ABSTRACT
Background
Sedentary lifestyle has become prevalent in our community. Recent data showed controversy on the effect of regular exercise on left ventricular compliance and myocardial relaxation.
Hypothesis
We sought to assess whether physical inactivity is an independent predictor of diastolic dysfunction in or community, after adjustment for several covariates.
Methods
Consecutive outpatients presenting to the echocardiography laboratory between July 2013 and June 2014 were prospectively enrolled. Clinical variables were collected prospectively at enrollment. Patients were considered physically active if they exercised regularly ≥3× a week, ≥30 minutes each time. The primary endpoint was presence of diastolic dysfunction.
Results
The final cohort included 1356 patients (mean age [SD] 52.9 [17.4] years, 51.3% female). Compared with physically active patients, the 1009 (74.4%) physically inactive patients were older, more often female, and had more comorbidities and worse diastolic function (51.3% vs 38.3%; P < 0.001). On univariate analysis, physical inactivity was associated with 70% increased odds of having diastolic dysfunction (odds ratio: 1.70, 95% confidence interval: 1.32‐2.18, P < 0.001). There was significant interaction between physical activity and left ventricular mass index (LVMI; P = 0.026). On multivariate analysis, patients who were physically inactive and had LVMI ≥ median had significantly higher odds of having diastolic dysfunction (odds ratio: 2.82, 95% confidence interval: 1.58‐5.05, P < 0.001).
Conclusions
In a large, prospectively enrolled cohort from a single tertiary center in the Middle East, physically inactive patients with increased LVMI had 2‐ to 3‐fold increased odds of having diastolic dysfunction after multivariate adjustment.
Introduction
Diastolic dysfunction is associated with significant morbidity, hospitalizations for heart failure (HF) symptoms, and all‐cause mortality, even among patients with normal left ventricular (LV) systolic function.1, 2, 3, 4, 5 There are several well‐known independent predictors of diastolic dysfunction, such as age, obesity, hypertension (HTN), and diabetes mellitus (DM), among others.2, 6 Oxidative stress worsens diastolic function, particularly in hypertensive patients, whereas regular exercise lowers its levels.7 Indeed, recent data showed that regular exercise improved LV compliance and myocardial tissue relaxation, reversed diastolic dysfunction, and slowed aging of the heart.8, 9 Other data however, showed limited effect on age‐related remodeling, diastolic function, and performance.10
Although the American Heart Association (AHA) endorses regular exercise ≥3× a week for 30 to 40 minutes each session, a sedentary lifestyle has become an epidemic in our community, with high economic burden.11 It is associated with physical deconditioning and obesity and often leads to insulin resistance, DM, and HTN. Physically inactive patients with normal LV systolic function often have poor functional capacity similar to those with diastolic dysfunction. Hence, we sought to assess whether physical inactivity is an independent predictor of diastolic dysfunction in our community and remains predictive after adjustment for several known covariates.
Methods
Patient Selection
Consecutive outpatients presenting to the echocardiography laboratory at the American University of Beirut Medical Center between July 2013 and June 2014 were prospectively enrolled into the database after obtaining informed consent. The study was approved by the institutional review board and complied with the Declaration of Helsinki. Patients with left ventricular ejection fraction (LVEF) <50%, severe valvular disease, mitral valve surgery, atrial fibrillation (AF) at the time of image acquisition, or congenital heart disease were excluded. Patients with history of AF who were in normal sinus rhythm at the time of the echocardiogram were included.
Clinical Variables
Clinical variables were collected prospectively at the time of enrollment; these included demographics (age, sex, country of origin, body mass index [BMI]), comorbidities (blood pressure, history of HTN, DM, hyperlipidemia, coronary artery disease [CAD], New York Heart Association [NYHA] class, chronic kidney disease [CKD], smoking, salt intake, physical activity, active cancer), and cardiac medications (aspirin, angiotensin‐converting enzyme inhibitor [ACEI], angiotensin receptor blocker [ARB], statins, and anticoagulants). Salt intake was estimated using an online calculator (https://www.projectbiglife.ca/sodium) and stratified as <2500 vs >2500 mg per day. Patients were considered physically active if they exercised regularly ≥3× a week, ≥30 minutes each time.
