flow‐mediated vasodilation in patients under statin therapy. Clin Cardiol. 2002;25:291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He FJ, Marciniak M, Carney C, et al. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension. 2010;55:681–688. [DOI] [PubMed] [Google Scholar]
- 16. Epstein M, Calhoun DA. Aldosterone blockers (mineralocorticoid receptor antagonism) and potassium‐sparing diuretics. J Clin Hypertens (Greenwich). 2011;13:644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown MJ, Williams B, Morant SV, et al; PATHWAY Studies Group . Effect of amiloride, or amiloride plus hydrochlorothiazide, versus hydrochlorothiazide on glucose tolerance and blood pressure (PATHWAY‐3): a parallel‐group, double‐blind randomised phase 4 trial. Lancet Diabetes Endocrinol. 2016;4:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Favre J, Gao J, Zhang AD, et al. Coronary endothelial dysfunction after cardiomyocyte‐specific mineralocorticoid receptor overexpression. Am J Physiol Heart Circ Physiol. 2011;300:H2035–H2043. [DOI] [PubMed] [Google Scholar]
- 19. Fraccarollo D, Berger S, Galuppo P, et al. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation. 2011;123:400–408. [DOI] [PubMed] [Google Scholar]
- 20. Lother A, Berger S, Gilsbach R, et al. Ablation of mineralocorticoid receptors in myocytes but not in fibroblasts preserves cardiac function. Hypertension. 2011;57:746–754. [DOI] [PubMed] [Google Scholar]
- 21. Montalescot G, Pitt B, Lopez de Sa E, et al; REMINDER Investigators . Early eplerenone treatment in patients with acute ST‐elevation myocardial infarction without heart failure: the Randomized Double‐Blind Reminder Study. Eur Heart J. 2014;35:2295–2302. [DOI] [PubMed] [Google Scholar]
- 22. Macdonald JE, Kennedy N, Struthers AD. Effects of spironolactone on endothelial function, vascular angiotensin‐converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart. 2004;90:765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 24. Fujimura N, Noma K, Hata T, et al; ROCK Study Group . Mineralocorticoid receptor blocker eplerenone improves endothelial function and inhibits rho‐associated kinase activity in patients with hypertension. Clin Pharmacol Ther. 2012;91:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Struthers A, Krum H, Williams GH. A comparison of the aldosterone‐blocking agents eplerenone and spironolactone. Clin Cardiol. 2008;31:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]


