Abstract
Background
The contribution of arterial endothelial dysfunction (ED) to increased cardiovascular disease (CVD) risk among Blacks is not known.
Hypothesis
We investigated whether peripheral arterial ED explains racial disparity in CVD events.
Methods
Data from the Heart Strategies Concentrating on Risk Evaluation (Heart SCORE) study was used. Endothelial dysfunction was assessed by the Framingham reactive hyperemia index (fRHI), measured using pulse amplitude tonometry (PAT). Lower values of fRHI indicate more severe ED. The primary outcome of interest was combined CVD events and all‐cause mortality.
Results
1454 individuals (62% female, 40% Black, mean age 59 ± 8 years) had available data on fRHI (mean [SD]: 0.74 [0.46]). Over a mean follow‐up period of 8.0 ± 2.4 years (11,186 person‐years), 116 events were observed. Black race, male sex, smoking, diabetes, blood pressure, triglycerides, C‐reactive protein, and interleukin‐6 were inversely correlated with fRHI in univariate models. In an unadjusted Cox regression model, fRHI was associated with 20% lower risk of the primary outcome events (hazard ratio [HR] per 1‐SD higher fRHI: 0.80, 95% confidence interval [CI]: 0.66‐0.97). However, this association was no longer significant after adjustment for CVD risk factors (HR: 0.90, 95% CI: 0.74‐1.11). In an age‐ and sex‐adjusted model, Blacks had 1.68 (95% CI: 1.16‐2.43) higher risk of primary outcome compared with Whites. This association was not significantly attenuated by addition of fRHI to the multivariable models.
Conclusion
Black race is associated with increased risk of CVD events and mortality independent of its associations with ED, as measured by PAT.
Introduction
Racial differences in mortality and cardiovascular disease (CVD) morbidity pose challenges for health care in the United States and worldwide.1, 2, 3, 4 Identification of the roles of CVD risk factors and markers of subclinical atherosclerosis in race‐related differences in CVD outcomes may elucidate pathophysiologic mechanisms of racial disparities of CVD and guide preventive strategies.
Arterial endothelial dysfunction (ED) is a systemic phenomenon associated with vascular inflammation, lipid deposition, and thrombosis.5, 6 It is characterized by decreased arterial nitric oxide (NO) bioavailability.6 Endothelial dysfunction can be assessed using invasive methods, such as coronary epicardial vasoreactivity and plethysmography of the forearm circulation; or noninvasive methods, such as flow mediated dilation (FMD) and digital pulse amplitude tonometry (PAT).7, 8 Studies have reported a strong correlation between coronary and peripheral artery ED,6, 9, 10 demonstrating the utility of noninvasive approaches in measurement of ED. Flow mediated dilation is one of the earlier developed methods for measuring peripheral conduit artery ED that has been studied in several populations.7, 8, 11 This technique measures the ability of the brachial artery to respond with dilatation (ie, endothelial nitric oxide release) during reactive hyperemia following occlusion of the arm with a blood pressure (BP) cuff. Its use in large‐scale studies is limited by its operator dependence and lack of standardization.7, 8 Pulse amplitude tonometry is a newer method of measuring peripheral artery microvascular ED that is automated and scalable, hence is useful for large epidemiological studies. It measures the finger arteriolar vasodilatory response to ischemia using finger plethysmography.7
Several epidemiological studies using FMD have reported that ED is associated with CVD risk factors and is an independent predictor of CVD risk.8, 12, 13, 14, 15, 16 Although prior multiracial studies investigating ED using FMD have not found significant interaction between ED and race,2 we previously demonstrated that Black race is independently associated with ED measured using PAT.17 However, whether race‐related differences in ED contribute to racial disparities in CVD events and mortality is not known. In the present study, we investigated whether peripheral artery ED measured by PAT and expressed as Framingham reactive hyperemia index (fRHI)18 explains, in part, disparities in CVD events between Blacks and Whites.
