ABSTRACT
Oxidants/antioxidants play an important role in cellular homeostasis. The human body has endogenous molecules that work as antioxidants, such as glutathione, superoxide dismutase, peroxidases, and catalase. Exogenous substances in the diet, such as β‐carotene, ascorbate, and vitamin E, are vital antioxidants. Of these, vitamin E is likely the most important antioxidant in the human diet, and many studies have been performed to elucidate its role in health and disease. Vitamin E is a family of several compounds, of which α‐tocopherol is the most widely known analog. α‐Tocopherol exhibits antioxidative property in vitro and inhibits oxidation of low‐density lipoprotein cholesterol. In addition, α‐tocopherol shows anti‐inflammatory activity and modulates expression of proteins involved in the uptake, transport, and degradation of atherogenic lipids. Though α‐tocopherol exhibits important antioxidant, anti‐inflammatory, and antiatherogenic features in vitro, α‐tocopherol supplements have failed to consistently reduce atherosclerosis‐related events in human trials. The conflicting results have led to reconsideration of the importance previously given to α‐tocopherol and led to interest in other members of vitamin E family, especially γ‐tocopherol, which exerts a much more potent antioxidant, anti‐inflammatory, and cardioprotective effect than α‐tocopherol. This reconsideration has been backed by solid laboratory and clinical research. We suggest that the absence of γ‐tocopherol in traditional preparations may be one reason for the lack of consistent salutary effects of vitamin E preparations in clinical trials. This review summarizes our current understanding of tocopherols as antioxidant molecules and emerging evidence of an important role of γ‐tocopherol in the pathophysiology of atherosclerosis‐related cardiovascular disease.
Introduction
As the role of oxidative stress in atherosclerosis‐related cardiovascular disease (CVD) became evident, a number of trials were conducted with α‐tocopherol, a natural antioxidant, in the therapy of these diseases states. Unfortunately, the salutary effects of α‐tocopherol in terms of reduction in CVD events seen in early epidemiological studies were not seen in large trials. Some studies even showed adverse effects of commercial vitamin E, whose major constituent is α‐tocopherol. Here, we summarize the controversial role of tocopherols in atherosclerosis‐related CVD. We also point to the evidence from studies that highlight a very important role of different constituents of vitamin E, especially γ‐tocopherol, in cardiovascular (CV) health.
Atherosclerosis: An Overview
Atherosclerosis is a progressive inflammatory disease characterized by excessive deposition of cholesterol in the arterial wall. Despite major advances in the management of CVD, it still remains the leading cause of morbidity and mortality globally. From the time of Leonardo da Vinci, who first described atherosclerosis as “excessive nourishment and hardening” of the arteries, to Duff and McMillan and others, who formulated the lipid hypothesis in the 1950s, the understanding of atherogenesis has come a long way.1, 2, 3 Steinberg et al, in the early 1980s, suggested a novel concept that oxidized low‐density lipoprotein (LDL) particles, and not the native LDL (n‐LDL), play a crucial role in atherogenesis.4, 5, 6 This concept was later confirmed by other investigators.7, 8, 9
The endothelial cells, smooth muscle cells (SMCs), and macrophages are sources of oxidant species, which cause endothelial injury by the oxidative modification of phospholipids and cholesterol. The oxidized LDL particles migrate to subendothelial layers, where they are taken up by macrophages via a variety of scavenger receptors, and later on they change into foam cells.10, 11, 12, 13 Foam cells secrete growth factors that induce SMC proliferation and migration, which, along with macrophages, convert fatty streaks to more advanced atherosclerotic lesions and ultimately to atherosclerotic lesions that protrude into the arterial lumen.7, 8 These steps are shown graphically in Figure 1.
Figure 1.
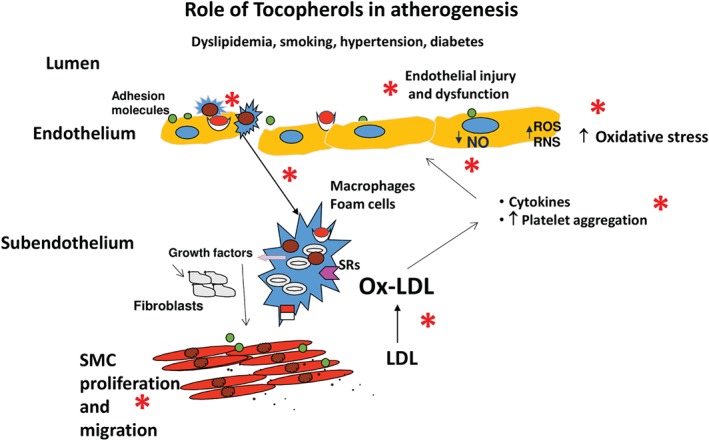
Oxidative stress in atherogenesis: role of tocopherols. Postulated steps in atherogenesis. *Steps at which the tocopherols act to decrease oxidative stress. They decrease SMC proliferation, decrease platelet aggregation, increase NO availability, decrease endothelial dysfunction, and decrease lipid peroxidation. Abbreviations: LDL, low‐density lipoprotein; NO, nitric oxide; Ox‐LDL, oxidized low‐density lipoprotein; RNS, reactive nitrogen species; ROS, reactive oxygen species; SMC, smooth muscle cell.
