ABSTRACT
Background
Practice guidelines recommend an early invasive strategy for high‐risk non‐ST‐segment elevation myocardial infarction (NSTEMI) patients, but international differences in the use of invasive strategies are unknown.
Hypothesis
Profiling NSTEMI patient management in the United States (U.S.) and South Korea could provide insight into how patients are triaged for an early invasive strategy in different health care environments and geographical regions.
Methods
We evaluated the use of angiography and revascularization for NSTEMI patients treated at revascularization‐capable hospitals (2007–2010) in both the ACTION Registry‐GWTG (U.S.: n = 133,835; 433 hospitals) and KAMIR/KorMI Registry (South Korea: n = 7,901; 72 hospitals).
Results
Compared with South Korean patients, U.S. NSTEMI patients more commonly had established cardiovascular risk factors, disease, and prior cardiovascular events and procedures. From 2007–2010, the use of angiography for NSTEMI patients rose steadily in both countries, but the use of revascularization only rose in South Korea. Patients from South Korea more commonly underwent angiography and revascularization. Percutaneous coronary intervention was the most common type of revascularization in both countries, but coronary artery bypass grafting was less common in South Korea. The use of both angiography and revascularization was incrementally lower with a higher predicted mortality risk for patients from both countries, but greater differences between low‐ and high‐risk patients occurred in the U.S.
Conclusions
The profile, characteristics, and use of angiography and revascularization for NSTEMI patients in the U.S. vs South Korea differed substantially from 2007–2010, underscoring the heterogeneity of NSTEMI patients and treatment selection among different countries.
Introduction
Multiple clinical trials have demonstrated the long‐term benefit of early invasive strategy (early angiography with the provisional use of revascularization determined by the angiographic findings) for non–ST‐segment elevation myocardial infarction (NSTEMI) patients, and practice guidelines recommend an invasive strategy for high‐risk NSTEMI patients.1, 2 Nevertheless, clinical characteristics associated with a higher predicted risk of mortality in NSTEMI patients, such as advanced age and comorbidities, may dissuade clinicians from selecting an invasive strategy. As a result, the use of invasive strategies in clinical practice can be nuanced and challenging.
International comparisons provide unique insights into differences in patient characteristics, distribution of cardiovascular (CV) risk factors, socioeconomic environments, and the influence of health care financing on clinical practice and use of guideline‐recommended therapy. Although there are many differences between the United States (U.S.) and South Korea, both countries have had increased access to invasive cardiac procedures over the last decade and commonly utilize an invasive strategy for NSTEMI patients. Profiling the management of NSTEMI patients in clinical practice among patients from the U.S. and South Korea can provide insight into how patients are selected and triaged for an early invasive strategy in different health care environments and geographical regions.
We evaluated the use of an invasive strategy and the profile of NSTEMI patients in the U.S. and South Korea by leveraging unique concurrent data from the Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With The Guidelines (ACTION Registry‐GWTG)3 and the Korea Acute Myocardial Infarction Registry (KAMIR)/Korea Working Group on Myocardial Infarction (KorMI) Registry.4
Methods
Patients
We examined data (January 2007 to December 2010) on 264 251 NSTEMI patients in the ACTION Registry‐GWTG and 17 622 NSTEMI patients in the KAMIR/KorMI Registry. Both registries enrolled consecutive patients at participating sites. We excluded patients with ST‐segment elevation myocardial infarction, those from sites without percutaneous coronary intervention (PCI) capabilities, those transferred to other hospitals, and those without full data collection (due to the use of a restricted data‐collection form, only in the ACTION Registry‐GWTG). After these exclusions, our final analysis population consisted of 133 835 NSTEMI patients from 433 hospitals in the ACTION Registry‐GWTG and 7901 NSTEMI patients from 72 hospitals in the KAMIR/KorMI Registry.
Our study complies with the Declaration of Helsinki and was approved by the research and publications committees of the ACTION Registry‐GWTG and the KAMIR/KorMI Registry. Data were collected anonymously without informed consent, and the data‐collection process for each registry was approved by the local institutional review boards or ethics committees of the participating hospitals.
Statistical Analysis
Data were collected separately from each registry and reported in a descriptive fashion without combining databases and without doing formal statistical comparisons between the 2 populations; however, statistical tests were performed for independently assessing time trends within each registry. Definitions of common variables were similar and verified before the statistical analyses were separately performed within each registry.