Echocardiography
Patients were imaged in the left lateral decubitus position with commercially available systems (Philips Electronics, Andover, MA; GE Medical Systems, Milwaukee, WI). Left ventricular mass was calculated using the American Society of Echocardiography (ASE) quantitative guidelines and was indexed to body surface area. Left ventricular ejection fraction was assessed by semiquantitative manner using the biplane Simpson method, and left atrial volume index was assessed in accordance with published guidelines.12 Right ventricular systolic function was assessed using the systolic longitudinal function with tissue Doppler imaging (S′); abnormal function was defined as S′ <10 cm/s. Diastolic function was assessed in a standardized method and in accordance with the most recently published guidelines by the ASE using a combination of echocardiographic variables (transmitral inflow pattern, mitral annular velocities with tissue Doppler imaging, left atrial volume index, and pulmonary venous flow pattern).13 Two level‐III trained and echocardiography board‐certified cardiologists (W.A., A.A.) reviewed all the cases and graded the diastolic function in a blinded manner; in case of a discrepancy, the images were reviewed by a third cardiologist. Diastolic function was labeled as normal or abnormal (diastolic dysfunction). Diastolic dysfunction was then categorized as mild (grade 1, impaired relaxation), moderate (grade 2, pseudonormal), or severe (grade 3, restrictive).13
Statistical Analysis
The primary endpoint was abnormal diastolic function (grade I–III). Continuous variables were expressed as mean (SD) and compared by use of the unpaired Student t test or Wilcoxon rank‐sum test, as appropriate. Categorical variables were expressed as frequency (percentages) and compared by use of the Fisher exact test or Pearson χ2 test, as appropriate. Diastolic function grade was also compared using the Mann–Whitney U test to better reflect burden of disease.
Multivariate regression analysis model was performed to determine the independent predictors of abnormal diastolic function. Significant univariates (P < 0.1) or clinically relevant ones were entered into the model. Variables with collinearity were entered into the model one at a time. To control for potential interaction between physical activty and LV mass index (LVMI), a composite variable of 4 categories was developed as follows: (1) physically active and LVMI < median (reference); (2) physically active and LVMI ≥ median; (3) physically inactive and LVMI < median; and (4) physically inactive and LVMI ≥ median. The final model adjusted for age, sex, BMI, CAD, DM, systolic blood pressure (SBP), active cancer, smoking, NYHA class, CKD, abnormal right ventricular systolic function, high‐salt diet, ACEI/ARB, β‐blocker and anticoagulant use, LV size, and the composite variable of LVMI and physical activity. We chose SBP rather than history of HTN in the model because the former carries more statistically significant weight as a continuous variable as opposed to a dichotomous variable, and because an SBP reading seemed clinically more relevant, particularly when some patients might be unaware of existing HTN or have poorly controlled blood pressure despite treatment. The LVEF was not entered into the model because all patients had a normal LVEF, ≥50%. Finally, left atrial volume index and other diastolic parameters were not entered into the models because of confounding effect with diastolic function grading. For better illustration, a predicted probability plot was performed using the β coefficients from the multivariate regression analysis to represent the probability of diastolic dysfunction as a function of age and stratified by physical‐activity status.
In exploratory, hypothesis‐generating analyses, we studied the association between physical activity and abnormal diastolic function in predefined subgroups of sex, age, BMI, several comorbidities (CAD, CKD, HTN, smoking, active cancer, NYHA class I–II vs III–IV), salt diet (low vs high), key cardiac medications (ACEI/ARB, β‐blockers, and anticoagulants), in addition to LV size and mass. An adjusted odds ratio (OR) was obtained with 95% confidence interval (CI) along with interaction P values, and a corresponding forest plot was made.
All statistical tests were 2‐sided. A P value <0.05 was set a priori and considered statsitically significant. All statsitical analyses were performed with SPSS software, version 22 (IBM Corp., Armonk, NY).
Results
The cohort consisted of 1356 patients, mean age (SD) 52.9 (17.4) years, 51.3% female, and 16.6% with DM (Table 1). The majority of patients were physically inactive (n = 1009, 74.4%), were older, more often female, and had more comorbidities.
Table 1.