Later, fibrosis and calcification occur in these lesions, yielding a fibrous cap that surrounds a lipid‐rich core. Sometimes the fibrous plaque ruptures with the formation of thrombi. Subsequent reduction in blood flow is thought to be the cause of acute coronary syndromes. The role of inflammation in atherogenesis has also been reviewed recently by Pant et al, who concluded that oxidative stress and inflammation are intertwined processes.14
Oxidative Stress and Atherosclerosis: Role of Reactive Oxygen and Reactive Nitrogen Species
The state of oxidative stress in the body is produced by reactive oxygen species (ROS), which are byproducts of the aerobic cellular metabolism. This release of ROS beyond the body's ability to scavenge them, resulting in “oxidative stress,” has been implicated in the pathophysiology of atherosclerotic disease process.15, 16 Oxidative stress contributes to atherogenesis by mechanisms that are multifactorial, complex, and intricate, and not necessarily linked to LDL oxidation. For example, ROS such as superoxide anion can rapidly inactivate nitric oxide (NO) and form reactive nitrogen species (RNS), which injure endothelial cells and create a prothrombotic and pro‐inflammatory environment.17
Excessive formation of ROS activates intrinsic cell death pathways such as apoptosis.15 Reactive oxygen species also participate in diverse pathophysiological signaling pathways leading to mitochondrial DNA damage, activation of redox‐sensitive pathways, NLRP3 inflammasome activation, and expression of toll‐like receptors.18, 19
Furchgott et al first showed that endothelial cells play a vital role in the relaxation of arterial SMCs in response to acetylcholine.20 Later this endothelium‐derived relaxing factor was found to be NO, which, besides being a potent vasodilator, has antithrombotic and anti‐inflammatory properties.21, 22, 23, 24, 25, 26 The endothelial injury with decreased production of NO and oxidative stress leading to the formation of RNS is central to the concept of endothelial injury and atherosclerosis.21 Especially important is the role of superoxide radical, which can combine with NO to form peroxynitrite, a potent free radical, and RNS.21 Peroxynitrite oxidizes tetrahydrobiopterin (BH4), which is a major cofactor for the activation of endothelial nitric oxide synthase (eNOS).27 In fact, relative deficiency of BH4 has been proposed to contribute to endothelial dysfunction in some conditions.28
Almost all cells in the body have both enzymatic and nonenzymatic mechanisms to protect against the toxic effects of ROS and RNS. The enzymatic mechanisms for cellular protection include the antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase. The nonenzymatic antioxidants include glutathione, ascorbate, and tocopherols.
Role of Vitamin Tocopherols in Atherosclerosis
Tocopherols: Pharmacology
Natural vitamin E consists of a family of 8 different compounds, 4 tocopherols and 4 tocotrienols. They are named α‐, β‐, γ‐, and δ‐tocopherol and α‐, β‐, γ‐, and δ‐tocotrienol. All these members of the vitamin E family have a head known as a chromanol ring and tail called a phytyl tail.29 The chromanol ring has a hydroxyl group and 2 methyl groups, whose position is different in each type of tocopherol. The difference between tocopherols and tocotrienols lies in the tail region, as the latter have 3 double bonds in their phytyl tails. All tocopherols and tocotrienols are potent antioxidants with lipoperoxyl radical‐scavenging activities. The oxidant species–scavenging action of the tocopherols is caused by donating the hydrogen ion from the phenol group on the chromanol ring. The structural differences between different tocopherols are shown in Figure 2.
Figure 2.
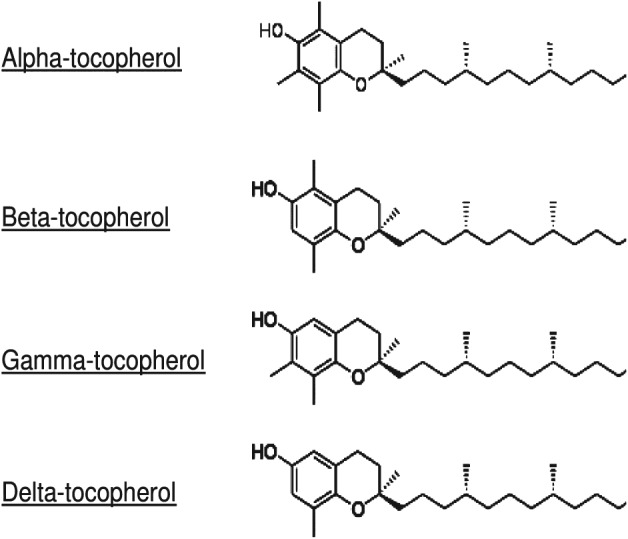
Chemical structure of the different tocopherols.
Until recently, most research on tocopherols has focused mainly on α‐tocopherol, because it is the predominant form of vitamin E and low intake of α‐tocopherol is associated with peripheral neuropathy and ataxia.30 There is accumulating information on a role for all members of the vitamin E family in health and disease. Here, we will focus primarily on tocopherols in the context of atherosclerosis and related CV disorders.
The natural sources of vitamin E are fruits, vegetables, and nuts. α‐Tocopherol is found in peanuts, almonds, and sunflower seeds, and γ‐tocopherol is predominantly found in walnuts, pecans, pistachios, and sesame seeds.29 The main sources of β‐tocopherol and δ‐tocopherol are corn, corn oil, and rapeseed oil. Other good sources of δ‐tocopherol are tomato seeds, rice germ, and soybean oil. γ‐Tocopherol derived from corn oil and soybean oil is the major constituent of vitamin E in the American diet.