Continuous variables were presented as medians (25th, 75th percentiles) and categorical data as percentage values. Time trends in the use of angiography and revascularization by year were evaluated separately within each registry. Baseline demographics, CV factors, comorbidities, angiographic findings, in‐hospital medications, and in‐hospital mortality rates were calculated for the overall cohorts and for patients who did (vs did not) undergo angiography. A modified version of the ACTION Registry‐GWTG mortality risk score was calculated to estimate an individual patient's risk. Among variables in the original ACTION Registry‐GWTG mortality risk score model,5 the variable baseline troponin (Tn) ratio was not included, as peak Tn level, not baseline Tn level, was collected in the KAMIR/KorMI Registry. The overall use of angiography and revascularization, as well as time trends in the use of these procedures by various levels of in‐hospital predicted mortality risk (≤20, 20–30, 30–40, >40) using the modified ACTION Registry‐GWTG score, was evaluated separately in both registries. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
The median age of patients from both registries was 67 years. Patients from the U.S. were more commonly female; had higher body mass indices; and more commonly had established CV risk factors, prior cardiac procedures, and prior cardiovascular disease (CVD), compared with patients from South Korea (Table 1). At presentation, congestive heart failure or cardiogenic shock were more common in South Korean patients, whereas baseline creatinine values were similar in patients from both countries. The in‐hospital mortality rate was 4.0% in US patients vs 4.5% in Korean patients.
Table 1.
Baseline Characteristics, Presentation, and Uses of In‐hospital Procedures and Medications
| United States | South Korea | |
|---|---|---|
| No. of patients | 133 835 | 7901 |
| No. of participating hospitals | 433 | 72 |
| Demographics | ||
| Median age, y (1Q, 3Q) | 67 (56, 78) | 67 (56, 75) |
| Female sex, % | 38.5 | 33.0 |
| Median BMI, kg/m2 (1Q, 3Q) | 28.4 (24.8, 32.8) | 23.8 (21.8, 25.8) |
| Risk factors, % | ||
| Current smoker | 30.1 | 40.9 |
| History of HTN | 76.3 | 55.1 |
| DM | 35.1 | 31.6 |
| Dyslipidemia | 62.5 | 13.8 |
| Prior CVD/procedures, % | ||
| Prior MI | 28.9 | 7.5 |
| Prior CHF | 16.8 | 3.3 |
| Prior PCI | 25.3 | 7.2 |
| Prior CABG | 19.1 | 1.4 |
| Cerebrovascular disease | 14.8 | 9.0 |
| PAD | 12.4 | 1.1 |
| Initial vital signs | ||
| Median SBP, mm Hg (1Q, 3Q) | 145 (125, 166) | 130 (110, 150) |
| Median heart rate, bpm (1Q, 3Q) | 83 (70, 98) | 77 (67, 89) |
| ECG at presentation, % | ||
| ST depression or transient elevation | 26.3 | 21.0 |
| T‐wave changes | 14.1 | 15.8 |
| No ST‐ or T‐wave changes | 58.8 | 52.1 |
| Signs/symptoms of CHF | 20.2 | 27.5 |
| Cardiogenic shock | 1.8 | 2.7 |
| Laboratory findings | ||
| Median Cr, mg/dL (1Q, 3Q) | 1.1 (0.9, 1.4) | 1.0 (0.8, 1.2) |
| In‐hospital mortality, % | 4.0 | 4.5 |
| Extent of coronary disease in patients who underwent angiography, % | ||
| 1‐vessel disease | 27.7 | 33.4 |
| 2‐vessel disease | 28.7 | 27.2 |
| 3‐vessel disease | 34.8 | 30.6 |
| Medications, % | ||
| Aspirin | 96.8 | 94.4 |
| P2Y12 inhibitors | 74.1 | 92.3 |
| GP IIb/IIIa inhibitors | 33.1 | 8.0 |
| Anticoagulants | ||
| Heparin | 84.8 | 80.7a |
| Bivalirudin | 16.7 | NAa |
| Fondaparinux | 0.4 | NAa |
| Argatroban | 0.1 | 0.1a |
| β‐Blocker | 92.6 | 80.0 |
| ACEI/ARB | 68.5 | 82.0 |
| Statin | 85.1 | 77.6 |
| In‐hospital procedures, % | ||
| Angiography | 79.6 | 91.3 |
| PCI | 47.4 | 74.8 |
| DES among PCI with stent | 67.2 | 92.7 |
| CABG | 11.4 | 4.4 |
| Total revascularization | 58.1 | 77.9 |
Abbreviations: 1Q, first quartile; 3Q, third quartile; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; Cr, creatinine; CVD, cardiovascular disease; DES, drug‐eluting stent; DM, diabetes mellitus; ECG, electrocardiogram; GP, glycoprotein; HTN, hypertension; MI, myocardial infarction; NA, not available; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
Results from 2007 to 2010, total revascularization was either PCI or CABG.
