Abstract
Remote ischemic preconditioning (RIPC) has been studied in models of different cardiovascular entities. Recently, a beneficial effect of RIPC on incidence of atrial fibrillation (AF) in postsurgical patients has been suggested. However, the potential impact of RIPC on electrophysiological‐ and thrombogenesis‐related parameters in the setting of paroxysmal nonvalvular AF has not been investigated. The aim of the study is to answer the following questions: (1) Does RIPC have impact on inducibility of AF in patients with known paroxysmal AF? If yes, what are the direct electrophysiological mechanisms of this phenomenon, and could RIPC be implemented to reduce AF burden? (2) Does RIPC have the potential to minimize thrombogenic effects of simulated episodes of AF? If so, what are inhibited components of thrombogenesis and can this be used to reduce thromboembolic risk related to paroxysmal AF? The presented study is a 2‐arm, randomized, placebo‐controlled, double‐blinded, single‐center trial in a cohort of 146 patients with paroxysmal AF referred for AF ablation in sinus rhythm. The study will collect electrophysiological data such as variability of P‐wave morphology, atrial refractory period, conduction times, and inducibility/sustainability of AF. Furthermore, AF‐induced prothrombotic processes will be analyzed by quantification of platelet aggregates, analysis of platelet function, and measurement of thrombogenesis‐related plasma markers. Moreover, the study will provide a unique bio‐database for further analysis of molecular and genetic mechanisms responsible for observed results.
Keywords: atrial fibrillation, remote ischemic preconditioning, Electrophysiology, ablation, Stroke prevention
1. Introduction
Remote ischemic preconditioning (RIPC) has been shown to have significant impact on various physiological and genetic parameters in different experimental models. Several studies demonstrated pleiotropic effects of RIPC in a setting of acute myocardial ischemia in nonsurgical patients,1 renoprotective and neuroprotective effects,2, 3 and an impact on hemostasis parameters.4
Recent publications suggested a possible beneficial effect of RIPC on the incidence of atrial fibrillation (AF). Candilio et al observed a significant reduction (54%) in incidence of new‐onset AF after cardiac surgery.5 The results still demonstrated that the cardioprotective effect on atrial myocardium is not fully understood, and they also suggest a possible additive antiarrhythmic effect of RIPC.
The observed decrease in AF incidence might be explained on a molecular and genetic basis. In a recent study, Slagsvold et al also observed a significant reduction of AF in the RIPC group undergoing bypass surgery; they related it to a preservation of mitochondrial respiration and modulation in expression of several microRNAs (miRNAs), endogenous short RNA sequences that regulate further gene expression.6 Those particles recently gained scientific recognition because they might provide novel insights into the molecular basis of the pathogenesis of AF and have potential value as biomarkers due to relative stability and feasible detection in plasma and serum.
Although those initial reports are very encouraging, the possible impact of RIPC on electrophysiological properties of the left atrium has not yet been studied in patients with paroxysmal nonvalvular AF (NVAF).
Finally, the possible favorable effect of RIPC in patients with AF could be also expected in terms of thromboembolic protection.7 Recent analysis of platelet activation and reactivity induced by catheter ablation of paroxysmal AF demonstrated a significantly lower increase in all platelet variables, including monocyte‐platelet aggregate formation and ADP‐induced platelet response. These results imply that RIPC depletes platelet activation and reactivity prior to ablation. However, no studies exist that systematically analyze the influence of RIPC on prothrombotic mechanisms associated with AF.
The aim of this study is to examine the effect of RIPC on physiological parameters in paroxysmal NVAF.
The protocol is designed to answer of the following questions: (1) Does RIPC have an impact on inducibility of AF in patients with known paroxysmal AF? If yes, what are the direct electrophysiological mechanisms of this phenomenon, and could RIPC be implemented to reduce AF burden? (2) Does RIPC have the potential to minimize thrombogenic effects of simulated episodes of AF? If so, what are inhibited components of thrombogenesis, and can this be used to reduce thromboembolic risk related to paroxysmal AF?
2. Methods
2.1. Study Design
The presented study is a 2‐arm, randomized, placebo‐controlled, double‐blinded, single‐center trial in a cohort of 146 patients with paroxysmal AF referred for AF ablation in sinus rhythm.
The protocol is methodologically and conceptually divided into 3 stages:
-
Comparison of electrophysiological properties:
Variability of P‐wave morphology.