Tocopherols are absorbed along with dietary fat in the intestine and are secreted as chylomicron particles. The chylomicron particles bound tocopherols are transported to adipose tissue, skin, muscle, bone marrow, and brain. α‐Tocopherol is preferentially bound to α‐tocopherol transfer protein, which protects it from catabolic enzymes in the liver. Other tocopherols, especially γ‐, β‐, and δ‐tocopherol, undergo ω‐hydroxylation, oxidation, and β‐oxidation in the liver to generate 13′ hydroxychromanols/carboxychromanols,29 which have potent antioxidant properties and strong radical‐scavenging action. These metabolites have been shown to inhibit cyclooxygenase (COX)‐2 and 5‐lipoxygenase pathways more strongly than the unmetabolized forms. This might be the reason for stronger anti‐inflammatory and antioxidant action of γ‐tocopherol than α‐tocopherol.29 γ‐Tocopherol has a unique unsubstituted C‐5 position to trap electrophiles, including RNS. α‐Tocopherol has a methyl group at this position and as such does not possess this property (Figure 2). As a result, γ‐tocopherol is superior to α‐tocopherol in detoxifying nitroso compounds and peroxynitrite via formation of 5‐nitro‐γ‐tocopherol.31, 32
The major effects of tocopherols on the biology of atherosclerosis are shown in Figure 1. α‐Tocopherol decreases lipid peroxidation, monocyte proatherogenicity, and platelet aggregation.33 It can modulate inflammatory response by inhibiting 5‐lipoxygenase, which in turn decreases monocyte released interleukin‐1β release. It also decreases monocyte adhesion to endothelial cells in vitro, possibly by inhibiting nuclear factor‐κB activation.34 α‐Tocopherol inhibits protein kinase C (PKC)‐mediated monocyte superoxide production, SMC proliferation, and platelet aggregation and adhesion.35, 36 Another key function regulated by α‐tocopherol is vascular homeostasis. Normal vascular function requires availability of NO and responsiveness to NO. α‐Tocopherol enhances NO production, and its supplementation in hypercholesterolemic men and smokers preserves endothelium‐dependent vasorelaxation.37 Notably, all these properties are exhibited by γ‐tocopherol as well, and in a more potent fashion.
There are other nonantioxidant functions of tocopherols that were first highlighted by Boscoboinik et al in the 1990s.38 They found that proliferation of vascular SMCs was inhibited by physiologically relevant concentrations of α‐tocopherol via inhibition of PKC.38 Less is known about the β‐ and δ‐tocopherols, but δ‐tocopherol is also considered to have potent anti‐inflammatory and antioxidant action. It also has unique actions of inhibition of COX‐1, COX‐2, and 5‐lipoxygenase. The salient features of different tocopherols are described in Table 1.
Table 1.
Properties of Different Tocopherols
| α‐Tocopherol | Decreases lipid peroxidation, monocyte proatherogenicity, and platelet aggregation.33, 34 |
| Modulates inflammatory response by inhibiting 5‐lipoxygenase, which in turn decreases monocyte IL‐1β release.33, 34 | |
| Decreases monocyte adhesion to ECs in vitro, possibly by inhibiting NF‐κB activation.34 | |
| Inhibits PKC‐mediated monocyte superoxide production, SMC proliferation, and platelet aggregation and adhesion.35, 36 | |
| γ‐Tocopherol | Has most of the effects of α‐tocopherol and is more potent.58, 69, 71 |
| Has additional effect of removing peroxynitrite‐derived RNS.27 | |
| Acts in vivo as a trap for membrane‐soluble electrophilic NOs and other electrophilic mutagens.31 | |
| Has an unique unsubstituted C‐5 position to trap electrophiles, which are enhanced during inflammation. As a result, γ‐tocopherol is superior to α‐tocopherol in detoxifying NO2 and peroxynitrite via formation of 5‐nitro‐γ‐tocopherol.31 | |
| Inhibits SMC proliferation.34 | |
| Decreases platelet aggregation and delays intra‐arterial thrombus formation.58, 62 | |
| β‐Tocopherol | Has anti‐inflammatory and antioxidant action, but less is known about it. |
| δ‐Tocopherol | Has potent anti‐inflammatory and antioxidant action. Inhibits COX‐1, COX‐2, and 5‐lipoxygenase. Inhibits SMC proliferation. |
Abbreviations: COX, cyclooxygenase; EC, endothelial cell; IL‐1β, interleukin‐1β; NF‐κB, nuclear factor κB; NO, nitrogen oxide; NO2, nitroso compounds; PKC, protein kinase C; RNS, reactive nitrogen species; SMC, smooth muscle cell.
Role of Tocopherols in Atherosclerosis‐Related Disorders; Failure of α‐Tocopherol
In the early 1990s, a potential beneficial role of antioxidants in the therapy of atherosclerosis‐related CVD was postulated. This postulate was based on the premise that the adverse effects of oxidative stress, such as peroxidation of lipid molecules as a result of excess ROS/RNS production, could be countered by dietary supplementation with α‐tocopherol.
Indeed, Reaven et al39 in 1993 studied the role of dietary antioxidant supplementation in humans and found that α‐tocopherol supplementation (1600 mg/d) caused greater protection of LDL from oxidation in in vitro assays. They found that in subjects that took α‐tocopherol, LDL susceptibility to oxidation was decreased by 50%.39 The observations that intake of fresh fruits and vegetables, which are rich in antioxidants, protected against CVD events40, 41 provided further support for the use of antioxidants in patients with CVD. This hypothesis also gained support from the success, although limited and variable, in reduction of atherosclerosis in animal models of atherosclerosis with the use of α‐tocopherol.40, 41, 42
Subsequently, clinical trials were conducted with the expectation that compounds such as α‐tocopherol would reduce atherosclerosis‐related events without adverse effects. Table 2 highlights some of the major clinical trials conducted with α‐tocopherol.
