ABSTRACT
Background
Previous research demonstrates greater survival among coronary artery disease (CAD) patients who engage in cardiac rehabilitation. No national prospective studies, however, have examined the effects of objectively measured free‐living physical activity on mortality among CAD patients, which is important because only 25% of eligible cardiac patients participate in cardiac rehabilitation. Therefore, the purpose of this study was to examine the association between objectively measured free‐living physical activity on all‐cause mortality among a national sample of CAD patients.
Hypothesis
We hypothesize that free‐living physical activity will be inversely associated with all‐cause mortality risk among CAD patients.
Methods
Data from the 2003 to 2006 National Health and Nutrition Examination Survey were used, with follow‐up through 2011. Physical activity was assessed over 7 days during waking hours using the ActiGraph 7164 accelerometer.
Results
Among the 256 CAD adults (representative of 6.5 million CAD patients in the United States), 68 died over the follow‐up period (26.56%). The median follow‐up period was 76.5 months (interquartile range = 62–91 months). After adjustment, for every 60‐minute increase in daily free‐living physical activity, CAD patients had a 16% reduced risk of all‐cause mortality (hazard ratio: 0.84, 95% confidence interval: 0.72‐0.97).
Conclusions
Free‐living objectively measured physical activity is associated with greater survival among CAD patients in the United States. If confirmed by future research, development of strategies to not only increase participation in supervised cardiac rehabilitation, but also increase participation in free‐living physical activity, are needed.
Introduction
Cardiac rehabilitation, inclusive of progressive increase in physical activity, is associated with a 20% to 30% reduction in mortality risk among those with coronary artery disease (CAD).1, 2, 3, 4 Despite this, only about 25% of eligible cardiac patients participate in cardiac rehabilitation.5 Thus, investigation of the effects of free‐living physical activity on mortality among those diagnosed with CAD is warranted. No epidemiological prospective cohort studies have examined the effects of free‐living, accelerometer‐assessed physical activity on mortality among CAD patients, which was the purpose of this brief study.
Methods
Design and Participants
Data were extracted from the 2003 to 2006 National Health and Nutrition Examination Survey (NHANES) (only available cycles with accelerometry data at the time of this writing). Data from participants in these cycles were linked to death certificate data from the National Death Index. Person‐months of follow‐up were calculated from the date of the interview until date of death or censoring on December 31, 2011, whichever came first.
In the 2003 to 2006 NHANES cycles, 10 020 adults 20+ years of age were enrolled. Among these 10 020 adults, 464 had a physician diagnosis of CAD. After excluding those with missing covariate data, 380 had a physician diagnosis of CAD. Lastly, after excluding those with missing or insufficient accelerometry data (<4 days of 10+ h/d of monitoring), 256 had a physician diagnosis of CAD. Analyses are based on data from these 256 CAD patients (defined below) (age range, 22–85 years) who provided complete data for the study variables; based on the sampling design and use of sampling weights, these 256 CAD patients represent 6.5 million CAD patients in the United States.
The NHANES is an ongoing survey conducted by the Centers for Disease Control and Prevention that uses a representative sample of noninstitutionalized United States civilians selected by a complex, multistage, stratified, clustered probability design. The multistage design consists of 4 stages, including the identification of counties, segments (city blocks), random selection of households within the segments, and random selection of individuals within the households. Procedures were approved by the National Center for Health Statistics review board. Consent was obtained from all participants prior to data collection. Further information on NHANES methodology and data collection is available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm).
Measurement of CAD
Participants who answered “yes” to the following question were considered to have CAD: “Has a doctor or other health professional ever told you that you had coronary artery disease?” Although less accurate than hospital records and other forms of objective measurement, self‐report of physician diagnosis of morbidity has demonstrated evidence of validity. For example, as shown by Bergmann et al,6 and when compared to hospitalized confirmed diagnosis, sensitivity (true positivity) of self‐reported heart disease was 84% among American adults.