Baseline Characteristics Stratified by Physical Activity
| All Patients, N = 1356 | Physically Active, N = 347 | Physically Inactive, N = 1009 | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Mean age, y (SD) | 52.9 (17.4) | 49.7 (19.0) | 54.1 (16.7) | <0.001 |
| Female sex | 696 (51.3) | 149 (42.9) | 547 (54.2) | <0.001 |
| BMI, kg/m2 (SD) | 28.3 (5.6) | 26.7(4.5) | 28.9 (5.9) | <0.001 |
| Comorbidities | ||||
| CAD | 278 (20.5) | 63 (18.2) | 215 (21.3) | 0.21 |
| DM | 225 (16.6) | 28 (8.1) | 197 (19.5) | <0.001 |
| HTN | 577 (42.6) | 126 (36.3) | 451 (44.7) | 0.006 |
| SBP, mm Hg (SD) | 133.0 (17.4) | 133.1 (17.9) | 132.9 (17.3) | 0.86 |
| Hyperlipidemia | 411 (30.3) | 99 (28.5) | 312 (30.9) | 0.40 |
| Smoking history | 635 (46.8) | 156 (45.0) | 479 (47.5) | 0.42 |
| Active cancer | 245 (18.1) | 42 (12.1) | 203 (20.1) | 0.001 |
| NYHA class ≥ II | 781 (57.6) | 117 (33.7) | 664 (65.8) | <0.001 |
| High salt intake | 754 (55.6) | 164 (47.3) | 590 (58.5) | <0.001 |
| CKD | 97 (7.2) | 26 (7.5) | 71 (7.0) | 0.78 |
| Medications | ||||
| ASA | 282 (20.8) | 64 (18.4) | 218 (21.6) | 0.21 |
| ACEI/ARB | 418 (30.8) | 99 (28.5) | 319 (31.6) | 0.28 |
| β‐Blocker | 461 (34) | 110 (31.7) | 351 (34.8) | 0.30 |
| Statin | 410 (30.2) | 109 (31.4) | 301 (29.8) | 0.58 |
| Anticoagulant | 42 (3.1) | 8 (2.3) | 34 (3.4) | 0.32 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, aspirin; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation.
Data are presented as n (%) unless otherwise noted.
Almost half of the cohort had abnormal diastolic function. The LV parameters including dimension, systolic function, diastolic parameters, and grading are summarized in Table 2 and stratified by the physical‐activity status. Physically inactive patients had more diastolic dysfunction as compared with physically active patients (51.3% vs 38.3%; P < 0.001).
Table 2.
Diastolic Parameters Stratified by Physical Activity
| All Patients, N = 1356 | Physically Active, N = 347 | Physically Inactive, N = 1009 | P Value | |
|---|---|---|---|---|
| LVEDD indexed, mm/m (SD) | 27.7 (2.8) | 27.5 (2.6) | 27.7 (2.9) | 0.13 |
| LVEDD ≥27 mm/m (median) | 715 (52.7) | 182 (52.4) | 533 (52.8) | 0.90 |
| LVMI, g/m2 (SD) | 47.5 (12.8) | 47.7 (12.0) | 47.5 (13.0) | 0.84 |
| LVMI ≥47 g/m2 (median) | 679 (50.1) | 181 (52.2) | 498 (49.4) | 0.37 |
| LVEF, % | 60.5 (4.1) | 60.5 (3.7) | 60.4 (4.2) | 0.76 |
| RV S′, cm/s | 13.8 (2.6) | 13.7 (2.5) | 13.8 (2.7) | 0.57 |
| RV S′ <10 cm/s | 24 (1.8) | 3 (0.9) | 21 (2.1) | 0.14 |
| LA volume index, mL/m2 (SD) | 24.6 (7.3) | 23.9 (6.1) | 24.8 (7.7) | 0.047 |
| E, cm/s (SD) | 76.5 (18.9) | 77.9 (18.7) | 76.0 (18.9) | 9.12 |
| A, cm/s (SD) | 74.0 (22.2) | 69.7 (20.5) | 75.9 (22.4) | <0.001 |
| E/A ratio (SD) | 1.3 (0.6) | 1.2 (0.4) | 1.3 (0.7) | 0.81 |
| DT, ms (SD) | 213 (47) | 208 (44) | 215 (48) | 0.036 |
| e′, cm/s (SD) | 10.6 (3.7) | 11.5 (4.0) | 10.3 (3.6) | <0.001 |
| E/e′ | 7.96 (3.37) | 7.46 (2.89) | 8.13 (3.50) | 0.001 |
| Diastolic function | <0.001a | |||
| Normal | 703 (52) | 214 (61.7) | 489 (48.7) | |
| Grade I | 531 (39.3) | 108 (31.1) | 423 (42.1) | |
| Grade II | 105 (7.8) | 24 (6.9) | 81 (8.1) | |
| Grade III–IV | 13 (1.0) | 1 (0.3) | 12 (1.2) |
Abbreviations: A, late diastolic filling velocity; E, early diastolic filling velocity; e′, early diastolic mitral annular velocity; DT, deceleration time; LA, left atrial; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; RV, right ventricular; S′, systolic velocity; SD, standard deviation.
