ABSTRACT
Background
Central obesity has been recognized as a main risk factor for cardiovascular (CV) events. Three popular central obesity indices are waist circumference, waist‐to‐hip ratio (WHR), and waist‐to‐height ratio; abdominal volume index and conicity index are 2 recent novel obesity indices. The main aim of this study is to determine the performance of these indices to best predict 10‐year CV events.
Hypothesis
Some obesity indices can be used to predict cardiovascular risk.
Methods
In total, 3199 subjects (age range, 40–79 years) were enrolled in this cross‐sectional study. The American College of Cardiology/American Heart Association and Framingham risk score tools were used to estimate the 10‐year CV events. Receiver operating characteristic curve analysis was used to determine the optimal discriminator(s) among the central obesity measures in the estimation of a 10‐year risk of CV events ≥7.5%, ≥10%, and ≥20% separately.
Results
Among the 5 central obesity indices, conicity index showed the most discriminatory power in estimation of a 10‐year CV risk. In men, based on the American College of Cardiology/American Heart Association tool, the areas under the curve (AUCs) were from 0.671 to 0.682 based on the 3 above thresholds, whereas with the Framingham tool, AUCs were from 0.651 to 0.659. In women, all AUCs were >0.7. Our results also showed WHR to be an almost comparable discriminator of CV disease risk in the Iranian study population.
Conclusion
Conicity index and WHR had a more discriminatory accuracy for 10‐year CV events compared with the other obesity indices.
Introduction
Cardiovascular (CV) diseases are the leading cause of death worldwide.1 The prevalence of CV diseases varies geographically and culturally. The Middle East and areas in Eastern Europe possibly contribute to the highest CV death rates in the world, with Iran probably bearing a greater affliction relative to other countries in this region.2
Although the rates of fatal and nonfatal ischemic heart diseases have decreased, their overall burden has increased due to population growth and aging in most countries between 1990 and 2010.3 Several powerful CV risk‐assessment tools were developed to assist clinicians in the assessment of CV disease at the individual level and also to help health policymakers estimate its burden in future years at community level.4, 5 One of the best‐known tools is the Framingham instrument, although the American College of Cardiology and American Heart Association (ACC/AHA) have jointly introduced a new instrument recently to assess 10‐year risk of CV events.4, 5 The Framingham and ACC/AHA tools use identical variables but different approaches to assess the risk of CV events over the next decade.
Neither the Framingham nor the ACC/AHA instruments have included obesity indices to assess the risk of CV events, despite well‐documented evidence for the association between central obesity and CV diseases.6, 7, 8 To measure central obesity, various indices have been suggested; among them, waist circumference (WC), waist‐to‐hip ratio (WHR), and waist‐to‐height ratio (WHtR) are regarded as the most popular indices that are widely applied in clinical settings. More recent indices, abdominal volume index (AVI) and conicity index (CI), which are calculated on simple data such as weight, height, WC, and hip circumference (HC), have also been introduced.9, 10, 11 Because of the undeniable association between central obesity and CV disease, this study sought to determine and compare the discriminatory performance of the 5 mentioned indices of central obesity—WC, WHR, WHtR, AVI, and CI—as instruments of screening to best estimate 10‐year CV risk in men and women based on 2 risk‐prediction tools (Framingham and ACC/AHA) in northern Iran.
Methods
For this cross‐sectional study, we used the baseline data of a larger study, a population‐based cohort that was started in September 2008 in Amol, a densely populated city of northern Iran. In total, 6140 subjects, age 10 to 90 years, participated in the main cohort study. Sampling has been described elsewhere.12 All participants gave informed consent for the study, which was approved by the Ethics Committee of Iran University of Medical Sciences. From the 6140 participants in the main cohort study, based on our inclusion criteria for this cross‐sectional study (age 40–79 years), the data of 3199 subjects were analyzed. A schematic diagram of the study population is demonstrated in Figure 1.
Figure 1.