Methods
Study Population
Heart Strategies Concentrating on Risk Evaluation (Heart SCORE) is an ongoing community‐based prospective cohort study with approximately equal representation of Blacks and Whites (44% Blacks, 56% Whites), aiming to investigate racial disparities in CVD outcomes. The methods of Heart SCORE have been described previously.17, 19, 20 Eligibility criteria included age 45 to 75 years at study entry, residence in the greater Pittsburgh metropolitan area, ability to undergo baseline and annual follow‐up visits, and absence of known comorbidities expected to limit life expectancy to <5 years. The present report is based on prospective analysis of data from 1454 individuals who underwent measurement of ED at baseline. The institutional review board at the University of Pittsburgh approved the study protocol, and all study subjects provided written informed consent.
Data Collection
Detailed demographic and medical histories were collected at the baseline visit. Race was self‐reported. Lifestyle characteristics were assessed using self‐developed questionnaires.
To assess smoking history, participants were classified as “never,” “former,” and “current” smoker based on responses from a self‐reported questionnaire. Physical examination included measurement of vital signs and anthropometric measures of body fat distribution. Experienced research nurses measured BP 2 times after 5 minutes of rest in a seated position by using a manual sphygmomanometer (Welch Allyn, Skaneateles Falls, NY) and an appropriately sized cuff. The average of the 2 readings was taken. Hypertension was defined as systolic BP >140 mm Hg or diastolic BP >90 mm Hg, or current treatment with antihypertensive medication. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Diabetes mellitus (DM) was defined as fasting glucose >126 mg/dL based on clinical guidelines at the time of study initiation or a history of previously diagnosed DM treated with diet, oral agents, and/or insulin.
Laboratory assessments of lipoprotein levels and particle sizes were performed on venous blood drawn after fasting for 8 hours. The vertical autoprofile technique (VAP; Atherotech, Birmingham, AL) was used to quantify the amount of cholesterol contained in each lipoprotein particle subfraction. This method was also used to quantify concentrations of proatherogenic, small dense low‐density lipoprotein particles. Fasting blood glucose was measured using the glucose oxidase method. Measurement of high‐sensitivity C‐reactive protein (hsCRP) was performed using an immunoturbidimetric assay on the Roche P Modular system (Roche Diagnostics, Indianapolis, IN), using reagents and calibrators from DiaSorin (Stillwater, MN). Serum interleukin‐6 (IL‐6) concentrations were measured using commercially available ELISA assay kits (RD Systems, Minneapolis, MN). Measurements were performed using a standard curve provided with the ELISA kits; samples were read spectrophotometrically in a microtiter plate reader. To confirm reproducibility as reported by the kit manufacturer, a random subset of samples (10%) was assayed in duplicate.
Assessment of Endothelial Dysfunction
Endothelial function was measured using an Endo‐PAT2000 device (Itamar Medical, Caesarea, Israel) using the protocol described by the Framingham study, as previously reported.17, 18 In brief, digital pulse amplitude was measured using the PAT device placed on the tip of each index finger; one was the study finger and the other served as the control finger. Baseline PAT signal was measured for 5 minutes on both fingers. Arterial flow was then interrupted on one side (the study finger) by applying occlusive pressure on the study arm. After 5 minutes, the cuff pressure was abruptly deflated and PAT signal was measured on both (study and control) fingers for the subsequent 5 minutes. The data were recorded electronically and analyzed using a computerized, automated algorithm (Itamar Medical). Pulse amplitude response to hyperemia was calculated from the hyperemic fingertip as the ratio of the post‐occlusion pulse amplitude to the baseline pulse amplitude. This result was divided by corresponding ratio in the control hand to give the PAT ratio (also known as reactive hyperemia index [RHI]). The fRHI was calculated as the natural log‐transformation of the RHI.13, 18 The reproducibility of the PAT test has been previously reported by McCrea.21
Lower fRHI indicates that hyperemic response is blunted and suggests a lower endothelial‐dependent function. With regard to the timing of fRHI measurement in this cohort, 80% of the PAT measurements were performed at the baseline visit; 10% of the measurements were done at the second visit, 1 year after study entry; and the remaining 10% were performed between 1 and 2 years after study entry. Sensitivity analyses were conducted to determine if the later measurement of endothelial function in the minority of the subjects could affect the results.