Data are presented as n (%) unless otherwise noted.
Using χ2 and Mann–Whitney U test.
On univariate analysis, physical inactivity was associated with 70% increased odds of having diastolic dysfunction (OR: 1.70, 95% CI: 1.32‐2.18, P < 0.001). Because of significant interaction between physical activity and LVMI (P = 0.026), a composite of the 2 variables was formed with 4 categories (physically active [yes/no], LVMI ≥ median [yes/no]), as detailed in the Methods section. On multivariate analysis and after adjusting for baseline demographics, comorbidities, and medications, patients who were physically inactive and with LVMI ≥ median had significantly higher odds of having diastolic dysfunction (OR: 2.82, 95% CI: 1.58‐5.05, P < 0.001), whereas those with normal LVMI did not. Also, advanced age, increased BMI, DM, elevated SBP, anticoagulation use, and small LV end‐diastolic diameter were associated with increased odds of having diastolic dysfunction (Table 3). The predicted probability of diastolic dysfunction was significantly higher among physically inactive patients across all age groups (Figure 1).
Table 3.
Independent Predictors of Abnormal Diastolic Function
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Physical activity/LVMI category | ||||
| Physically active, LVMI < median | Ref | Ref | ||
| Physically active, LVMI ≥ median | 1.97 (1.26‐3.06) | 0.03 | 1.28 (0.66‐2.49) | 0.468 |
| Physically inactive, LVMI < median | 1.22 (0.83‐1.78) | 0.31 | 0.96 (0.55‐1.66) | 0.879 |
| Physically inactive, LVMI ≥ median | 5.10 (3.48‐7.48) | <0.001 | 2.82 (1.58‐5.05) | <0.001 |
| Age, y | 1.14 (1.13‐1.16) | <0.001 | 1.13 (1.11‐1.14) | <0.001 |
| Female sex | 1.02 (0.82‐1.26) | 0.88 | 0.99 (0.70‐1.40) | 0.95 |
| BMI | 1.07 (1.05‐1.10) | <0.001 | 1.04 (1.004‐1.07) | 0.029 |
| CAD | 4.14 (3.08‐5.56) | <0.001 | 0.83 (0.54‐1.27) | 0.38 |
| DM | 6.79 (4.71‐9.78) | <0.001 | 1.85 (1.14‐2.98) | 0.012 |
| SBP | 1.03 (1.027‐1.04) | <0.001 | 1.011 (1.001‐1.02) | 0.033 |
| Cancer | 0.76 (0.58‐1.01) | 0.055 | 1.11 (0.74‐1.65) | 0.62 |
| Smoking | 1.49 (1.21‐1.85) | <0.001 | 1.31 (0.95‐1.81) | 0.10 |
| NYHA class II–IV | 2.23 (1.79‐2.78) | <0.001 | 1.26 (0.90‐1.75) | 0.18 |
| CKD | 7.22 (4.05‐12.85) | <0.001 | 1.47 (0.65‐3.29) | 0.36 |
| RV S′ <10 cm/s | 2.13 (1.22‐7.98) | 0.017 | 1.03 (0.97‐1.10) | 0.27 |
| High salt intake | 0.90 (0.73‐1.12) | 0.35 | 1.33 (0.97‐1.84) | 0.080 |
| Medication use | ||||
| ACEI/ARB | 3.82 (2.98‐4.89) | <0.001 | 1.15 (0.79‐1.67) | 0.46 |
| β‐Blocker | 3.80 (2.99‐4.83) | <0.001 | 1.08 (0.75‐1.55) | 0.67 |
| Anticoagulation | 4.80 (2.21‐10.4) | <0.001 | 3.55 (1.26‐10.0) | 0.017 |
| LVEDD/height | 1.02 (0.98‐1.06) | 0.30 | 0.91 (0.85‐.097) | 0.03 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; LVEDD, left ventricular end‐diastolic diameter; LVMI, left ventricular mass index; NYHA, New York Heart Association; OR, odds ratio; Ref, reference; RV, right ventricular; S′, systolic velocity; SBP, systolic blood pressure.