Table 2.
Clinical Trials of α‐Tocopherol in CV Health and Atherosclerosis
| CHAOS demonstrated that treatment with α‐tocopherol (400–800 mg/d) reduced the risk of nonfatal MI in patients with angiographically proven coronary atherosclerosis.43 |
| The SPACE study in patients with CKD showed that α‐tocopherol (800 mg/d) administration significantly reduced the composite endpoint of MI (fatal and nonfatal), ischemic stroke, PVD (excluding AV fistula), and UA.44 |
| The GISSI trial showed α‐tocopherol administration had no effect on CV outcomes in patients with acute MI.46 |
| The HOPE study showed that 400 IU of α‐tocopherol administered daily for 4 to 6 years had no beneficial effects on CV outcomes in a high‐risk older patient population.49 |
Abbreviations: AV, arteriovenous; CHAOS, Cambridge Heart Antioxidant Study; CKD, chronic kidney disease; CV, cardiovascular; GISSI, Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico; HOPE, Heart Outcomes Prevention Evaluation; MI, myocardial infarction; PVD, peripheral vascular disease; SPACE, Secondary Prevention With Antioxidants of Cardiovascular Disease in End‐stage Renal Disease; UA, unstable angina.
In 1996, the Cambridge Heart Antioxidant Study (CHAOS) demonstrated that α‐tocopherol (400–800 mg/d) significantly reduced the risk of nonfatal myocardial infarction (MI) in patients with angiographically proven coronary atherosclerosis; however, there was a nonsignificant excess of CV deaths in the α‐tocopherol group.43
Subsequently, the Secondary Prevention With Antioxidants of Cardiovascular Disease in End‐stage Renal Disease (SPACE) study, reported in 2000, showed that the administration of α‐tocopherol (800 mg/d) significantly reduced the composite endpoint, consisting of MI (fatal and nonfatal), ischemic stroke, peripheral vascular disease (excluding arteriovenous fistula), and unstable angina.44
The Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study reported in 2003 was designed to study the effect of 136 IU of α‐tocopherol plus 250 mg of vitamin C twice daily on the progression of carotid atherosclerosis in patients with hypercholesterolemia. Dietary supplementation with α‐tocopherol and vitamin C was found to significantly decrease the rate of progression of carotid intima‐media thickness. This beneficial effect was confined to men only.45
However, in another large‐scale randomized trial, the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI) trial, α‐tocopherol administration had no effect on CV outcomes in patients with acute MI.46 Rapola et al also observed not only lack of benefit of α‐tocopherol (50 mg/d), β‐carotene (20 mg/d), or both, but a significant increase in CV deaths in patients with previous MI who received β‐carotene alone or both α‐tocopherol and β‐carotene.47, 48 Similar to the results of the GISSI trial, the Heart Outcomes Prevention Evaluation (HOPE) study49 found that 400 IU of α‐tocopherol administered daily had no beneficial effects on CV outcomes in a high‐risk older patient population.
Thus, on balance, these studies show no definitive evidence of benefit of α‐tocopherol for secondary prevention of CV events.50
Controversy Regarding the Role of Different Components of Vitamin E; Role of γ‐Tocopherol
Despite the conflicting results in clinical trials on the role of α‐tocopherol in CVD, work in this field continues (Table 3). In particular, there has been a shift in emphasis previously given to α‐tocopherol toward other members of vitamin E family, especially γ‐tocopherol, based on in vitro and in vivo studies.
Table 3.
Studies With γ‐Tocopherol Affecting the Biology of Atherosclerosis
| Christen et al and Jiang et al postulated that γ‐tocopherol acts in vivo as a trap for membrane‐soluble electrophilic NOs and other electrophilic mutagens, forming stable carbon‐centered adducts through the nucleophilic 5‐position.31, 51 |
| γ‐Tocopherol supplementation maintained and improved vascular endothelial function impaired by postprandial hyperglycemia and smoking, probably by decreasing pro‐inflammatory mediators TNF‐α and myeloperoxidase.72, 73, 74 |
| Devaraj et al found that γ‐tocopherol supplementation alone and in combination with α‐tocopherol, but not α‐tocopherol alone, reduced biomarkers of oxidative stress in patients with metabolic syndrome.71 |
| Vucinic et al found that dietary supplementation with γ‐tocopherol or a γ‐tocopherol‐rich mixture, but not α‐tocopherol, attenuated exercise‐increased coagulation and platelet aggregation.66 |
| Liu et al showed that a mixed tocopherol preparation (mixture of α‐, γ‐, and δ‐tocopherols) was more potent than α‐tocopherol alone in inhibiting platelet aggregation in humans, lipid peroxidation in human erythrocytes, and inactivation of cNOS in human leukocytes after 8 weeks of tocopherol supplementation.61, 62, 63 |
| Cooney et al showed that γ‐tocopherol was more potent than α‐tocopherol in decreasing nitrosative stress, and it reacted with nitrogen dioxide to produce NO, whereas α‐tocopherol formed an intermediate analog, such as quinone, in vitro.32 |
| Ohrvall et al suggested that it is the plasma levels of γ‐tocopherol, and not α‐tocopherol, that are low in patients with CAD.64 |
| 13′ hydroxychromanols/carboxychromanols, major metabolites of ω‐hydroxylation and β‐oxidation of γ‐tocopherol, have potent antioxidant properties and strong radical‐scavenging actions. These metabolites also inhibit COX‐2 and 5‐lipoxygenase pathways.29 They also contribute to the more potent antioxidant action of γ‐tocopherol. |
Abbreviations: CAD, coronary artery disease; COX, cyclooxygenase; NO, nitrogen oxide; cNOS, endothelial nitric oxide synthase; TNF‐α, tumor necrosis factor‐α.