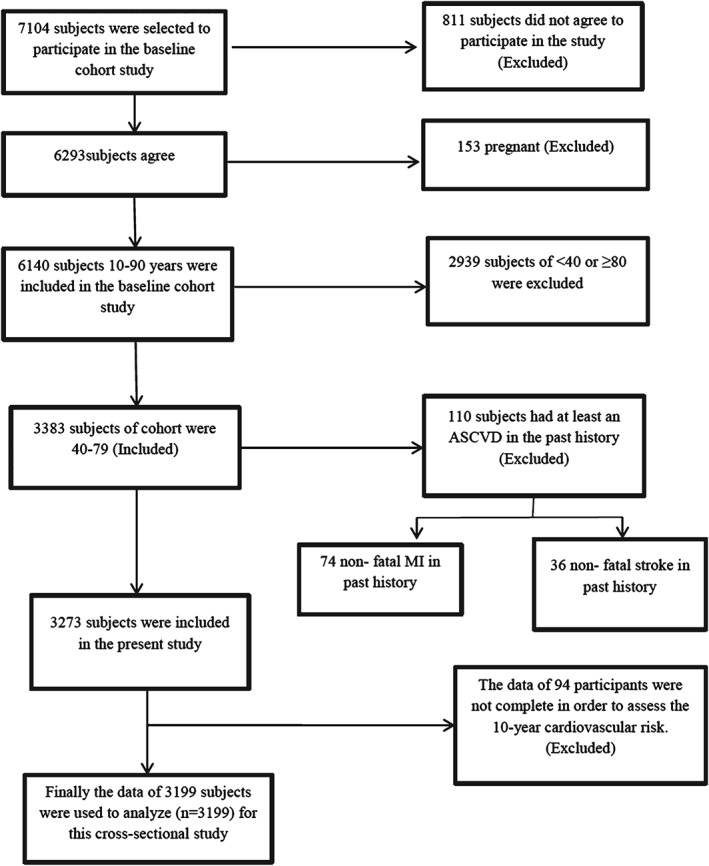
A schematic diagram of the study participants. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; MI, myocardial infarction.
Trained health care providers measured blood pressure and anthropometric data, including weight, height, WC, and HC. Before weight measurement, calibration of weighing scales was performed with 5‐kg weights. Moreover, the removal of excess clothes and shoes was recommended to assure accurate measurements. Height was measured while the participants were standing against a wall with their heels and buttocks in contact with the wall. Waist circumference was determined, in duplicate, at the midpoint between the lowest costal ridge and the upper border of the iliac crest. In the event of a >2‐cm discrepancy, then a third measurement was performed and the average of the 2 nearest values was reported as WC. Hip circumference was measured at the largest circumference between waist and knee. Both WC and HC were done with a nonstretchable and accurately calibrated scale with 0.5‐cm precision.
Other indices of central obesity were calculated using following formulas:
Systolic and diastolic blood pressures were determined using a properly fitted cuff with participants in sitting position, with back supported and legs uncrossed.
A venous blood sample was drawn from each participant following 12‐hour fasting to assess fasting blood sugar (FBS) and lipid profiles. All tests, including FBS, triglycerides (TG), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), and total cholesterol, were assessed enzymatically using the BS‐200 auto analyzer (Mindray, Nanshan, Shenzhen, China). Ten percent of the blood samples were evaluated by the Iranian National Reference Laboratory, with the coefficients of variation being between 1.7% and 3.8% of all laboratory values.
To estimate the 10‐year risk for CV events, the risks were calculated separately for men and women based on ACC/AHA equations and Framingham risk scores. In the ACC/AHA approach, race‐specific and sex‐specific multivariate equations were used to estimate the 10‐year risk for a first severe atherosclerotic cardiovascular disease (ASCVD) event, including coronary heart disease (CHD), death, nonfatal myocardial infarction, and fatal or nonfatal stroke, in non‐Hispanic African Americans and non‐Hispanic American whites age 40 to 79 years. We used the sex‐specific non‐Hispanic American white version of pooled cohort multivariate equations to calculate 10‐year risk for a first severe ASCVD event. To estimate the prevalence of a 10‐year CV risk ≥7.5%, ≥10%, and ≥20%, each calculated risk was converted to a dichotomous scale based on thresholds of 7.5%, 10%, and 20%.