Measurement of Free‐Living Objectively Measured Physical Activity
Free‐living physical activity was assessed during all waking hours using the ActiGraph 7164 accelerometer (ActiGraph, Pensacola, FL). SAS (version 9.2; SAS Institute Inc., Cary, NC) was used to reduce accelerometry data to those with ≥4 days of ≥10 h/d of monitored data and integrate it into 1‐minute time intervals. Nonwear time was identified as ≥60 consecutive minutes of 0 activity counts, with allowance for 1 to 2 minutes of activity counts between 0 and 100. Activity counts/minute ≥100 were used as the threshold to determine time spent at physical activity across the valid days (ie, days with at least 10+ hours of monitoring).7 The average physical activity level across these valid days was calculated for each participant. This activity count/minute cut‐point of 100 allows for the quantification of time spent engaging in any ambulatory‐based movements (ie, nonsedentary behaviors).7 Intensity‐specific effects (eg, effects of vigorous intensity) was not possible, as these CAD patients, for example, engaged in little vigorous‐intensity (cut‐point of 5999+ counts/minute) physical activity (mean, 0.31 min/d; standard error, 0.1). The ActiGraph accelerometer has demonstrated evidence of reliability and validity.8, 9 For example, in a calibration study comparing ActiGraph‐derived activity counts with oxygen consumption, there was a significant positive association between activity counts and oxygen consumption (r > 0.8; P < 0.01).8 ActiGraph interinstrument reliability among adults in free‐living conditions is also very high (intraclass correlation coefficient = 0.98).9
Covariates
Covariates included age (continuous, years), gender, race–ethnicity (Mexican American, non‐Hispanic white, non‐Hispanic black, and other), serum cotinine (marker of active/passive smoking status, continuous, ng/mL), measured body mass index (kg/m2); poverty‐to‐income ratio (continuous); C‐reactive protein (CRP, continuous; mg/dL), cholesterol medication use (yes/no), blood pressure medication use (yes/no), and comorbid illness (ordinal variable). As a measure of socioeconomic status, the poverty‐to‐income ratio was assessed. The poverty‐to‐income ratio is calculated by dividing the family income by the poverty guidelines, which is specific to the family size, year assessed, and state of residence. High‐sensitivity CRP was used as a marker of systemic inflammation. Blood samples were obtained to assess high‐sensitivity CRP, using latex‐enhanced nephelometry, with samples taken prior to physical activity assessment. The comorbid illness variable indicated the summed number of morbidities for each participant, based on physician diagnosis of arthritis, chronic obstructive pulmonary disease, congestive heart failure, heart attack, stroke, overweight/obese, and hypertension.
Analysis
Statistical analyses were performed via procedures from survey data using Stata (version 12, StataCorp, College Station, TX). To account for oversampling, nonresponse, noncoverage, and to provide nationally representative estimates, all analyses included the use of survey sample weights, clustering, and primary sampling units. Cox proportional hazard models were used to examine the association between physical activity and all‐cause mortality. Schoenfeld residuals were used to verify the proportional hazards assumption. Kaplan‐Meier survival analysis was used to generate the Kaplan‐Meier survival curve across 2 physical activity groups: those engaging in ≥300 minutes/day of total physical activity (light to vigorous) (N = 100) and those <300 minutes/week of total physical activity (N = 156). This threshold was chosen because our previous work demonstrates that this threshold is favorably associated with various cardiovascular disease risk factors.7
Results
Table 1 displays baseline characteristics of CAD adults compared to NHANES participants without CAD. In the 2003 to 2006 NHANES, and after excluding those with missing data on the study variables, 5575 remained, and among these 256 had CAD. When comparing the 256 CAD patients to the 5319 non‐CAD patients, as expected, the CAD patients were less active, older, more likely to be male and non‐Hispanic white, had a lower socioeconomic status (SES) (lower poverty‐to‐income ratio score), had more comorbidities, and were more likely to be on cholesterol and hypertensive medications (Table 1).
Table 1.