Clinical Outcome
Participants were assessed for incident hospitalization and CVD outcomes by semiannual questionnaires and during annual follow‐up study visits. Incident CVD events were predefined as nonfatal myocardial infarction, acute coronary syndrome, stroke, coronary revascularization (eg, percutaneous coronary intervention, coronary artery bypass graft surgery), or cardiac death. The primary outcome of interest was combined CVD events and all‐cause mortality. Furthermore, we performed subsidiary analyses by restricting the outcome to incident CVD events (excluding mortality due to noncardiac causes). Events were confirmed and classified by reviews of medical records, including death certificates obtained from the Commonwealth of Pennsylvania, performed by an investigator blinded to results of risk factor, biomarker, and PAT assessments.
Statistical Analysis
We assessed the correlates of fRHI by summarizing several sociodemographic, biophysical, and biochemical variables within tertiles of fRHI; test parameters were z values from regression of fRHI on each covariate. Linear regression analysis was used to assess the association between fRHI and race. Survival analyses were used to examine the associations of fRHI and Black race with incident clinical outcomes. Kaplan‐Meier plots were used to visually compare survivor function by race and categories of fRHI. The log‐rank test was used to determine the statistical significance of differences between survivor curves. Hazard ratios (HRs) were determined from Cox regression models. The association of fRHI with outcome was determined for 1‐SD change in the independent variable. For comparison, the association was also assessed for tertiles of fRHI. The potential role of fRHI in explaining the association between Black race and clinical outcomes was assessed by adding fRHI to a Cox model containing race, progressively adjusted for other CVD risk factors. Adjustment variables included age, sex, smoking status, BP, DM, BMI, total cholesterol, and high‐density lipoprotein cholesterol (HDL‐C). We assessed potential effect modification of the association between ED and clinical outcomes by race by putting interaction terms between fRHI and race. The assumptions of the proportionality of hazards were evaluated using Schoenfeld residuals. Sensitivity analyses were performed to assess if the lag between the baseline visit and date of endothelial function measurement in a minority of subjects could affect the results. These included (1) adjusting for categorical variable indicating the time of measurement (ie, baseline, within 1 year, within 2 years), and (2) changing the baseline to the time of measurement of endothelial function, in the survival analyses models. All analyses were performed using Stata software, version 11 (StataCorp LP, College Station, TX). P values <0.05 were considered statistically significant.
Results
Baseline Characteristics and Correlates of Framingham Reactive Hyperemia Index and Race
Baseline characteristics of the participants by race are shown in Table 1. In total, 1454 individuals (62% female, 40% Black) had available data on fRHI. The mean (SD) age and fRHI of participants were 59 ± 8 years and 0.74 ± 0.46, respectively. White participants were older than Blacks (60 vs 58 years) and were composed of fewer females (59% vs 67%), current smokers (7% vs 12%), and individuals with DM (6% vs 17%) and hypertension (30% vs 55%). Black participants had higher BMI (32 vs 29 kg/m2) and higher levels of hsCRP (0.60 vs 0.12 log‐mg/L) and IL‐6 (0.72 vs 0.34 log‐pg/mL; Table 1).
Table 1.