Receiver operating characteristic (ROC) curves were plotted with the use of each value of the central obesity index as a possible cutoff point to compute related sensitivities and false‐positive rates. The reference variables were considered 10‐year risk of CV diseases ≥7.5%, ≥10%, and ≥20%. Then 3 ROC analyses were conducted based on each of above 3 thresholds, as reference variables, separately. The plotted points formed the ROC curves and the areas under the curve (AUCs) were computed to determine the discriminatory accuracy of each of the 5 obesity measures (WC, WHR, WHtR, AVI, and CI) in the diagnosis of the individuals with 10‐year risk ≥7.5%, ≥10%, and ≥20%. Receiver operating characteristic analyses were performed on reference variables computed using both ACC/AHA and Framingham approaches separately.
All statistical analyses were conducted using Stata software, version 12 (StataCorp, College Station, TX). The rocreg (ROC regression) package of Stata software was used to create the ROC curves and related comparisons.
Results
Association of Demographic Data, Anthropometric Measurements, Anthropometric Indices, and Markers of Metabolic Impact According to Sex
The demographic details, anthropometric measurements, and laboratory and blood pressure data of participants are presented in Table 1. There was a preponderance of men (n = 1824; 57%), with age, weight, and height being significantly higher (P < 0.05) than in women. Among the central obesity indices, CI showed no sex differences (P = 0.443). However, WHR in men (P < 0.001) and WC, WHtR, and AVI in women were significantly higher (P < 0.001). Women also showed significantly higher HC and markers of metabolic impact: diastolic blood pressure (DBP), systolic blood pressure (SBP), FBS, TG, total cholesterol, LDL‐C, and HDL‐C (P < 0.05).
Table 1.
Baseline Demographic, Anthropometric, and Metabolic Characteristics of Study Participants Age 40 to 79 Years
| Characteristics | Mean ± SD in Total Study Population (n = 3201) | Mean ± SD in Men (n = 1826) | Mean ± SD in Women (n = 1375) | P Valuea |
|---|---|---|---|---|
| Age, y | 54.68 ± 10.08 | 55.10 ± 10.41 | 54.15 ± 9.59 | 0.003 |
| Weight, kg | 75.83 ± 13.78 | 76.76 ± 14.04 | 74.62 ± 13.33 | <0.001 |
| Height, cm | 161.75 ± 9.70 | 167.45 ± 7.54 | 154.32 ± 6.89 | <0.001 |
| WC, cm | 94.90 ± 11.42 | 93.80 ± 11.20 | 96.32 ± 11.53 | <0.001 |
| HC, cm | 104.19 ± 9.70 | 101.01 ± 7.77 | 108.34 ± 10.35 | <0.001 |
| DBP, mm Hg | 78.63 ± 13.07 | 78.08 ± 13.09 | 79.34 ± 13.01 | 0.004 |
| SBP, mm Hg | 120.40 ± 17.72 | 119.51 ± 17.07 | 121.57 ± 18.48 | 0.004 |
| FBS, mg/dL | 107.88 ± 41.96 | 103.36 ± 35.58 | 113.76 ± 48.55 | <0.001 |
| TG, mg/dL | 151.37 ± 102.89 | 149.56 ± 98.71 | 153.76 ± 107.96 | <0.001 |
| TC, mg/dL | 193.91 ± 42.78 | 188.50 ± 41.30 | 200.95 ± 43.52 | <0.001 |
| LDL‐C, mg/dL | 114.65 ± 31.38 | 111.86 ± 30.91 | 118.28 ± 31.57 | <0.001 |
| HDL‐C, mg/dL | 43.12 ± 11.84 | 42.23 ± 11.65 | 44.27 ± 12.03 | <0.001 |
| BMI, kg/m2 | 28.89 ± 5.04 | 27.14 ± 4.27 | 31.18 ± 5.05 | <0.001 |
| WHR | 0.911 ± 0.077 | 0.928 ± 0.0736 | 0.890 ± 0.0781 | <0.001 |
| WHtR | 0.589 ± 0.0802 | 0.561 ± 0.0690 | 0.625 ± 0.0786 | <0.001 |
| AVI | 18.37 ± 4.39 | 17.92 ± 4.19 | 18.97 ± 4.56 | <0.001 |
| CI | 1.275 ± 0.0891 | 1.274 ± 0.0805 | 1.275 ± 0.099 | 0.443 |
Abbreviations: AVI, abdominal volume index; BMI, body mass index; CI, conicity index; DBP, diastolic blood pressure; FBS, fasting blood sugar; HC, hip circumference; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglycerides; WC, waist circumference; WHR, waist‐to‐hip ratio; WHtR, waist‐to‐height ratio.