Figure 1.
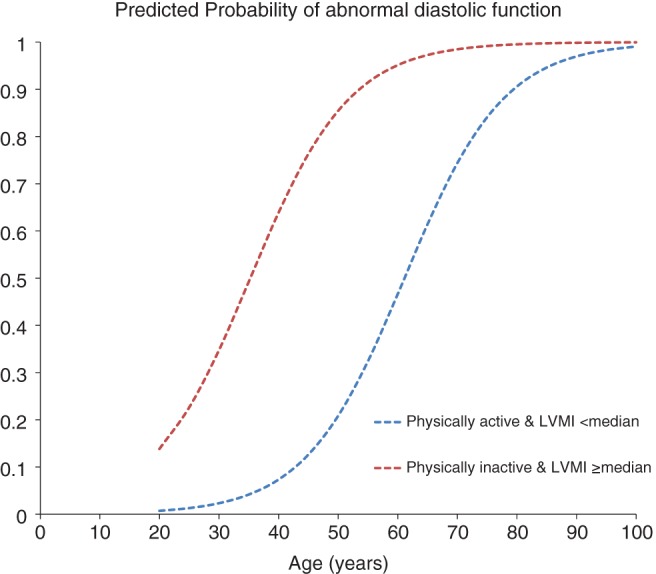
Predicted probability of abnormal diastolic function. Probability plot of having abnormal diastolic function stratified by age and by physical‐activity status. Plot was performed using the data from the multivariate regression analysis (Table 3). The mean value for continuous variables and median value for categorical variables were used (female sex = 1, BMI = 28.3 kg/m2, CAD = 0, DM = 0, SBP = 133 mm Hg, active cancer = 0, smoking = 0, NYHA class II–IV = 1, CKD = 0, RV S′ <10 cm/s = 0, high salt intake = 0, use of ACEI/ARB = 0, use of β‐blockers = 0, use of anticoagulants = 0, LVEDD = 27.7 mm/m). Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; DM, diabetes mellitus; LVEDD, left ventricular end‐diastolic diameter; LVMI, left ventricular mass index; NYHA, New York Heart Association; RV, right ventricular; SBP, systolic blood pressure.
The study was not sufficiently powered for subgroup analyses. However, we performed exploratory analyses examining the effect of physical‐activity status (active vs inactive) in a priori defined subgroups and tested for interactions between these subgroup strata and physical‐activity status as a determinant of diastolic dysfunction. Physical inactivity was associated with diastolic dysfunction irrespective of age or blood pressure. There was a trend of worse diastolic function when stratified by sex, BMI, active cancer status, NYHA class, and salt intake (Figure 2).
Figure 2.

Association between sedentary lifestyle and abnormal diastolic function: subgroup analysis of the entire cohort with adjusted HRs. Adjustment was done for all the variables listed in Table 3. Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; HR, hazard ratio.