Baseline Characteristics of Participants by Race
| Variable | White | Black | P Value | ||
|---|---|---|---|---|---|
| N | Value | N | Value | ||
| fRHI | 835 | 0.80 (0.46) | 579 | 0.66 (0.44) | <0.001 |
| Age, y | 835 | 60 (8) | 579 | 58 (8) | <0.001 |
| Female sex, n (%) | 835 | 492 (59) | 579 | 387 (67) | 0.003 |
| Current smoker, n (%) | 834 | 57 (7) | 579 | 69 (12) | 0.001 |
| Hx of DM, n (%) | 829 | 47 (6) | 578 | 96 (17) | <0.001 |
| Hx of HTN, n (%) | 833 | 252 (30) | 579 | 319 (55) | <0.001 |
| SBP, mm Hg | 834 | 133 (18) | 578 | 141 (19) | <0.001 |
| DBP, mm Hg | 833 | 79 (10) | 578 | 83 (10) | <0.001 |
| Fasting glucose, mg/dL | 832 | 96 (18) | 579 | 103 (34) | <0.001 |
| BMI, kg/m2 | 825 | 29 (5) | 573 | 32 (6) | <0.001 |
| Waist‐hip ratio | 775 | 0.89 (0.09) | 538 | 0.89 (0.09) | 0.96 |
| Log hsCRP, log‐mg/L | 796 | 0.12 (1.16) | 541 | 0.60 (1.23) | <0.001 |
| Log IL‐6, log‐pg/mL | 791 | 0.34 (0.74) | 528 | 0.72 (0.67) | <0.001 |
| TC, mg/dL | 833 | 217 (42) | 575 | 209 (45) | 0.002 |
| HDL‐C, mg/dL | 833 | 57 (15) | 575 | 58 (15) | 0.09 |
| TG, mg/dL | 833 | 135 (85) | 575 | 110 (58) | <0.001 |
| LDL‐C, mg/dL | 833 | 145 (35) | 575 | 140 (40) | 0.015 |
| sdLDL, mg/dL | 833 | 50 (20) | 575 | 44 (20) | <0.001 |
| IDL‐C, mg/dL | 833 | 18 (9) | 575 | 17 (8) | 0.11 |
| VLDL‐C, mg/dL | 833 | 15 (11) | 575 | 11 (8) | <0.001 |
| Non–HDL‐C, mg/dL | 833 | 160 (39) | 575 | 151 (42) | <0.001 |
| Total CAC volume | 335 | 184 (324) | 270 | 124 (443) | 0.057 |
| Max of CIMT | 415 | 0.80 (0.19) | 199 | 0.83 (0.18) | 0.091 |
Abbreviations: BMI, body mass index; BP, blood pressure; CAC, coronary artery calcium; CIMT, carotid intima‐media thickness; DBP, diastolic blood pressure; DM, diabetes mellitus; fRHI, Framingham reactive hyperemia index; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; HTN, hypertension; Hx, history; IDL‐C, intermediate‐density lipoprotein cholesterol; IL‐6, interleukin‐6; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation; sdLDL, small dense low‐density lipoprotein; TC, total cholesterol; TG, triglycerides; VLDL‐C, very low‐density lipoprotein cholesterol.
Data are expressed as mean (SD) unless otherwise noted.
For the characteristics of the participants by tertiles of fRHI, see Supporting Information, Table 1, in the online version of this article. In a univariate model, females and individuals with higher HDL‐C had higher fRHI. Black race and several traditional and emerging cardiovascular risk factors including smoking, DM, systolic and diastolic BP, BMI, and levels of triglycerides, small dense low‐density lipoprotein, CRP, and IL‐6 were inversely associated with fRHI. The mean fRHI among Whites was 0.80 ± 0.46, whereas the corresponding value in Blacks was 0.66 ± 0.44. The significant Black‐White difference in fRHI persisted after adjustment for age, sex, and CVD risk factors. (Data available from authors upon request.)
Framingham Reactive Hyperemia Index and Clinical Events
Over a mean follow‐up period of 8.0 ± 2.4 years (11 186 person‐years), 116 primary events were observed. Figure 1 shows the event‐free survival rates of participants by tertiles of fRHI for the primary outcome. Individuals in the top and middle tertiles of fRHI had better survival than those in the bottom tertile (trend P value: 0.01). In an unadjusted Cox regression model, higher fRHI values were associated with 20% lower risk of combined CVD and mortality events (HR per 1‐SD higher fRHI: 0.80, 95% CI: 0.66‐0.97). However, this association was no longer significant after adjustment for CVD risk factors (HR: 0.90, 95% CI: 0.74‐1.11; Table 2). Similar associations were observed for analyses comparing tertiles of fRHI (see Supporting Information, Table 2, in the online version of this article).
Figure 1.