Significance level for difference between men and women was P < 0.05.
Table 2 shows the prevalence of 10‐year risk ≥7.5%, ≥10%, and ≥20% based on estimations of instruments of ACC/AHA and Framingham, of which the former estimations were significantly higher than the latter in both sexes (P < 0.05). However, the κ coefficients were found to show substantial agreement (>0.7) between the estimation of both tools for the 10‐year risk of ≥7.5% and ≥10% men compared with women. The agreement values between the 2 instruments were decreased from the risk estimation of ≥7.5% to 20% in both sexes.
Table 2.
Prevalence of 10‐Year CV Risk ≥7.5%, ≥10%, and ≥20% According to Sex and Age
| Risk Probability | 10‐Year ACC/AHA Risk (95% Confidence Interval) | 10‐Year Framingham Risk (95% Confidence Interval) | P Valuea |
|---|---|---|---|
| 10‐year risk ≥7.5% | |||
| Men | 61.9 (59.6‐64.1) | 58.1 (55.8‐0.60.4) | 0.0208 |
| κ = 0.8153, SE = 0.0233 | |||
| Women | 26.5 (24.1‐28.8) | 9.3 (7.8‐10.8) | <0.0001 |
| κ = 0.4295, SE = 0.0224 | |||
| 10‐year risk ≥10% | |||
| Men | 53.5 (51.2‐ 55.8) | 48.9 (46.6‐51.2) | 0.0057 |
| κ = 0.7756, SE = 0.0233 | |||
| Women | 20.1 (18.0‐22.2) | 5.7 (4.5‐6.9) | <0.0001 |
| κ = 0.3740, SE = 0.0213 | |||
| 10‐year risk ≥20% | |||
| Men | 28.1 (26.0‐30.2) | 14.4 (12.8‐16.0) | <0.0001 |
| κ = 0.4864, SE = 0.0215 | |||
| Women | 6. 8 (5.4‐8.1) | 1.0 (0.5‐1.5) | <0.0001 |
| κ = 0.2484, SE = 0.0178 | |||
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; CV, cardiovascular; SE, standard error.
κ is the agreement coefficient between the 2 tools for 10‐year CV risk of ≥7.5%, ≥10%, as well as 10‐year risk of ≥20%.
P values are for all comparisons between the ACC/AHA and Framingham tools using the 2‐sample proportion test to determine whether ACC/AHA and Framingham tools produce the same proportion of 10‐year risk of ≥7.5%, ≥0%, or ≥20%.
Areas Under the Curve for Obesity Indices With American College of Cardiology/American Heart Association and Framingham Tools
In general, with both risk‐assessment tools it was found that all 5 obesity indices for men and women yielded ROC curves of varying convexity relative to the reference line (Figures 2 and 3), suggesting discriminatory power of the indices.
Figure 2.
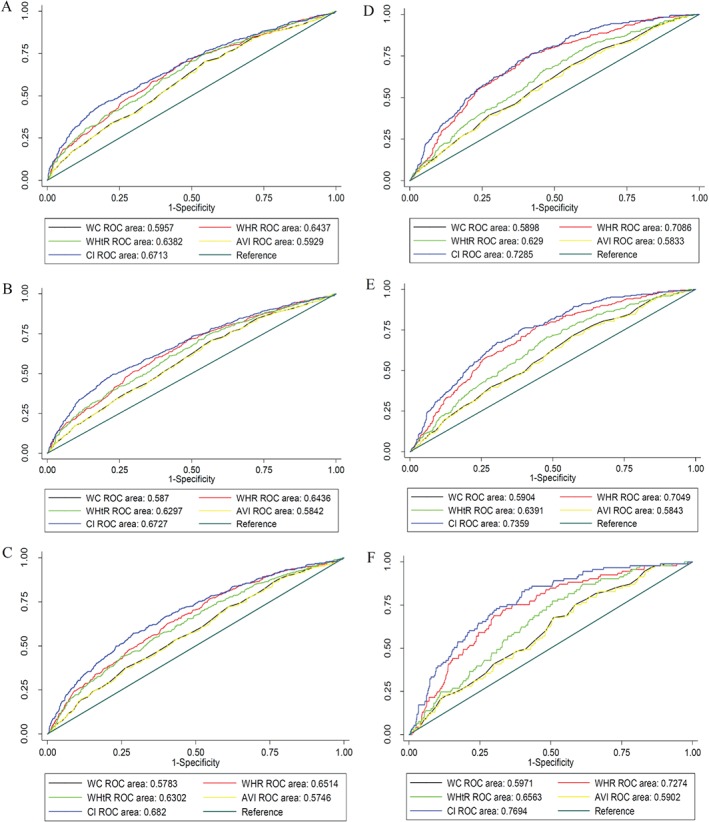
The ROC curves for discriminatory accuracy of central obesity indexes for 10‐year risk of CV disease events using the ACC/AHA tool. The A‐C graphs are related to 10‐year risks ≥7.5%, ≥10%, and ≥20%, respectively, in men, and D‐F are related to identical outcomes in women. Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; AVI, abdominal volume index; CI, conicity index; CV, cardiovascular; ROC, receiver operating characteristic; WC, waist circumference; WHR, waist‐to‐hip ratio; WHtR, waist‐to‐height ratio.