Christen et al31 and Jiang et al51 postulated that γ‐tocopherol acts in vivo as a trap for membrane‐soluble electrophilic nitrogen oxides and other electrophilic mutagens, forming stable carbon‐centered adducts. Jiang et al52, 53 showed that γ‐tocopherol was more potent than α‐tocopherol in reducing 8‐isoprostane, one of the markers of lipid peroxidation formation in activated macrophages. They showed that large doses of dietary α‐tocopherol actually displaced γ‐tocopherol in tissues. These observations were in accordance with the results of a previous study54 that showed administration of α‐tocopherol for 14 days resulted in a significant decrease in serum γ‐tocopherol levels in healthy subjects and in patients with end‐stage renal disease. It is likely that displacement of γ‐tocopherol from the lipid‐cell membranes by α‐tocopherol potentially causes the adverse effects seen in some clinical trials.55, 56
Tocopherols were found to decrease platelet aggregation in various studies. Freedman et al57 in 1996 published that α‐tocopherol inhibited platelet aggregation by a PKC‐dependent mechanism. Saldeen et al58 in 1999 observed that although both α‐tocopherol and γ‐tocopherol decreased platelet aggregation and delayed thrombus formation, γ‐tocopherol was significantly more potent than α‐tocopherol in these effects. Platelet aggregation is thought to be central to the atherosclerotic disease process.59, 60 Later, Liu et al showed that a mixed tocopherol preparation (mixture of α‐, γ‐ and δ‐tocopherols) given for 8 weeks was more potent than α‐tocopherol alone in inhibiting platelet aggregation in humans61 and lipid peroxidation in erythrocytes,62 and in activation of endothelial cNOS in leukocytes.63
Notably, in 1996, Ohrvall et al64 had suggested that it is the plasma levels of γ‐tocopherol, and not α‐tocopherol, that are low in patients with CVD. Kontush et al65 in 1999 published that plasma levels of γ‐tocopherol were significantly lower in CVD patients compared with control subjects. However, there was no decrease in plasma α‐tocopherol levels in these patients. Vucinic et al66 also found that dietary supplementation with γ‐tocopherol or a γ‐tocopherol‐rich mixture, but not α‐tocopherol, attenuated exercise‐increased coagulation and platelet aggregation. Cooney et al32 showed that γ‐tocopherol was more potent than α‐tocopherol in decreasing nitrosative stress, and it reacted with nitrogen dioxide to produce NO, whereas α‐tocopherol formed an intermediate analog, such as the quinone, in vitro.
As the critical role of oxidized LDL in atherogenesis began to unfold, it was identified that macrophages imbibe oxidized LDL via scavenger receptors CD36 and SR‐AI/II and change into foam cells. Ricciarelli et al67, 68 found that α‐tocopherol reduced the expression of CD36 and that its beneficial effect against atherosclerosis could be explained, at least in part, by its effect of lowering the uptake of oxidized lipoproteins with consequent reduction of foam‐cell formation. Li et al observed that γ‐tocopherol reduced oxidized LDL‐mediated apoptosis of vascular endothelial cells by inhibiting the activation of NF‐κB.69 This effect was also observed by de Nigris et al.70
Data on the superior effect of γ‐tocopherol in preventing endothelial injury, lipid peroxidation, and oxidative stress have been accumulating. Devaraj et al71 found that γ‐tocopherol alone and in combination with α‐tocopherol, but not α‐tocopherol alone, significantly reduced biomarkers of oxidative stress in patients with metabolic syndrome. A host of other investigators32, 72, 73 has demonstrated an important and potent inhibitory effect of γ‐tocopherol on oxidative stress and platelet aggregation. Others73, 74 have shown that γ‐tocopherol maintains and improves vascular endothelial function that is otherwise impaired by postprandial hyperglycemia and smoking, probably by decreasing pro‐inflammatory mediators tumor necrosis factor‐α and myeloperoxidase. Still others, Wade et al,75 described that dietary supplementation with α‐tocopherol (400 IU/d) oxidized high‐density lipoprotein (HDL)‐2 and HDL‐3, a clearly proatherogenic effect.
It is surprising that although the potentially beneficial effects of γ‐tocopherol were described by so many investigators as early as 1996, no major study has yet examined the role of γ‐tocopherol in patients with CVD.
γ‐Tocopherol Is a Better Candidate for Antioxidant Effects
From the above discussion, it is apparent that γ‐tocopherol is better than α‐tocopherol in preventing formation of lipid peroxides, counteracting the effect of RNS and oxidative stress in the pathophysiology of atherosclerosis. γ‐Tocopherol plays an important role in preserving endothelial function by protecting degradation of BH4, a key cofactor in the synthesis of NO. Further, γ‐tocopherol has superior pharmacokinetic properties as it undergoes omega hydroxylation, oxidation, and β‐oxidation in the liver to generate 13′ hydroxychromanols/carboxychromanols. These molecules have potent antioxidant properties and strong radical‐scavenging actions. These metabolites also inhibit COX‐2 and 5‐lipoxygenase pathways. α‐Tocopherol is primarily bound to α‐tocopherol transfer protein and does not undergo this metabolism in the body.