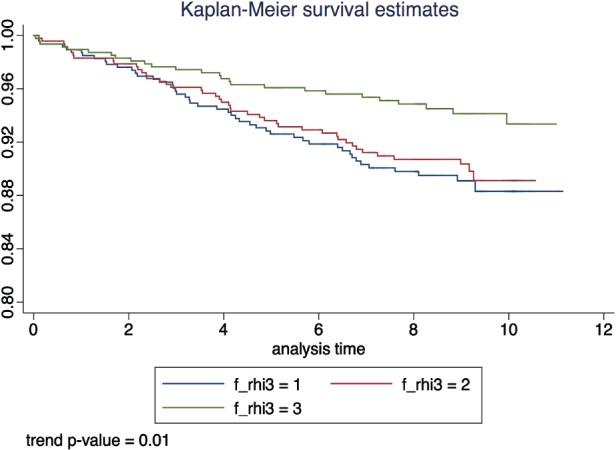
Kaplan‐Meier curve of primary study endpoint by tertiles of fRHI. Abbreviations: fRHI, Framingham reactive hyperemia index.
Table 2.
Association of 1‐SD Higher fRHI With Incident Outcomesa
| Adjustment | N | No. of Cases | HR (95% CI) per 1‐SD Higher fRHI | χ2 | P Value |
|---|---|---|---|---|---|
| Crude | 1397 | 115 | 0.80 (0.66‐0.97) | 5.2 | 0.02 |
| Model 1 | 1397 | 115 | 0.85 (0.70‐1.04) | 2.6 | 0.11 |
| Model 2 | 1393 | 115 | 0.90 (0.74‐1.10) | 1.0 | 0.31 |
| Model 3 | 1387 | 113 | 0.90 (0.74‐1.11) | 1.0 | 0.33 |
Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CI, confidence interval; DM, diabetes mellitus; fRHI, Framingham reactive hyperemia index; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol.
Model 1: Age, sex, and race. Model 2: model 1 + smoking status, SBP, and DM. Model 3: model 2 + TC and HDL‐C.
Clinical outcome included MI, stroke, ACS, or revascularization (CABG or PCI).
Race, Reactive Hyperemia Index, and Clinical Outcome
Figure 2 shows survivor function by race and demonstrates that Blacks have worse event‐free survival than Whites for the primary outcome (P = 0.05). The incidence of the primary outcome was 12.8 (9.8‐16.7) and 9.0 (7.0‐11.5) per 1000 person‐years in Blacks and Whites, respectively. In an age‐ and sex‐adjusted model, Blacks had 1.68 (1.16‐2.43) higher risk of combined CVD and mortality events. This association was attenuated by only 5% on adjustment for fRHI (HR: 1.60, 95% CI: 1.10‐2.34; Table 3). There was insignificant attenuation of the HRs on addition of fRHI to multivariable models progressively adjusted for conventional CVD risk factors (Table 3). Results of tests of interaction between fRHI and race on clinical outcomes were not statistically significant (see Supporting Information, Table 3, in the online version of this article).
Figure 2.
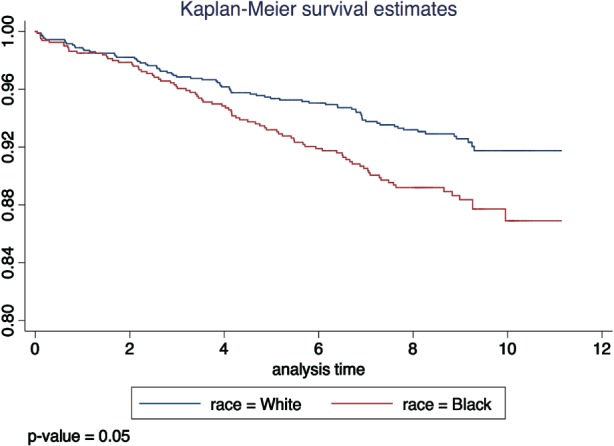
Kaplan‐Meier curve of primary study endpoint by race.
Table 3.
Effect of Adjustment for fRHI on the Association Between Race and Incident Clinical Outcomea
| Adjusted for Variable on the Left Column | Further Adjusted for fRHI | |||||
|---|---|---|---|---|---|---|
| Adjustment | N | No. of Cases | HR (95% CI), Black vs White | χ2 | HR (95% CI), Black vs White | χ2 |
| Crude | 1367 | 114 | 1.42 (0.98‐2.05) | 3.5 | 1.35 (0.93‐1.95) | 2.4 |
| Model 1 | 1367 | 114 | 1.68 (1.16‐2.43) | 7.5 | 1.60 (1.10‐2.34) | 6.1 |
| Model 2 | 1346 | 113 | 1.45 (0.98‐2.15) | 3.4 | 1.44 (0.97‐2.14) | 3.2 |
| Model 3 | 1341 | 111 | 1.54 (1.03‐2.31) | 4.5 | 1.53 (1.02‐2.29) | 4.2 |
Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CI, confidence interval; DM, diabetes mellitus; fRHI, Framingham reactive hyperemia index; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; TC, total cholesterol.