Figure 3.
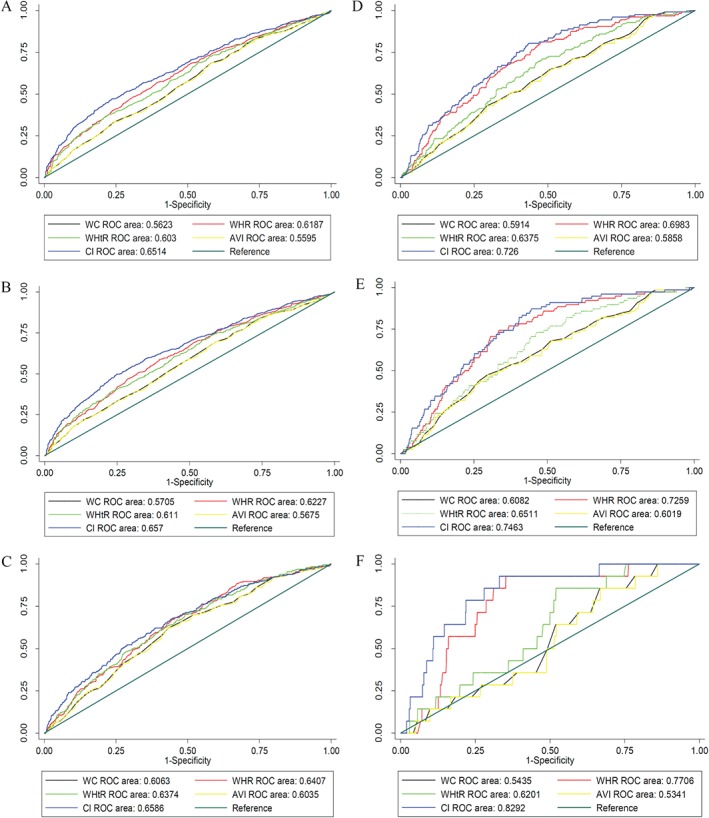
ROC curves for discriminatory accuracy of central indexes for 10‐year risk of CV diseases events using the Framingham tool. Panels A–C are related to 10‐year risks ≥7.5%, ≥10%, and ≥20%, respectively, in men, and panels D–F are related to identical outcomes in women. Abbreviations: AVI, abdominal volume index; CI, conicity index; CV, cardiovascular; WC, waist circumference; WHR, waist‐to‐hip ratio; WHtR, waist‐to‐height ratio.
Comparison of the Discriminatory Performance of Obesity Indices
Three ROC analyses were separately performed on reference variables that were calculated based on the ACC/AHA tool (according to risk thresholds of 7.5%, 10%, and 20%), and another 3 analyses were performed on reference variables that were calculated based on the Framingham tool. All analyses were performed on data of men and women separately.