Future Directions
In recent years, research on vitamin E has expanded from primarily focusing on α‐tocopherol to investigation of different tocopherols, their metabolism, and their antioxidant and anti‐inflammatory properties. It is important to recall that α‐tocopherol preparations were not effective in clinical trials and gave conflicting results. Now it is evident that both α‐tocopherol and γ‐tocopherol compete for the same transport proteins. Therefore, supplementation with α‐tocopherol may prevent uptake of dietary γ‐tocopherol. Additionally, α‐tocopherol supplementation may inhibit γ‐tocopherol levels in various tissues, such as heart, skin, aorta, and perirenal adipose tissue. With this knowledge of the superior pharmacologic activity of γ‐tocopherol, the time has come to explore the role of γ‐tocopherol in coronary heart disease.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Duff GL, McMillan GC. Pathology of atherosclerosis. Am J Med. 1951;11:92–108. [DOI] [PubMed] [Google Scholar]
- 2. Gofman JW, Jones HB, Lindgren FT, et al. Blood lipids and human atherosclerosis. Circulation. 1950;2:161–178. [DOI] [PubMed] [Google Scholar]
- 3. Gofman JW, Lindgren FT, Elliot H. Ultracentrifugal studies of lipoproteins of human serum. J Biol Chem. 1949;179:973–979. [PubMed] [Google Scholar]
- 4. Steinberg D. Research related to underlying mechanisms in atherosclerosis. Circulation. 1979;60:1559–1565. [DOI] [PubMed] [Google Scholar]
- 5. Henriksen T, Mahoney EM, Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981;78:6499–6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parthasarathy S, Quinn MT, Steinberg D. Is oxidized low density lipoprotein involved in the recruitment and retention of monocyte/macrophages in the artery wall during the initiation of atherosclerosis? Basic Life Sci. 1988;49:375–380. [DOI] [PubMed] [Google Scholar]
- 7. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 8. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. [DOI] [PubMed] [Google Scholar]
- 9. Kummerow FA, Olinescu RM, Fleischer L, et al. The relationship of oxidized lipids to coronary artery stenosis. Atherosclerosis. 2000;149:181–190. [DOI] [PubMed] [Google Scholar]
- 10. Mehta JL. Oxidized or native low‐density lipoprotein cholesterol: which is more important in atherogenesis? J Am Coll Cardiol. 2006;48:980–982. [DOI] [PubMed] [Google Scholar]
- 11. Mehta JL, Saldeen TG, Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol. 1998;31:1217–1225. [DOI] [PubMed] [Google Scholar]
- 12. Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. [DOI] [PubMed] [Google Scholar]
- 13. Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. [DOI] [PubMed] [Google Scholar]
- 14. Pant S, Deshmukh A, Gurumurthy GS, et al. Inflammation and atherosclerosis—revisited. J Cardiovasc Pharmacol Ther. 2014;19:170–178. [DOI] [PubMed] [Google Scholar]
- 15. Victor VM, Rocha M, Solá E, et al. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des. 2009;15:2988–3002. [DOI] [PubMed] [Google Scholar]
- 16. Hink U, Li H, Mollnau H, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. [DOI] [PubMed] [Google Scholar]
- 17. Landmesser U, Harrison DG. Oxidant stress as a marker for cardiovascular events: Ox marks the spot. Circulation. 2001;104:2638–2640. [PubMed] [Google Scholar]
- 18. Ding Z, Liu S, Wang X, et al. LOX‐1, mtDNA damage, and NLRP3 inflammasome activation in macrophages: implications in atherogenesis. Cardiovasc Res. 2014;103:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sugamura K, Keaney JF Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. [DOI] [PubMed] [Google Scholar]
- 21. Anderson TJ. Assessment and treatment of endothelial dysfunction in humans. J Am Coll Cardiol. 1999;34:631–638. [DOI] [PubMed] [Google Scholar]
- 22. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357(part 3):593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gutstein DE, Fuster V. Pathophysiology and clinical significance of atherosclerotic plaque rupture. Cardiovasc Res. 1999;41:323–333. [DOI] [PubMed] [Google Scholar]
- 24. Esper RJ, Nordaby RA, Vilariño JO, et al. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wennmalm A. Endothelial nitric oxide and cardiovascular disease. J Intern Med. 1994;235:317–327. [DOI] [PubMed] [Google Scholar]
- 26. Bretón‐Romero R, Acín‐Perez R, Rodríguez‐Pascual F, et al. Laminar shear stress regulates mitochondrial dynamics, bioenergetics responses and PRX3 activation in endothelial cells. Biochim Biophys Acta. 2014;1843:2403–2413. [DOI] [PubMed] [Google Scholar]
- 27. McCarty MF. Gamma‐tocopherol may promote effective NO synthase function by protecting tetrahydrobiopterin from peroxynitrite. Med Hypotheses. 2007;69:1367–1370. [DOI] [PubMed] [Google Scholar]