Atrial refractory period and conduction times.
Inducibility and sustainability of AF.
-
Analysis of AF‐induced prothrombotic processes:
Quantification of platelet aggregates and analysis of platelet function.
Measurement of thrombogenesis‐related plasma markers.
Collection of samples for further analysis of miRNAs, including explorative analysis.
The study is registered at http://www.ClinicalTrials.gov, no. NCT02779660.
2.2. Endpoints
The primary endpoints of the study are inducibility and sustainability of AF during electrophysiological study.
We consider irregular atrial activation in intracardial electrocardiograms lasting ≥30 seconds as induced AF, and AF lasting >10 minutes as sustained AF. Additionally, the duration of AF episodes, measured in seconds, will be compared between the groups. The differences in P‐wave morphology, atrial refractory periods, and conduction times measured during electrophysiological study will be analyzed.
Supplementary analysis will focus on the potential effect of RIPC on acute outcome of AF ablation.
To examine potential impact of RIPC on stroke risk, several markers (eg, P‐selectin, D‐dimer, and von Willebrand factor and platelets parameters) will be compared between the groups.
2.3. Sample Calculation
The trial is designed to be a proof‐of‐concept study, as there are no preliminary data regarding impact of RIPC on paroxysmal NVAF. Assuming AF inducibility of 90% in the control group and a 20% reduction in the RIPC group, the calculated sample size for a 95% confidence level of 0.95 and a power of 80% would be 146 patients (ie, 73 per arm).
2.4. Patients
After written consent, 146 consecutive patients referred for invasive AF treatment in sinus rhythm will be enrolled. Inclusion and exclusion criteria are summarized in the Table 1. The overview of the progress through the phases of the trial is depicted in a flow diagram (Figure 1).
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria for study participation: |
|---|
| Scheduled AF ablation procedure |
| SR in ECG at admission |
| Written informed consent |
| Exclusion criteria for study participation: |
| AF in ECG at the begin of EP study |
| Persistent or long‐standing type of AF |
| History of AF ablation |
| Use of antiplatelet agent |
| Suppressed level of TSH |
| SBP >200 mm Hg |
| Age <18 years |
| Pregnancy |
| Neoplastic disorders |
| Acute or systemic inflammation, autoimmune diseases |
Abbreviations: AF, atrial fibrillation; ECG, electrocardiogram; SBP, systolic blood pressure; SR, sinus rhythm; TSH, thyroid‐stimulating hormone.
Figure 1.
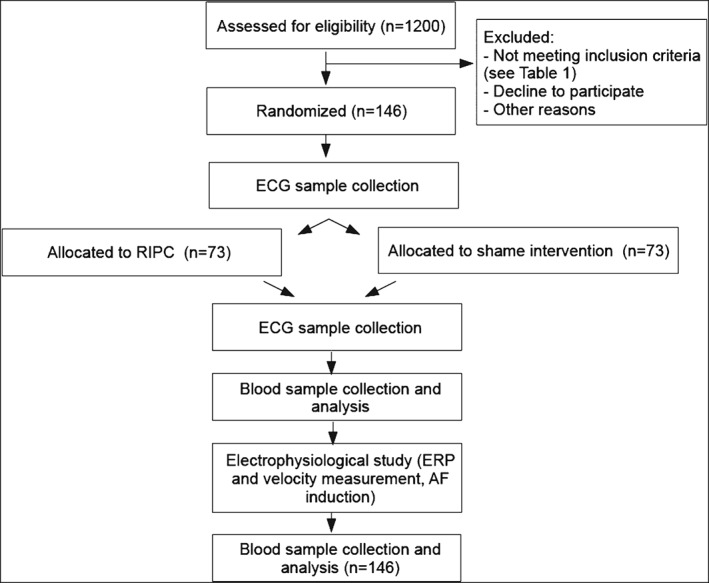
Flow diagram of the progress through the phases of the trial. Abbreviations: AF, atrial fibrillation; ECG, electrocardiogram; ERP, effective refractory period; RIPC, remote ischemic preconditioning.
2.5. Remote Ischemic Preconditioning
After randomization, patients will undergo 3 sessions of RIPC or a sham intervention: (1) on the preoperative day, (2) 1 hour before, and (3) directly before the blood‐sample collection and invasive measurement of electrophysiological parameters.