In men, using the ACC/AHA tool, the CI had significantly more discriminatory accuracy than other obesity indices (P values for all comparisons were <0.001). The AUCs of CI were 0.6713 (95% confidence interval: 0.64651‐0.69604), 0.6727 (95% confidence interval: 0.64816‐0.69714), and 0.6820 (95% confidence interval: 0.65459‐0.70949) to discriminate the individuals who had the 10‐year risk ≥7.5%, ≥10%, and ≥20%, respectively. On the other hand, using the Framingham risk score, CI showed a significantly higher performance (P < 0.05) than other obesity indices except for the discrimination of the 10‐year risk ≥ %20 when it was compared with WHR (P = 0.1089) and WHtR (P = 0.0936). The AUCs of CI were 0.6514 (95% confidence interval: 0.62638‐0.67641), 0.6570 (95% confidence interval: 0.63206‐0.68194), and 0.6586 (95% confidence interval: 0.62295‐0.69420) for 10‐year risk ≥7.5%, ≥10%, and ≥20%, respectively.
In women, using the ACC/AHA tool, the discriminatory power of CI was again significantly greater than that of the other obesity indices. The AUCs of CI were 0.7285 (95% confidence interval: 0.69951‐0.75741), 0.7359 (95% confidence interval: 0.70465‐0.76710), and 0.7694 (95% confidence interval: 0.72380‐0.81507) to discriminate the patients who had a 10‐year risk ≥7.5%, ≥10%, and ≥20%, respectively. Applying the Framingham approach, a significantly higher performance was also computed for CI compared with other obesity indices except WHR. The P values for all significant comparisons were <0.001. Finally, the AUCs of CI were 0.7260 (95% confidence interval: 0.68434‐0.76765), 0.7463 (95% confidence interval: 0.69701‐0.79568 l), and 0.8292 (95% confidence interval: 0.73849‐0.91991) in the discrimination of 10‐year risk ≥7.5%, ≥10%, and ≥20%, respectively. More details are displayed in Figures 2 and 3.
Discussion
Our results revealed that the central obesity indices have discriminatory power to estimate the risk of CV diseases. Among 5 central obesity indices, CI and WHR had the strongest discriminatory power in men and women. The present study also confirms that a large part of our participants, predominantly men, will be at risk of developing CV events over the next decade. Further, our data showed a high level of agreement between the Framingham and ACC/AHA approaches, particularly for the 10‐year risk ≥10% in men.
Although central obesity is not directly used to estimate risk of CV events in the 2 instruments used in the study, our findings confirm the relative merit of their discriminatory power in the estimation of these events. Previous studies have confirmed the association between obesity measures and CV diseases.13, 14, 15, 16 However, our study revealed that WC and AVI were the poorest discriminators, with WHtR having some discriminatory potential in the Framingham 10‐year risk ≥20% tool. In the case of the latter index, a meta‐analysis study of 88 000 individuals showed the statistical superiority of WHtR relative to WC and WHR in detecting CV risk factors in both men and women.17 With WC, previous studies on this measure and CV risk have produced conflicting results.18, 19, 20
Although identical measures were included in the WHR and AVI formula, the present study revealed that the latter does not seem to be a good discriminatory index for the 10‐year CV events. Despite the fact that identical variables are involved to calculate these 2 indices, different algebraic operations are used to adjust the WC in each of these indices. In the WHR, the inverse of the HC serves for adjustment; but in AVI, WC − HC is used, which means that a more rigorous approach was used to adjust the WC in WHR formula compared with AVI. Conicity index as a higher discriminator index has 1 or 2 additional measures, compared with other indices of obesity. However, despite this advantage, no sex‐discriminating body‐shape measure is included in the CI formula for the estimation of central obesity. Finally, although CI had the highest discriminatory power, WHR, with a lesser data requirement, portrayed itself as an almost comparable discriminator of CV disease risk in the Iranian study population of Amol. Other published literature also noted the comparative merit of WHR as a central obesity index that is associated with higher coronary risk.21, 22, 23, 24 However, the results of various studies with regard to the obesity indices used in predicting risk of CV diseases are inconsistent.16, 17, 25, 26, 27
The present study estimated a large part of our population, particularly men, will be at risk of developing CV events over the next decade; however, a lower risk was estimated by Framingham compared with the ACC/AHA approach. The recently introduced ACC/AHA tool additionally includes an estimation of first severe ASCVD events (defined as first occurrence of nonfatal myocardial infarction or CHD death, or fatal or nonfatal stroke) rather than just being limited to a CHD outcome alone, as in the Framingham tool, and this may account for higher estimation.5
Although the values of the κ coefficient were relatively high in men, this degree of agreement was relatively lower in women. As discussed above, the Framingham risk approach is more conservative than the ACC/AHA approach in the estimation of risk, and also our study did estimate a lower CV risk in women. As a result, when we converted the continuous risk to a dichotomous value, a greater portion of the women were classified as the low‐risk category according to the Framingham approach compared with that of ACC/AHA.