- 28. Stroes E, Kastelein J, Erkelens W, et al. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti‐inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sokol RJ. Vitamin E and neurologic deficits. Adv Pediatr. 1990;37:119–148. [PubMed] [Google Scholar]
- 31. Christen S, Woodall AA, Shigenaga MK, et al. Gamma‐tocopherol traps mutagenic electrophiles such as NO(x) and complements alpha‐tocopherol: physiological implications. Proc Natl Acad Sci U S A. 1997;94:3217–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cooney RV, Franke AA, Harwood PJ, et al. Gamma‐tocopherol detoxification of nitrogen dioxide: superiority to alpha‐tocopherol. Proc Natl Acad Sci U S A. 1993;90:1771–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaul N, Devaraj S, Jialal I. Alpha‐tocopherol and atherosclerosis. Exp Biol Med (Maywood). 2001;226:5–12. [DOI] [PubMed] [Google Scholar]
- 34. Devaraj S, Jialal I. The effects of alpha‐tocopherol on crucial cells in atherogenesis. Curr Opin Lipidol. 1998;9:11–15. [DOI] [PubMed] [Google Scholar]
- 35. Ricciarelli R, Tasinato A, Clément S, et al. Alpha‐tocopherol specifically inactivates cellular protein kinase C alpha by changing its phosphorylation state. Biochem J. 1998;334(part 1):243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keaney JF Jr, Simon DI, Freedman JE. Vitamin E and vascular homeostasis: implications for atherosclerosis. FASEB J. 1999;13:965–975. [DOI] [PubMed] [Google Scholar]
- 37. Heitzer T, Ylä Herttuala S, Wild E, et al. Effect of vitamin E on endothelial vasodilator function in patients with hypercholesterolemia, chronic smoking or both. J Am Coll Cardiol. 1999;33:499–505. [DOI] [PubMed] [Google Scholar]
- 38. Boscoboinik D, Szewczyk A, Hensey C, et al. Inhibition of cell proliferation by alpha‐tocopherol: role of protein kinase C. J Biol Chem. 1991;266:6188–6194. [PubMed] [Google Scholar]
- 39. Reaven PD, Khouw A, Beltz WF, et al. Effect of dietary antioxidant combinations in humans: protection of LDL by vitamin E but not beta‐carotene. Arterioscler Thromb. 1993;13:590–600. [DOI] [PubMed] [Google Scholar]
- 40. O'Keefe JH Jr, Lavie CJ Jr, McCallister BD. Insights into the pathogenesis and prevention of coronary artery disease. Mayo Clin Proc. 1995;70:69–79. [DOI] [PubMed] [Google Scholar]
- 41. Hennig B, Boissonneault GA, Wang Y. Protective effects of vitamin E in age‐related endothelial cell injury. Int J Vitam Nutr Res. 1989;59:273–279. [PubMed] [Google Scholar]
- 42. Wallert M, Schmölz L, Galli F, et al. Regulatory metabolites of vitamin E and their putative relevance for atherogenesis. Redox Biol. 2014;2:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stephens NG, Parsons A, Schofield PM, et al. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet. 1996;347:781–786. [DOI] [PubMed] [Google Scholar]
- 44. Boaz M, Smetana S, Weinstein T, et al. Secondary prevention with antioxidants of cardiovascular disease in end‐stage renal disease (SPACE): randomised placebo‐controlled trial. Lancet. 2000;356:1213–1218. [DOI] [PubMed] [Google Scholar]
- 45. Salonen RM, Nyyssönen K, Kaikkonen J, et al. Six‐year effect of combined vitamin C and E supplementation on atherosclerotic progression: ASAP study. Circulation. 2003;107:947–953. [DOI] [PubMed] [Google Scholar]
- 46.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial [published corrections appear in Lancet 2001;357:642 and Lancet 2007;369:106]. Lancet. 1999;354:447–455. [PubMed]
- 47. Rapola JM, Virtamo J, Ripatti S, et al. Effects of alpha‐tocopherol and beta carotene supplements on symptoms, progression, and prognosis of angina pectoris. Heart. 1998;79:454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rapola JM, Virtamo J, Ripatti S, et al. Randomised trial of alpha‐tocopherol and beta‐carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet. 1997;349:1715–1720. [DOI] [PubMed] [Google Scholar]
- 49. Yusuf S, Dagenais G, Pogue J, et al; Heart Outcomes Prevention Evaluation Study Investigators . Vitamin E supplementation and cardiovascular events in high‐risk patients. N Engl J Med. 2000;342:154–160. [DOI] [PubMed] [Google Scholar]
- 50. Vivekananthan DP, Penn MS, Sapp SK, et al. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta‐analysis of randomised trials [published correction appears in Lancet. 2004;363:662]. Lancet. 2003;361:2017–2023. [DOI] [PubMed] [Google Scholar]
- 51. Jiang Q, Lykkesfeldt J, Shigenaga MK, et al. Gamma‐tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med. 2002;33:1534–1542. [DOI] [PubMed] [Google Scholar]
- 52. Jiang Q, Ames BN. Gamma‐tocopherol, but not alpha‐tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. [DOI] [PubMed] [Google Scholar]