Remote ischemic preconditioning will be induced by the application of 3 short episodes (5 minutes) of forearm ischemia by cuff sphygmomanometer inflation (at 200 mm Hg) separated by 5 minutes of reperfusion. In the control group, the cuff will be inflated 3 times at 10 mm Hg for 5 minutes with 5‐minute intervals.
2.6. P‐Wave Variability
Prior to the first and after the last session of RIPC or sham intervention, a 12‐lead electrocardiogram (ECG) will be recorded, digitized, and stored for offline analysis. Beat‐to‐beat P‐wave morphology will be subsequently defined using a predefined classification algorithm.8 The most commonly observed P‐wave morphology in each sample would be defined as the dominant morphology. Finally, the P‐wave morphology will be defined as the percentage of P waves with nondominant morphology in the 10‐minute sample.
2.7. Preprocedural Imaging
Echocardiographic parameters such as anteroposterior left atrial diameter, left ventricular end‐diastolic diameters, left ventricular ejection fraction, and transmitral flow profile will be assessed prior to the procedure according to guidelines for standardized quality of chamber quantification.9 Additional preprocedural cardiac computed tomography (CT) imaging will be performed with a 64‐row multidetector helical system (Brilliance 64; Philips Medical Systems, Best, the Netherlands). End‐systolic imaging data will be recorded and used for 3‐dimensional cardiac reconstruction and assessment of atrial volume and anteroposterior left atrial diameter.
2.8. Electrophysiological Study
Intracardiac catheters will be positioned as follows: 10‐pole coronary sinus (CS) catheter (2–5‐2 mm interelectrode spacing), with the proximal bipole positioned at the CS ostium2; and a quadripolar catheter with 5‐mm interelectrode distance in the right ventricular apex. Bipolar intracardiac electrograms and a 12‐lead surface ECG will record simultaneously on a digital amplifier system.
Atrial effective refractory periods of both the right and left atrium will be evaluated at a pacing cycle length of 500 ms, with an 8‐beat drive followed by an extra stimulus with 10‐ms decrements. Furthermore, the conduction time along the CS in proximal to distal and distal to proximal directions at a cycle length of 500 ms will be analyzed.
Atrial fibrillation will be induced with pacing from CS at a cycle length of 300 ms for 20 seconds (if the induction does not succeed, the following adjustment of the sequence will be used: 250 ms, 200 ms, or cycle length of atrial refractoriness).10 After successful induction of sustained AF, an electrical cardioversion with 360 J will be conducted after a waiting period of 10 minutes.
The patient preparation, procedural settings, and AF ablation will be applied as previously described.11 In brief, patients are studied under deep propofol sedation with continuous invasive monitoring of arterial blood pressure and oxygen saturation. Nonfluoroscopic 3D catheter orientation, CT image integration, and tagging of the ablation sites will be performed using Ensite NavX, Ensite Velocity (St. Jude Medical, St. Paul, MN), or CARTO 3 (Biosense Webster, Diamond Bar, CA). Transseptal access and catheter navigation will be performed with a steerable sheath (Agilis; St. Jude Medical, St. Paul, MN). In all patients, circumferential left atrial ablation lines will be placed around the antrum of the ipsilateral pulmonary veins (irrigated tip catheter, preselected tip temperature of 48°C, and maximum power of 30–50 W). After circumferential line placement, voltage and pace mapping along the ablation line are used to identify and close gaps. The isolation of all pulmonary veins with bidirectional block will be verified with a multipolar circular mapping catheter and is defined as the procedural endpoint.
2.9. Analysis of MicroRNAs
Prior to the first session and after the last session of RIPC or sham intervention, a peripheral venous blood sample will be collected. Further analysis of miRNAs will only be conducted if a significant effect of RIPC on AF inducibility/sustainability and/or prothrombotic activation is observed. The analysis will be conducted as previously described.6 In brief, initial 20 samples of frozen plasma aliquots will be used for exploratory analysis of miRNAs on miRNA qPCR panels (752 human miRNAs on two 384‐well plates) including RNA extraction and qPCR‐based RNA quality control to assess the performance of samples prior to profiling. Screening of remaining plasma samples on custom miRNA qPCR panels will follow this stage. The panel content will be defined based on the results of the pilot screen (Exiqon, Vedbaek, Denmark).