Model 1: age and sex. Model 2: model 1 + smoking status, SBP, and DM. Model 3: model 2 + TC and HDL‐C.
Clinical outcome included MI, stroke, ACS, or revascularization (CABG or PCI).
Subsidiary analyses that restricted outcome to incident CVD events (excluding mortality due to noncardiac causes) had limited statistical power but revealed findings that are consistent with those of the primary analyses. Sensitivity analyses performed to assess any effect of the lag between the baseline and date of endothelial function measurement yielded similar results to the main analyses. (Data are available from the authors upon request.)
Discussion
Prospective evaluation of a racially diverse cohort of 1454 participants (40% Black) over a mean follow‐up period of 8.0 ± 2.4 years indicates that Black race is associated with increased risk of CVD events and mortality. However, this observation is not explained by our finding that Black race is independently associated with arterial ED measured by PAT. Specifically, our data indicate that ED is not independently associated with increased CVD risk after adjustment for CVD risk factors.
Although our study and others demonstrate that Blacks have more prevalent CVD risk factors, such as hypertension, obesity, and DM, than do Whites,22, 23, 24 racial disparities in CVD are not fully explained by differences in traditional risk factors. This established finding led to previous studies investigating the role of emerging CVD risk factors such as lipoprotein(a) and inflammatory factors as explanations for racial disparities in CVD. Our data confirm those from previous studies, which reported that CRP and IL‐6 are elevated in Blacks.25, 26 We also demonstrated race‐related differences in lipoprotein particle sizes. However, our results show that race‐related differences in these emerging CVD risk factors do not fully explain racial disparities in clinical event rates.
Arterial ED is both a risk factor for and an early marker of atherosclerosis. Endothelial dysfunction has been shown to strongly correlate with CVD risk factors in numerous studies.7 Available evidence indicates that ED is a marker of CVD burden and an indicator of the effect that CVD risk factors have on vascular health. Furthermore, laboratory studies demonstrate that ED is also a mediator and contributor to progression of atherosclerosis.7, 27, 28, 29 Consistent with prior reports, we found that ED is correlated with several CVD risk factors.
Several studies have reported that arterial ED assessed by measurement of endothelium‐dependent vasodilation of coronary and brachial arteries is independently associated with adverse CVD outcome.5, 8, 12, 15, 16, 30, 31, 32, 33 Most of these studies measured conduit artery (macrovascular) ED in epicardial coronary or brachial arteries.5, 8, 12, 15, 16, 30, 31, 32, 33 On the other hand, outcome data on arteriolar ED assessed using PAT‐measured fRHI are limited. One recent study of 323 Japanese patients with chronic kidney disease and a second study of 329 Europeans with unexplained chest pain who were followed over average periods of 2.5 and 7 years, respectively, reported that fRHI‐defined ED of finger arterioles measured by PAT was independently associated with adverse CVD events.5, 12 However, although our study confirmed that fRHI is correlated with several CVD risk factors,6, 13, 17 after adjusting for these risk factors, we found that fRHI was not independently associated with the primary outcome of CVD events or mortality. This finding may possibly be related to our study population, which was composed of a cohort of healthy individuals recruited from the community, whereas prior studies comprised patients with chronic kidney disease or chest pain.