Discussion
Physical inactivity is becoming an epidemic. In our community (and as shown in Table 1), almost three‐quarters of outpatients with normal LV systolic function acknowledged having a sedentary lifestyle. The latter leads to physical deconditioning and is often associated with obesity, metabolic syndrome, DM, and other cardiovascular risk factors (Table 1) that are common pathophysiological links to diastolic dysfunction. Although one cannot prove a cause‐effect relationship, recent data showed that regular exercise improves LV compliance and myocardial tissue relaxation, reverses diastolic dysfunction, and slows aging of the heart.8, 9 Indeed, physical activity reduces oxidative stress, which has been proposed as an additional mechanism to impaired myocardial relaxation.7 In another study, patients attaining the greatest increase in fitness and reduction in abdominal fat had a modest trend toward improved LV diastolic function.14 Similarly, weight loss improves not only physical fitness, but also diastolic function.15 However, the relationship between physical activity and diastolic function remains controversial, with studies showing limited effect of physical activity on age‐related remodeling, diastolic function, and performance.10, 16
In the current study, we sought to assess whether a relationship indeed exists between sedentary lifestyle and diastolic dysfunction, and whether it persists after adjusting for several covariates and potential confounders. On univariate analysis, physical inactivity was associated with 70% increased odds of having diastolic dysfunction (OR: 1.70, 95% CI: 1.32‐2.18, P < 0.001). There was an interaction between physical activity and LVMI (Figure 2); on multivariate adjustment, the association between physical inactivity and diastolic dysfunction was more pronounced among those with increased LVMI (OR: 2.82, 95% CI: 1.58‐5.05, P < 0.001), but not those with normal LVMI (Table 3). Although the explanation for this finding is not clear, one can speculate that increased LVMI may precede a change in LV compliance, leading to increased stiffness and diastolic impairment.
Another borderline interaction was found with DM (Figure 2). Physically inactive patients without DM were more likely to be have diastolic dysfunction. However, this should be cautiously interpreted given the borderline interaction P value and the inadequately powered small sample size in this subgroup analysis.
Aging is known to be the most powerful predictor of diastolic dysfunction, with a 3‐fold increase in odds for every 10 years of aging.3 Still, the predicted probability of diastolic dysfunction remained significantly higher among physically inactive patients across the full age spectrum of the study population (Figure 1).
The association between anticoagulation and diastolic dysfunction is not fully clear. Although we excluded patients with AF at the time of imaging, we did not exclude those with history of AF. Hence, one can assume that many patients were on anticoagulation because of prior history of AF, which is well known to be associated with left atrial remodeling and impaired diastolic function. Furthermore, the number of patients on anticoagulation was relatively small (42/1356), therefore introducing potential error and bias. Finally, patients on anticoagulation are often elderly with comorbidities, which could be a confounding variable with diastolic dysfunction.
Physically inactive subjects often have suboptimal functional capacity and can get symptomatic on average activity level. Indeed, the NYHA class was worse among those with sedentary lifestyle despite all having normal systolic function (66% vs 34% having NYHA class ≥ II, P < 0.001; Table 2). Diastolic dysfunction is a leading cause of HF symptoms among patients with preserved systolic function5 and is often unmasked during exercise. Although we assessed diastolic function at rest only, hence perhaps missing subclinical diastolic impairment, this serves our results and strengthens the current findings. More importantly, one needs to recognize that not only is sedentary lifestyle reversible, but also diastolic dysfunction, which in fact was shown to be dynamic.1, 5 In a recent prospective study, successful lifestyle modification with exercise in obese, prediabetic patients with HF and preserved ejection fraction resulted in improvement of diastolic LV function and functional capacity.17 Hence, though there is hope after all for turning things around, an aggressive implementation of healthy lifestyle with exercise and physical fitness in our community is much needed without further delay.
Study Limitations
This is one of the first studies that looked at the association between physical activity and diastolic dysfunction in a tertiary referral center from the Middle East. The sample size was relatively large and the data were collected prospectively. Furthermore, diastolic function was graded uniformly, read blindly, and verified independently by 2 level‐III echocardiographers. However, there are several limitations to the study. This is a single‐center study with referral and selection bias. The cohort consisted of outpatients with normal LV systolic function and with a high prevalence of those with active cancer presenting for pre‐chemotherapy cardiac assessment. In addition, physical activity was assessed using a simple questionnaire and dichotomized as active vs inactive. Because of the small number of patients that were active (<25% of the entire cohort), further stratification by the intensity and frequency of exercise pattern was not feasible. Furthermore, the high prevalence of physical inactivity could be related to the fact that a large percentage of patients had active cancer and were undergoing chemotherapy and likely to be anemic and frail; although we adjusted for active cancer, several confounders were likely missed in the process. Furthermore, diastolic function was assessed at 1 time point only; however, we have previously shown that diastolic function is not static, but rather dynamic, and varies with time in both directions.1 Finally, although more than half of patients had diastolic dysfunction, the majority of them had mild relaxation abnormality (grade I) and few had more advanced stages of disease.