2.10. Platelet Activation and Reactivity
Blood‐sample collection will be performed from the left atrium before induction and after termination of AF. Quantification of platelet aggregates and analysis of platelet function will be assessed by measuring platelet aggregates and the expression of the platelet receptors glycoprotein IIb/IIIa (CD41) and P‐selectin (CD62) by flow cytometry, as previously described.8, 9
For the purpose of analysis of plasma‐level thrombogenesis‐related plasma markers (P‐selectin, D‐dimer, and von Willebrand factor), commercially available specific enzyme‐linked immunosorbent assays will be used.
2.11. Timeline
Given the annual volume of approximately 1200 AF ablation procedures performed at Heart Center Leipzig, the number of eligible patients can be estimated at 300 per year. Therefore, the patient enrollment should be completed within 12 months after initiation, and final evaluation and reporting should be completed 4 months afterward.
Further analysis of the miRNAs will be initiated after proving the primary hypothesis and will be conducted within 3 months.
2.12. Data Accessibility and Publications
Patients included in the study will have their journals and results, including ECG and interrogation recordings, kept in the normal archive that each researcher has access to within their normal course of work. De‐identified blood samples will be stored at the Heart Center Leipzig. Identifiers are accessible only to researchers.
Results from the study will be submitted for publication and will only be published on a statistical group level. There will be no way to associate individual patients participating in the study with specific results or data.
2.13. Risk Assessment and Ethical Considerations
According to literature review, RIPC may rarely cause mild skin petechiae (4.4%) or arm twitching (0.1%) without any long‐term consequences. This is not associated with any additional risk or discomfort. Other components of the study (eg, blood sampling, ECG) do not cause any additional discomfort or risk above what is already associated with the normal procedures in the clinical routine.
For the study, special written consent is necessary and will be obtained. Special ethical aspects and demands of blood storage and analysis have been considered. Due to the experimental and innovative nature of the planned study, their results cannot be used to derive any information about any health‐threatening disease. Appropriate recommendations or therapeutic suggestions are therefore not possible and are not created at any time. Adverse events associated with this study will be reported to the Ethics Committee.
3. Discussion
This study will be the first description of the potential impact of RIPC on parameters related to paroxysmal NVAF. The potential effect of RIPC on AF inducibility and/or prothrombotic activity might be implemented as an additional treatment component to reduce AF burden and minimize thromboembolic risk.
Although RIPC has been studied in different models over the past decades, it is still not a fully understood phenomenon, as recently has been shown in multicenter randomized trials challenging the initial encouraging reports describing reduction of AF in surgical collective. The Remote Ischaemic Preconditioning for Heart Surgery (RIPHeart) study, focusing on a composite of death, myocardial infarction, stroke, or acute renal failure, has not detected a significant difference in incidence of in‐hospital AF.11 In contrast, the very similar Effect of Remote Ischaemic Preconditioning on Clinical Outcomes in Coronary Artery Bypass Grafting Surgery (ERICCA) trial reported a trend toward reduction of AF in the RIPC group (18% vs 14%; P = 0.07).12
However, it is important to note that patients included in those trials were frequently admitted for valve replacement/reconstruction, and therefore were more predisposed to AF. This might have reduced the protective effect of RIPC.
In previously described studies focusing on surgical patients, potential confounders such as underlying structural heart disease, the surgical procedure, and supportive therapy might have diminished the antiarrhythmic effect of RIPC. In a relatively healthy population of patients with paroxysmal NVAF, this possible antiarrhythmic effect of RIPC might be more significant. The influence of RIPC on ionic distribution along cellular compartments (eg, mitochondrion, endoplasmic reticulum) might be sufficient to reduce AF inducibility and sustainability. Moreover, arrhythmogenesis is partially triggered by temporal partial ischemia caused by rapid atrial activation and therefore can be inhibited by RIPC. However, the magnitude of those experimental findings must be verified.
Recent encouraging reports describing reduction of AF linked this observation with changes in expression of several miRNAs. In particular, the authors reported decreased level of miR‐1, whose overexpression has been implicated in promoting arrhythmias. As further miRNAs indicating different mechanisms might still be overlooked, our study aims to collect data for more exploratory analysis providing more insights into physiology of RIPC and its impact on AF.