Unweighted Baseline Characteristics Comparing CAD Patients to Those Without CAD, 2003–2006 NHANES
| Variable | Baseline Point Estimates (95% CI) | P Value a | |
|---|---|---|---|
| No CAD, n = 5319 | CAD, n = 256 | ||
| Total physical activity, min/d | 367.5 (364.5‐370.5) | 287.2 (274.4‐299.9) | <0.001 |
| Age, mean, y | 50.4 (49.9‐50.9) | 69.1 (67.7‐70.4) | <0.001 |
| Female, % | 51.2 (49.9‐52.5) | 29.6 (24.0‐35.2) | <0.001 |
| Non‐Hispanic white, % | 53.1 (51.7‐54.3) | 69.9 (64.2‐75.5) | <0.001 |
| Poverty‐to‐income ratio | 2.77 (2.72‐2.81) | 2.54 (2.36‐2.72) | 0.03 |
| Cotinine, mean ng/mL | 51.6 (48.3‐54.9) | 37.1 (25.4‐48.8) | 0.06 |
| CRP, mean mg/dL | 0.44 (0.42‐0.46) | 0.43 (0.36‐0.50) | 0.78 |
| Comorbidities, mean | 1.43 (1.40‐1.46) | 3.1 (2.88‐3.21) | <0.001 |
| Cholesterol medication, % | 13.7 (12.7‐14.6) | 53.1 (46.9‐59.2) | <0.001 |
| Hypertensive medication, % | 24.2 (23.1‐25.4) | 61.7 (55.7‐67.6) | <0.001 |
Abbreviations: CAD, coronary artery disease; CI, confidence interval; CRP, C‐reactive protein; NHANES, National Health and Nutrition Examination Survey.
An adjusted Wald test was used to examine statistical differences for continuous variables (eg, age); a design‐based likelihood ratio test was used for categorical variables (eg, gender).
Among the 256 CAD adults, 68 (n = 50 for men, n = 18 for women) died over the follow‐up period (26.56%, unweighted). The unweighted median follow‐up period was 76.5 months (interquartile range = 62–91), and the longest follow‐up period was 107 months (8.9 years). In the sample, 18 776 person‐months occurred, with a mortality incidence rate of 3.62 deaths per 1000 person‐months.
Table 2 displays the baseline characteristics of CAD adults stratified by mortality status at follow‐up. CAD patients alive at follow‐up had higher baseline physical activity, were younger, had a higher poverty‐to‐income ratio score (higher SES), and had fewer comorbidities.
Table 2.
Unweighted Baseline Characteristics of CAD Patients, 2003–2006 NHANES (N = 256)
| Variable | Baseline Point Estimates (95% CI) | P Value a | |
|---|---|---|---|
| Alive at Follow‐up, n = 188 | Deceased at Follow‐up, n = 68 | ||
| Total physical activity, min/d | 299.7 (285.4‐314.0) | 252.6 (226.4‐278.8) | 0.001 |
| Age, mean yrs | 67.3 (65.6‐68.9) | 74.0 (71.7‐76.3) | <0.001 |
| Female, % | 30.8 (24.1‐37.5) | 26.4 (15.8‐37.0) | 0.49 |
| Non‐Hispanic white, % | 70.2 (63.6‐76.7) | 69.1 (58.0‐80.2) | 0.10 |
| Poverty‐to‐income ratio | 2.67 (2.45‐2.88) | 2.19 (1.86‐2.53) | 0.02 |
| Cotinine, mean ng/mL | 35.1 (22.1‐48.2) | 42.5 (16.8‐68.1) | 0.58 |
| CRP, mean mg/dL | 0.39 (0.31‐0.47) | 0.52 (0.37‐0.67) | 0.11 |
| Comorbidities, mean | 2.9 (2.7‐3.1) | 3.4 (3.0‐3.8) | 0.004 |
| Cholesterol medication, % | 55.3 (48.1‐62.4) | 47.0 (35.0‐59.0) | 0.24 |
| Hypertensive medication, % | 60.1 (53.0‐67.1) | 66.1 (54.7‐77.5) | 0.38 |
Abbreviations: CAD, coronary artery disease; CI, confidence interval; CRP, C‐reactive protein; NHANES, National Health and Nutrition Examination Survey.
An adjusted Wald test was used to examine statistical differences for continuous variables (eg, age); a design‐based likelihood ratio test was used for categorical variables (eg, gender).