We previously reported that Black race is associated with ED independent of its associations with conventional CVD risk factors.17 Therefore, in the present study, we postulated that ED might provide a mechanistic explanation for the well‐established association between Black race and increased risk of CVD events.1, 2, 3, 4 However, our results indicate that Black race remained an independent predictor of CVD events after controlling for both ED and CVD risk factors. Therefore, ED measured by PAT did not provide a pathophysiologic explanation for the higher CVD risk observed for Black individuals in our study. This may be due to lack of power of our study to detect an existing effect. It is also important to note that these findings pertain to endothelial‐dependent vascular function as measured by PAT and do not necessarily translate to endothelial‐independent vascular function, macrovascular ED as measured by FMD, or direct assessment of coronary‐artery vasoreactivity. This might be particularly relevant, as microvascular ED may be an early indicator of risk of disease and hence may require longer follow‐up periods and/or larger sample size to detect effect, whereas macrovascular ED may be more important in people with existing atherosclerosis.7
Study Limitations
Our study provided a large amount of data on endothelial function involving >1400 participants from a generally healthy community‐based population. Forty percent of the subjects were Black, which permitted a robust assessment of race‐related CVD disparities. Our methods measured endothelial function using PAT, which has been reported to be a more reliable and scalable approach than previous endeavors.21, 34 However, PAT has not been studied as well as some of the other methods of measuring ED, such as FMD, and may be affected by non–endothelial‐dependent factors. Hence, it would be important to demonstrate similar findings by determining ED using other measurement methods in the same population.
We also acknowledge other limitations of our study. First, our data suggest that increased CVD risk associated with Black race is not related to an association between Black race and ED. However, with only 116 events of the primary outcome and wide CI of our estimates, we cannot definitively conclude that ED does not meaningfully contribute at all to racial disparities in CVD. Second, in the minority of the subjects where there was some delay between baseline date and measurement date of endothelial function, we assumed that these measurements are reflective of baseline status. Sensitivity analyses by adjusting for time of PAT measurement or changing the baseline to date of PAT measurement yielded very similar results, indicating our assumptions were valid. Third, due to our sample size, we were unable to reliably examine individual components of the primary composite outcome, such as ischemic heart disease, stroke, or mortality events separately to assess for heterogeneity. However, we performed subsidiary analyses of a composite of CVD events and cardiac mortality (instead of all‐cause mortality), which yielded comparable results, albeit limited by power. We were also unable to assess heterogeneity of effect by clinically important categories such as different age groups or by sex, for similar reasons. Finally, a repeat measurement of PAT in the same individuals would have provided information about the reliability and long‐term variability of this marker.
Conclusion
Our data indicate that Black race is independently associated with ED and a higher risk of adverse clinical outcomes. However, ED as measured by PAT does not appear to explain observed race‐related differences in CVD outcomes in the present study. Further larger studies of ED using multiple measurement methods are needed to fully understand the role of this marker in racial disparities.
Supporting information
Supplementary table 1: Baseline correlates of Framingham reactive hyperemia index (fRHI)
Supplementary table 2: Association of thirds of fRHI with incident outcomes*
Supplementary table 2: Association of 1‐SD higher fRHI with incident outcomes* by race
This study was funded by the Pennsylvania Department of Health (ME‐02‐384) and National Institutes of Health (R01HL089292).