- 53. Jiang Q, Elson‐Schwab I, Courtemanche C, et al. Gamma‐tocopherol and its major metabolite, in contrast to alpha‐tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97:11494–11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Himmelfarb J, Kane J, McMonagle E, et al. Alpha and gamma‐tocopherol metabolism in healthy subjects and patients with end‐stage renal disease. Kidney Int. 2003;64:978–991. [DOI] [PubMed] [Google Scholar]
- 55. Handelman GJ, Machlin LJ, Fitch K, et al. Oral alpha‐tocopherol supplements decrease plasma gamma‐tocopherol levels in humans. J Nutr. 1985;115:807–813. [DOI] [PubMed] [Google Scholar]
- 56. Baker H, Handelman GJ, Short S, et al. Comparison of plasma alpha and gamma‐tocopherol levels following chronic oral administration of either all‐rac‐alpha‐tocopheryl acetate or RRR‐alpha‐tocopheryl acetate in normal adult male subjects. Am J Clin Nutr. 1986;43:382–387. [DOI] [PubMed] [Google Scholar]
- 57. Freedman JE, Farhat JH, Loscalzo J, et al. Alpha‐tocopherol inhibits aggregation of human platelets by a protein kinase C–dependent mechanism. Circulation. 1996;94:2434–2440. [DOI] [PubMed] [Google Scholar]
- 58. Saldeen T, Li D, Mehta JL. Differential effects of alpha‐ and gamma‐tocopherol on low‐density lipoprotein oxidation, superoxide activity, platelet aggregation and arterial thrombogenesis [published correction appears in J Am Coll Cardiol. 2000;35:263]. J Am Coll Cardiol. 1999;34:1208–1215. [DOI] [PubMed] [Google Scholar]
- 59. Conti CR, Mehta JL. Acute myocardial ischemia: role of atherosclerosis, thrombosis, platelet activation, coronary vasospasm, and altered arachidonic acid metabolism. Circulation. 1987;75(6 part 2):V84–V95. [PubMed] [Google Scholar]
- 60. Fuster V, Badimon L, Badimon JJ, et al. The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med. 1992;23;326:242–250. [DOI] [PubMed] [Google Scholar]
- 61. Liu M, Wallmon A, Olsson‐Mortlock C, et al. Mixed tocopherols inhibit platelet aggregation in humans: potential mechanisms. Am J Clin Nutr. 2003;77:700–706. [DOI] [PubMed] [Google Scholar]
- 62. Liu M, Wallin R, Wallmon A, et al. Mixed tocopherols have a stronger inhibitory effect on lipid peroxidation than alpha‐tocopherol alone. J Cardiovasc Pharmacol. 2002;39:714–721. [DOI] [PubMed] [Google Scholar]
- 63. Liu M, Wallin R, Saldeen T. Effect of mixed tocopherols on eNOS, SOD and PKC in leukocytes in human subjects. Nutr Res. 2002;22:1253–1263. [Google Scholar]
- 64. Ohrvall M, Sundlöf G, Vessby B. Gamma, but not alpha, tocopherol levels in serum are reduced in coronary disease patients. J Intern Med. 1996;239:111–117. [DOI] [PubMed] [Google Scholar]
- 65. Kontush A, Spranger T, Reich A, et al. Lipophilic antioxidants in blood plasma as markers of atherosclerosis: the role of alpha‐carotene and gamma tocopherol. Atherosclerosis. 1999;144:117–122. [DOI] [PubMed] [Google Scholar]
- 66. Vucinic L, Singh I, Spargo FJ, et al. Gamma‐tocopherol supplementation prevents exercise‐induced coagulation and platelet aggregation. Thromb Res. 2010;125:196–199. [DOI] [PubMed] [Google Scholar]
- 67. Ricciarelli R, Azzi A. Regulation of recombinant PKC alpha activity by protein phosphatase 1 and protein phosphatase 2A. Arch Biochem Biophys. 1998;355:197–200. [DOI] [PubMed] [Google Scholar]
- 68. Ricciarelli R, Zingg JM, Azzi A. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation. 2000;102:82–87. [DOI] [PubMed] [Google Scholar]
- 69. Li D, Saldeen T, Mehta JL. Gamma‐tocopherol decreases ox‐LDL‐mediated activation of nuclear factor‐kappaB and apoptosis in human coronary artery endothelial cells. Biochem Biophys Res Commun. 1999;259:157–161. [DOI] [PubMed] [Google Scholar]
- 70. de Nigris F, Franconi F, Maida I, et al. Modulation by alpha‐ and gamma‐tocopherol and oxidized low‐density lipoprotein of apoptotic signaling in human coronary smooth muscle cells. Biochem Pharmacol. 2000;59:1477–1487. [DOI] [PubMed] [Google Scholar]
- 71. Devaraj S, Leonard S, Traber MG, et al. Gamma‐tocopherol supplementation alone and in combination with alpha‐tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic Biol Med. 2008;44:1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mah E, Noh SK, Ballard KD, et al. Supplementation of a γ‐tocopherol‐rich mixture of tocopherols in healthy men protects against vascular endothelial dysfunction induced by postprandial hyperglycemia. J Nutr Biochem. 2013;24:196–203. [DOI] [PubMed] [Google Scholar]
- 73. Bruno RS, Traber MG. Vitamin E biokinetics, oxidative stress and cigarette smoking. Pathophysiology. 2006;13:143–149. [DOI] [PubMed] [Google Scholar]
- 74. Singh I, Turner AH, Sinclair AJ, et al. Effects of gamma‐tocopherol supplementation on thrombotic risk factors. Asia Pac J Clin Nutr. 2007;16:422–428. [PubMed] [Google Scholar]
- 75. Wade L, Nadeem N, Young IS, et al. α‐Tocopherol induces proatherogenic changes to HDL2 and HDL3: an in vitro and ex vivo investigation. Atherosclerosis. 2013;226:392–397. [DOI] [PubMed] [Google Scholar]