In an unadjusted model, for every 60 minutes/day increase in physical activity, CAD patients had a 24% reduced risk of all‐cause mortality (hazard ratio [HR]: 0.74, 95% confidence interval [CI]: 0.61‐0.89, P = 0.003). After adjustment (Table 3), for every 60 minutes/day increase in free‐living physical activity, CAD patients had a 16% reduced risk of all‐cause mortality (HR: 0.84, 95% CI: 0.72‐0.97). Notably, when duration of CAD (mean 9.3 years, 95% CI: 7.9‐10.7) was added as a covariate, results were unchanged (HR = 0.85, 95% CI: 0.73‐0.99). The proportional hazards assumption was not violated (P = 0.36) and the Harrell C concordance statistic for the model was 0.75. The Kaplan‐Meier survival curve among those ≥300 minutes/day and <300 minutes/day of total physical activity is shown in the Figure 1.
Table 3.
Weighted Cox Proportional Hazard Model Examining the Association Between Physical Activity and All‐Cause Mortality Among CAD Patients, 2003–2006 NHANES (N = 256)
| All‐Cause Mortality | ||
|---|---|---|
| Hazard Ratio (95% CI) | P Value | |
| Physical activity, 60 min/d increase | 0.84 (0.72‐0.97) | 0.02 |
| Covariates | ||
| Age, 1 yr increase | 1.05 (1.02‐1.08) | 0.01 |
| Female vs male | 0.72 (0.38‐1.37) | 0.31 |
| Race–ethnicity | ||
| Mexican American vs non‐Hispanic white | 1.58 (0.61‐4.13) | 0.33 |
| Non‐Hispanic black vs non‐Hispanic white | 0.83 (0.32‐2.13) | 0.69 |
| Other vs non‐Hispanic white | 2.56 (0.70‐9.30) | 0.14 |
| Poverty‐to‐income ratio, 1 unit increase | 0.82 (0.63‐1.08) | 0.17 |
| CRP, 1 mg/dL increase | 1.33 (0.93‐1.91) | 0.10 |
| Cotinine, 50 ng/mL increase | 1.11 (0.98‐1.27) | 0.08 |
| Comorbid illness, 1 illness increase | 1.21 (0.96‐1.53) | 0.09 |
| Cholesterol medication, yes vs no | 0.57 (0.58‐1.96) | 0.22 |
| Hypertensive medication, yes vs no | 1.06 (0.58‐1.96) | 0.82 |
Abbreviations: CAD, coronary artery disease; CI, confidence interval; CRP, C‐reactive protein; NHANES, National Health and Nutrition Examination Survey.
Figure 1.
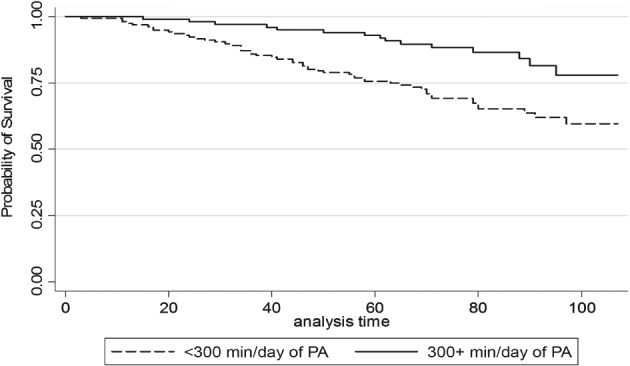
Kaplan‐Meier survival curve across physical activity (PA) level (above or below 300 minutes/day of total physical activity, light to vigorous). The top line represents those who achieved at least 300 minutes/day of total physical activity, with the bottom line representing those not achieving this threshold. Analysis time is in months.
Discussion
Approximately 1 of every 15 Americans 18 years old and older has CAD, which is the leading cause of death for people of most racial and ethnic groups in the United States.10 Attainment of ideal cardiovascular health metrics (inclusive of physical activity) has been shown to be low in different populations,11, 12 hence the need to continually improve on available methods to get individuals to enhance cardiovascular health.