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Stewart JA, Dundas R, Howard RS, et al. Ethnic differences in incidence of stroke: prospective study with stroke register. BMJ. 1999;318:967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lynch GF, Gorelick PB. Stroke in African Americans. Neurol Clin. 2000;18:273–290. [DOI] [PubMed] [Google Scholar]
- 3. Association AH. Heart Disease and Stroke Statistics: 2006 Update. Dallas, TX: American Heart Association; 2006. [Google Scholar]
- 4. Mensah GA, Mokdad AH, Ford ES, et al. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. [DOI] [PubMed] [Google Scholar]
- 5. Hirata Y, Sugiyama S, Yamamoto E, et al. Endothelial function and cardiovascular events in chronic kidney disease. Int J Cardiol. 2014;173:481–486. [DOI] [PubMed] [Google Scholar]
- 6. Brant LC, Hamburg NM, Barreto SM, et al. Relations of digital vascular function, cardiovascular risk factors, and arterial stiffness: the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil) cohort study. J Am Heart Assoc. 2014;3:e001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cardona A, Kondapally Seshasai SR, Davey J, et al. A meta‐analysis of published studies of endothelial dysfunction does not support its routine clinical use. Int J Clin Pract. 2015;69:649–658. [DOI] [PubMed] [Google Scholar]
- 9. Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 10. Matsuzawa Y, Sugiyama S, Sugamura K, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688–1696. [DOI] [PubMed] [Google Scholar]
- 11. Kullo IJ, Malik AR, Santos S, et al. Association of cardiovascular risk factors with microvascular and conduit artery function in hypertensive subjects. Am J Hypertens. 2007;20:735–742. [DOI] [PubMed] [Google Scholar]
- 12. Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. [DOI] [PubMed] [Google Scholar]
- 13. Hamburg NM, Keyes MJ, Larson MG, et al. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ras RT, Streppel MT, Draijer R, et al. Flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta‐analysis. Int J Cardiol. 2013;168:344–351. [DOI] [PubMed] [Google Scholar]
- 15. Yeboah J, Crouse JR, Hsu FC, et al. Brachial flow‐mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. [DOI] [PubMed] [Google Scholar]
- 16. Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow‐mediated dilation for incident cardiovascular events in a population‐based study: the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2009;120:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulukutla SR, Venkitachalam L, Bambs C, et al. Black race is associated with digital artery endothelial dysfunction: results from the Heart SCORE study. Eur Heart J. 2010;31:2808–2815. [DOI] [PubMed] [Google Scholar]
- 18. Hamburg NM, Palmisano J, Larson MG, et al. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aiyer AN, Kip KE, Marroquin OC, et al. Racial differences in coronary artery calcification are not attributed to differences in lipoprotein particle sizes: the Heart Strategies Concentrating on Risk Evaluation (Heart SCORE) Study. Am Heart J. 2007;153:328–334. [DOI] [PubMed] [Google Scholar]
- 20. Bambs C, Kip KE, Dinga A, et al. Low prevalence of “ideal cardiovascular health” in a community‐based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011;123:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCrea CE, Skulas‐Ray AC, Chow M, et al. Test‐retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med. 2012;17:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ix JH, Allison MA, Denenberg JO, et al. Novel cardiovascular risk factors do not completely explain the higher prevalence of peripheral arterial disease among African Americans: the San Diego Population Study. J Am Coll Cardiol. 2008;51:2347–2354. [DOI] [PubMed] [Google Scholar]
- 23. Markus H, Kapozsta Z, Ditrich R, et al. Increased common carotid intima‐media thickness in UK African Caribbeans and its relation to chronic inflammation and vascular candidate gene polymorphisms. Stroke. 2001;32:2465–2471. [DOI] [PubMed] [Google Scholar]
- 24. Beltrán‐Sánchez H, Harhay MO, Harhay MM, et al. Prevalence and trends of metabolic syndrome in the adult US population, 1999–2010. J Am Coll Cardiol. 2013;62:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paalani M, Lee JW, Haddad E, et al. Determinants of inflammatory markers in a bi‐ethnic population. Ethn Dis. 2011;21:142–149. [PMC free article] [PubMed] [Google Scholar]
- 26. Virani SS, Brautbar A, Davis BC, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lavi S, Bae JH, Rihal CS, et al. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95:1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lerman A, Edwards BS, Hallett JW, et al. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997–1001. [DOI] [PubMed] [Google Scholar]
- 29. Stone GW, Maehara A, Lansky AJ, et al; PROSPECT Investigators . A prospective natural‐history study of coronary atherosclerosis [published correction appears in N Engl J Med 2011;365:2040]. N Engl J Med. 2011;364:226–235. [DOI] [PubMed] [Google Scholar]
- 30. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. [DOI] [PubMed] [Google Scholar]
- 31. Targonski PV, Bonetti PO, Pumper GM, et al. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–2809. [DOI] [PubMed] [Google Scholar]
- 32. Suwaidi JA, Hamasaki S, Higano ST, et al. Long‐term follow‐up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 33. Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long‐term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. [DOI] [PubMed] [Google Scholar]
- 34. Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1: Baseline correlates of Framingham reactive hyperemia index (fRHI)
Supplementary table 2: Association of thirds of fRHI with incident outcomes*
Supplementary table 2: Association of 1‐SD higher fRHI with incident outcomes* by race