The impact of RIPC on platelet activation in a setting of AF ablation has been documented recently.7 A similar protective effect might be expected in a scenario of spontaneous episodes of paroxysmal AF, which are typically followed by extensive platelet activation and boost of coagulation processes leading to thrombi formation and possible embolic complications.13, 14
In addition, the collected data will be complemented by further parameters, including anatomical and functional description of the left atrium and other heart chambers obtained by multimodal imaging and direct electroanatomical mapping, as well as an acute outcome of the ablation. These data could be used for future projects focusing on different biomarkers, genome analysis, and ablation in patients with AF.
3.1. Acknowledgments
The authors thank Mr. Patrik Meyer for his help with manuscript preparation.
Kosiuk J, Milani R, Ueberham L, Uhe T, Stegmann C, Hindricks G and Bollmann A. Effect of Remote Ischemic Preconditioning on Electrophysiological and Biomolecular Parameters in Nonvalvular Paroxysmal Atrial Fibrillation (RIPPAF Study): Rationale and Study Design of a Randomized, Controlled Clinical Trial, Clin Cardiol 2016;39(11):631–635.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Eitel I, Stiermaier T, Rommel KP, et al. Cardioprotection by combined intrahospital remote ischaemic preconditioning and postconditioning in ST‐elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J. 2015;36:3049–3057. [DOI] [PubMed] [Google Scholar]
- 2. Zarbock A, Schmidt C, van Aken H, et al; Renal RIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high‐risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. [DOI] [PubMed] [Google Scholar]
- 3. Nikkola E, Laiwalla A, Ko A, et al. Remote ischemic conditioning alters methylation and expression of cell cycle genes in aneurysmal subarachnoid hemorrhage. Stroke. 2015;46:2445–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Battipaglia I, Scalone G, Milo M, et al. Upper‐arm intermittent ischaemia reduces exercise‐related increase of platelet reactivity in patients with obstructive coronary artery disease. Heart. 2011;97:1298–1303. [DOI] [PubMed] [Google Scholar]
- 5. Candilio L, Malik A, Ariti C, et al. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart. 2015;101:185–192. [DOI] [PubMed] [Google Scholar]
- 6. Slagsvold KH, Rognmo O, Høydal M, et al. Remote ischemic preconditioning preserves mitochondrial function and influences myocardial microRNA expression in atrial myocardium during coronary bypass surgery. Circ Res. 2014;114:851–859. [DOI] [PubMed] [Google Scholar]
- 7. Stazi A, Scalone G, Laurito M, et al. Effect of remote ischemic preconditioning on platelet activation and reactivity induced by ablation for atrial fibrillation. Circulation. 2014;129:11–17. [DOI] [PubMed] [Google Scholar]
- 8. Huo Y, Holmqvist F, Carlson J, et al. Variability of P‐wave morphology predicts the outcome of circumferential pulmonary vein isolation in patients with recurrent atrial fibrillation. J Electrocardiol. 2015;48:218–225. [DOI] [PubMed] [Google Scholar]
- 9. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 10. Kumar S, Kalman JM, Sutherland F, et al. Atrial fibrillation inducibility in the absence of structural heart disease or clinical atrial fibrillation: critical dependence on induction protocol, inducibility definition, and number of inductions. Circ Arrhythm Electrophysiol. 2012;5:531–536. [DOI] [PubMed] [Google Scholar]
- 11. Eitel C, Hindricks G, Sommer P, et al. Circumferential pulmonary vein isolation and linear left atrial ablation as a single‐catheter technique to achieve bidirectional conduction block: the pace‐and‐ablate approach. Heart Rhythm. 2010;7:157–164. [DOI] [PubMed] [Google Scholar]
- 12. Meybohm P, Bein B, Brosteanu O, et al; RIPHeart Study Collaborators. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. [DOI] [PubMed] [Google Scholar]
- 13. Hausenloy DJ, Candilio L, Evans R, et al; ERICCA Trial Investigators. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. [DOI] [PubMed] [Google Scholar]
- 14. Akar JG, Jeske W, Wilber DJ. Acute onset human atrial fibrillation is associated with local cardiac platelet activation and endothelial dysfunction. J Am Coll Cardiol. 2008;51:1790–1793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Patients included in the study will have their journals and results, including ECG and interrogation recordings, kept in the normal archive that each researcher has access to within their normal course of work. De‐identified blood samples will be stored at the Heart Center Leipzig. Identifiers are accessible only to researchers.
Results from the study will be submitted for publication and will only be published on a statistical group level. There will be no way to associate individual patients participating in the study with specific results or data.