In theory, the ACC/AHA instrument has several advantages over Framingham tool. For instance, in the calculation of final risk probability, the ACC/AHA approach uses the exponential function, and as a consequence it gives the continuous values for risk estimation. The range of probability in the ACC/AHA approach varies continuously from 0 to 1, but in the Framingham approach it varies discretely to 0.3. The continuous values in risk estimation that range from 0 to 1 without any limitation can lead to a more precise estimation of risks at both the individual and community levels. More precise estimations can help decision‐makers to consider and intervene with more timely and efficient health strategies to implement preventive, therapeutic, and rehabilitative programs against the burden of CV events in the future. In clinical practice it also helps clinicians have a better estimation of clinical status of their patients.
Given the lack of data, particularly with regard to the recent ACC/AHA guidelines, we used Caucasian race as suggested to estimate the 10‐year ASCVD risk.5 To our knowledge, our study is the first attempt to delineate the ASCVD risk among a representative sample of a North Iranian population. Our study group averages according to sex and age can serve as reliable estimations of absolute risk and can potentially be applied to individual patients in practice, providing them with intervening choices for initiating preventive strategies alongside potential public health gains in tackling major national CV health imperatives in developing countries.
Study Limitations
Our study had some limitations, the most crucial being that it was of a cross‐sectional design. Our approach requires validation with the use of prospective studies. Further, the study was confined to participants in Amol, Northern Iran, hence this may limit generalizing our findings to other regions of the Iranian population. However, this may have the advantage of minimizing any confounding variables regarding variations in medical care and access, varying socioeconomic strata, and others. Another important limitation linked to our cross‐sectional design is that our study did not accommodate for temporality. Hence, a time relationship of whether risk factors of CV disease follow enhanced adiposity, or vice versa, could not be established as would be possible with a prospective study.
Conclusion
Conicity index had the most discriminatory accuracy for the 10‐year CV events compared with the other obesity indices. In clinical practice, this index can be measured using a few simple and routine measurements. In the same way, the WHR, with a requirement for even fewer measurements in clinical approaches, revealed a relatively good discriminatory power.
This study was funded by Gastrointestinal and Liver Disease Research Center, Firoozgar Hospital, Iran University of Medical Sciences, Tehran, Iran.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Murray CJ, Vos T, Lozano R, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 2. Talaei M, Sarrafzadegan N, Sadeghi M, et al. Incidence of cardiovascular diseases in an Iranian population: the Isfahan Cohort Study. Arch Iran Med. 2013;16:138–144. [PubMed] [Google Scholar]
- 3. Moran AE, Forouzanfar MH, Roth GA, et al. The Global Burden of Ischemic Heart Disease in 1990 and 2010: The Global Burden of Disease 2010 Study. Circulation. 2014;129:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Heart, Lung, and Blood Institute , National Institutes of Health, US Department of Health and Human Services Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm. Published May 2001. NIH publication 01–3670. [Google Scholar]
- 5. Goff DC Jr, Lloyd‐Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(25 suppl 2):S74–S75]. Circulation. 2014;129(25 suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 6. van Dis I, Geleijnse JM, Kromhout D, et al. Do obesity and parental history of myocardial infarction improve cardiovascular risk prediction? Eur J Prev Cardiol. 2013;20:793–799. [DOI] [PubMed] [Google Scholar]
- 7. Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe [published correction appears in N Engl J Med. 2010;362:2433]. N Engl J Med. 2008;359:2105–2120. [DOI] [PubMed] [Google Scholar]