Previous research demonstrated that cardiac rehabilitation is associated with a 20% to 30% reduction in mortality risk among those with CAD.1, 2, 3, 4 The effects of free‐living physical activity behavior on mortality risk among those with CAD has yet to be examined. This is worthy of investigation, as only about 25% of eligible cardiac patients participate in cardiac rehabilitation.5 Structured home‐based exercise has been shown to also be efficacious in improving cardiac function13; however, this study goes further to analyze the all‐cause mortality benefit of unstructured physical activity in CAD patients. The results of the present study suggest that, independent of age, time since diagnosis of CAD, and other confounders, greater engagement in free‐living physical activity was associated with reduced all‐cause mortality among those with a physician diagnosis of CAD. Importantly, however, like most observational studies, multiple time‐period assessments were not made across the follow‐up period. Thus, it was not possible to take into account changes that may have occurred over time (eg, new therapies added to the patient's regimen, access to care), which may have influenced the physical activity–mortality relationship.
Given the beneficial effects of cardiac rehabilitation, efforts are needed to increase participation in cardiac rehabilitation, with specific guidelines on exercise‐based cardiac rehabilitation provided elsewhere.3 However, present findings of an association of free‐living physical activity on total mortality among those with CAD underscores the importance of developing strategies to increase free‐living physical activity among the majority (75%) of cardiac patients not engaging in cardiac rehabilitation.
Major strengths of this study include the prospective study design, objective measure of physical activity, and novel examination of free‐living physical activity on all‐cause mortality among a national sample of adults with CAD. Limitations of this study include the inability to examine cause‐specific deaths (due to sample size considerations) and the specific type of ischemic heart disease of each patient (not assessed in NHANES). Other limitations include the reliance of subjective assessment of CAD, the lack of multiple time‐period assessments during the follow‐up period, and the inability to determine what percent of the study population engaged in a cardiac rehabilitation program.
Conclusion
Our findings suggest that free‐living physical activity is associated with reduced all‐cause mortality risk among CAD patients. If confirmed by future research, development of strategies to not only increase participation in supervised cardiac rehabilitation, but also increase participation in free‐living physical activity, are needed.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Suaya JA, Stason WB, Ades PA, et al. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54:25–33. [DOI] [PubMed] [Google Scholar]
- 2. O'Connor GT, Buring JE, Yusuf S, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–244. [DOI] [PubMed] [Google Scholar]
- 3. Heran BS, Chen JM, Ebrahim S, et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goel K, Lennon RJ, Tilbury RT, et al. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123:2344–2352. [DOI] [PubMed] [Google Scholar]
- 5. Suaya JA, Shepard DS, Normand SL, et al. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. [DOI] [PubMed] [Google Scholar]
- 6. Bergmann MM, Byers T, Freedman DS, et al. Validity of self‐reported diagnoses leading to hospitalization: a comparison of self‐reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147:969–977. [DOI] [PubMed] [Google Scholar]
- 7. Loprinzi PD, Walker JF, Lee H. Association between physical activity and inflammatory markers among U.S. adults with chronic obstructive pulmonary disease. Am J Health Promot. 2014;29:81–88. [DOI] [PubMed] [Google Scholar]
- 8. Kelly LA, McMillan DG, Anderson A, et al. Validity of actigraphs uniaxial and triaxial accelerometers for assessment of physical activity in adults in laboratory conditions. BMC Med Phys. 2013;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McClain JJ, Sisson SB, Tudor‐Locke C. Actigraph accelerometer interinstrument reliability during free‐living in adults. Med Sci Sports Exerc. 2007;39:1509–1514. [DOI] [PubMed] [Google Scholar]
- 10.Summary health statistics for U.S. adults: National Health Interview Survey 2012 [Table 1]. DHHS publication no. 2014–1588. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2014.
- 11. O'Flynn AM, McHugh SM, Madden JM, et al. Applying the ideal cardiovascular health metrics to couples: a cross‐sectional study in primary care. Clin Cardiol. 2015;38:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ogunmoroti O, Younus A, Rouseff M, et al. Assessment of American Heart Association's ideal cardiovascular health metrics among employees of a large healthcare organization: the Baptist Health South Florida Employee Study. Clin Cardiol. 2015;38:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sparks KE, Shaw DK, Eddy D, et al. Alternatives for cardiac rehabilitation patients unable to return to a hospital‐based program. Heart Lung. 1993;22:298–303. [PubMed] [Google Scholar]