Conclusion
In a large prospectively enrolled cohort from a single tertiary center in the Middle East, physical inactivity was associated with increased odds of diastolic dysfunction among patients with increased LVMI, even after multivariate adjustment. The magnitude of the problem is large given the very high prevalence of sedentary lifestyle in our community and the significant comorbidity associated with diastolic dysfunction.
Antoine Abchee, MD, and Wael AlJaroudi, MD, contributed equally to this work.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Aljaroudi W, Alraies MC, Halley C, et al. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125:782–788. [DOI] [PubMed] [Google Scholar]
- 2. AlJaroudi WA, Thomas JD, Rodriguez LL, et al. Prognostic value of diastolic dysfunction: state‐of‐the‐art review. Cardiol Rev. 2014;22:79–90. [DOI] [PubMed] [Google Scholar]
- 3. AlJaroudi WA, Alraies MC, Halley C, et al. Incremental prognostic value of diastolic dysfunction in low risk patients undergoing echocardiography: beyond framingham score. Int J Cardiovasc Imaging. 2013;29:1441–1450. [DOI] [PubMed] [Google Scholar]
- 4. Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082–1087. [DOI] [PubMed] [Google Scholar]
- 5. Kane GC, Karon BL, Mahoney DW, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aljaroudi W, Halley C, Houghtaling P, et al. Impact of body mass index on diastolic function in patients with normal left ventricular ejection fraction. Nutr Diabetes. 2012;2:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dekleva M, Celic V, Kostic N, et al. Left ventricular diastolic dysfunction is related to oxidative stress and exercise capacity in hypertensive patients with preserved systolic function. Cardiology. 2007;108:62–70. [DOI] [PubMed] [Google Scholar]
- 8. Hollekim‐Strand SM, Bjørgaas MR, Albrektsen G, et al. High‐intensity interval exercise effectively improves cardiac function in patients with type 2 diabetes mellitus and diastolic dysfunction: a randomized controlled trial. J Am Coll Cardiol. 2014;64:1758–1760. [DOI] [PubMed] [Google Scholar]
- 9. Levy WC, Cerqueira MD, Abrass IB, et al. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation. 1993;88:116–126. [DOI] [PubMed] [Google Scholar]
- 10. Jakovljevic DG, Papakonstantinou L, Blamire AM, et al. Effect of physical activity on age‐related changes in cardiac function and performance in women. Circ Cardiovasc Imaging. 2015;8:e002086. [DOI] [PubMed] [Google Scholar]
- 11. Oldridge NB. Economic burden of physical inactivity: healthcare costs associated with cardiovascular disease. Eur J Cardiovasc Prev Rehabil. 2008;15:130–139. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 13. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 14. Stewart KJ, Ouyang P, Bacher AC, et al. Exercise effects on cardiac size and left ventricular diastolic function: relationships to changes in fitness, fatness, blood pressure and insulin resistance. Heart. 2006;92:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rider OJ, Francis JM, Tyler D, et al. Effects of weight loss on myocardial energetics and diastolic function in obesity. Int J Cardiovasc Imaging. 2013;29:1043–1050. [DOI] [PubMed] [Google Scholar]
- 16. Korzeniowska‐Kubacka I, Bilińska M, Michalak E, et al. Influence of exercise training on left ventricular diastolic function and its relationship to exercise capacity in patients after myocardial infarction. Cardiol J. 2010;17:136–142. [PubMed] [Google Scholar]
- 17. Ritzel A, Otto F, Bell M, et al. Impact of lifestyle modification on left ventricular function and cardiopulmonary exercise capacity in patients with heart failure with normal ejection fraction and cardiometabolic syndrome: a prospective interventional study. Acta Cardiol. 2015;70:43–50. [DOI] [PubMed] [Google Scholar]