- 8. Wilson PW, D'Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. [DOI] [PubMed] [Google Scholar]
- 9. Guerrero‐Romero F, Rodríguez‐Morán M. Abdominal volume index: an anthropometry‐based index for estimation of obesity is strongly related to impaired glucose tolerance and type 2 diabetes mellitus. Arch Med Res. 2003;34:428–432. [DOI] [PubMed] [Google Scholar]
- 10. Valdez R. A simple model‐based index of abdominal adiposity. J Clin Epidemiol. 1991;44:955–956. [DOI] [PubMed] [Google Scholar]
- 11. Valdez R, Seidell JC, Ahn YI, et al. A new index of abdominal adiposity as an indicator of risk for cardiovascular disease: a cross‐population study. Int J Obes Relat Metab Disord. 1993;17:77–82. [PubMed] [Google Scholar]
- 12. Zamani F, Sohrabi M, Alipour A, et al. Prevalence and risk factors of cholelithiasis in Amol city, northern Iran: a population‐based study. Arch Iran Med. 2014;17:750–754. [PubMed] [Google Scholar]
- 13. Whitlock G, Lewington S, Sherliker P, et al; Prospective Studies Collaboration . Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yusuf S, Hawken S, Ounpuu S, et al; INTERHEART Study Investigators . Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case–control study. Lancet. 2005;366:1640–1649. [DOI] [PubMed] [Google Scholar]
- 15. Dudina A, Cooney MT, Bacquer DD, et al; SCORE Investigators . Relationships between body mass index, cardiovascular mortality, and risk factors: a report from the SCORE investigators. Eur J Cardiovasc Prev Rehabil. 2011;18:731–742. [DOI] [PubMed] [Google Scholar]
- 16. Schneider HJ, Friedrich N, Klotsche J, et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab. 2010;95:1777–1785. [DOI] [PubMed] [Google Scholar]
- 17. Lee CM, Huxley RR, Wildman RP, et al. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta‐analysis. J Clin Epidemiol. 2008;61:646–653. [DOI] [PubMed] [Google Scholar]
- 18. Tarastchuk JC, Guérios EE, Bueno Rda R, et al. Obesity and coronary intervention: should we continue to use body mass index as a risk factor [article in English, Portuguese]? Arq Bras Cardiol. 2008;90:284–289. [DOI] [PubMed] [Google Scholar]
- 19. Shidfar F, Alborzi F, Salehi M, et al. Association of waist circumference, body mass index and conicity index with cardiovascular risk factors in postmenopausal women. Cardiovasc J Afr. 2012;23:442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pitanga FJ, Lessa I. Anthropometric indices of obesity as an instrument of screening for high coronary risk in adults in the city of Salvador‐Bahia [article in Portuguese]. Arq Bras Cardiol. 2005;85:26–31. [DOI] [PubMed] [Google Scholar]
- 21. Al‐Lawati JA, Barakat NM, Al‐Lawati AM, et al. Optimal cut‐points for body mass index, waist circumference and waist‐to‐hip ratio using the Framingham coronary heart disease risk score in an Arab population of the Middle East. Diab Vasc Dis Res. 2008;5:304–309. [DOI] [PubMed] [Google Scholar]
- 22. Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle‐aged and older US men. Am J Epidemiol. 1995;141:1117–1127. [DOI] [PubMed] [Google Scholar]
- 23. Silventoinen K, Jousilahti P, Vartiainen E, et al. Appropriateness of anthropometric obesity indicators in assessment of coronary heart disease risk among Finnish men and women. Scand J Public Health. 2003;31:283–290. [DOI] [PubMed] [Google Scholar]
- 24. Zhou Z, Hu D, Chen J. Associating between obesity indices and blood pressure or hypertension: which index is the best? Public Health Nutr. 2009;12:1061–1071. [DOI] [PubMed] [Google Scholar]
- 25. Paniagua L, Lohsoonthorn V, Lertmaharit S, et al. Comparison of waist circumference, body mass index, percent body fat and other measures of adiposity in identifying cardiovascular disease risks among Thai adults. Obes Res Clin Pract. 2008;2:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ashwell M, Hsieh SD. Six reasons why the waist‐to‐height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56:303–307. [DOI] [PubMed] [Google Scholar]
- 27. van Dis I, Kromhout D, Geleijnse JM, et al. Body mass index and waist circumference predict both 10‐year nonfatal and fatal cardiovascular disease risk: study conducted in 20 000 Dutch men and women aged 20–65 years. Eur J Cardiovasc Prev Rehabil. 2009;16:729–734. [DOI] [PubMed] [Google Scholar]