Use of Angiography, Revascularization, and In‐hospital Medication
The overall use of angiography (US: 79.6%, 95% confidence interval [CI]: 79.4%‐79.8% vs South Korea: 91.3%, 95% CI: 90.7%‐91.9%) and revascularization (58.1%, 95% CI: 57.8%‐58.3% vs 77.9%, 95% CI: 77.0%‐78.9%) was less common in the U.S. vs South Korea (Table 1).
Among the patients who underwent angiography, the presence of 2‐ and 3‐vessel disease was more common in US patients, whereas 1‐vessel disease was more common in South Korean patients. For both countries, the most common method of revascularization was PCI. For patients undergoing stent placement during PCI, drug‐eluting stent use was less common in the U.S. (67.2%, 95% CI: 66.8%‐67.6% vs 92.7%, 95% CI: 92.1%‐93.4%), whereas the use of coronary artery bypass grafting (CABG) was more common in the U.S. (11.4%, 95% CI: 11.2%‐11.5% vs 4.4%, 95% CI: 3.9%‐4.8%).
In the U.S., the use of angiography consistently increased slightly from 2007 to 2010, whereas in South Korea, there was a more significant increase during this time (1‐sided P value for Cochran‐Armitage trend test for U.S. and South Korea: <0.0001 and <0.001, respectively; see Supporting Information, Table 1, in the online version of this article). Similarly, there was no change in the use of revascularization from 2007 to 2010 in the U.S. compared with a significant temporal increase in South Korea (1‐sided P value for Cochran‐Armitage trend test for U.S. and South Korea: 0.3071 and <0.001, respectively).
Among South Korean patients, P2Y12 antagonists and angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers were more frequently used, whereas glycoprotein IIb/IIIa inhibitors, anticoagulants, β‐blockers, and statins were more commonly used among US patients.
Comparison of Baseline Characteristics by Use of Angiography
In both countries, patients who did not undergo angiography were older and more commonly had prior CVD and comorbidities compared with patients who underwent angiography (Table 2). Additionally, the use of angiography was less frequent in female and elderly (age ≥75 years) patients, and in patients with DM, in both the U.S. and South Korea, but the proportion of patients undergoing angiography was higher in South Korea in each of these subgroups (see Supporting Information, Table 2, in the online version of this article).
Table 2.
Baseline Characteristics by Use of Angiography and Countries
| United States | South Korea | |||
|---|---|---|---|---|
| Angiography | No Angiography | Angiography | No Angiography | |
| Proportion of patients, % (n) | 79.6 (106 539) | 20.4 (27 202) | 91.3 (7262) | 8.7 (695) |
| Demographics | ||||
| Median age, y (1Q, 3Q) | 64 (54, 74) | 81 (70, 88) | 66 (56, 74) | 76 (68, 82) |
| Female sex, % | 35.3 | 51.1 | 31.7 | 47.4 |
| Median BMI, kg/m2 (1Q, 3Q) | 28.8 (25.3, 33.2) | 26.1 (22.5, 30.6) | 23.9 (22.0, 26.0) | 22.3 (20.1, 24.4) |
| Risk factors (%) | ||||
| Current smoker | 33.9 | 15.5 | 41.8 | 31.1 |
| HTN | 74.3 | 84.1 | 54.7 | 58.9 |
| DM | 33.1 | 43.0 | 31.1 | 37.2 |
| Dyslipidemia | 63.3 | 59.0 | 14.2 (18.8) | 11.8 (16.2) |
| Prior CVD/procedures, % | ||||
| Prior MI | 26.5 | 38.0 | 6.8 | 14.8 |
| Prior CHF | 11.4 | 37.5 | 2.5 | 11.8 |
| Prior PCI | 26.3 | 21.6 | 7.2 | 7.3 |
| Prior CABG | 17.5 | 25.1 | 1.1 | 3.6 |
| Cerebrovascular disease | 11.9 | 26.7 | 8.4 | 15.9 |
| PAD | 10.6 | 19.5 | 1.0 | 1.7 |
| Initial vital signs | ||||
| Median SBP, mm Hg (1Q, 3Q) | 147 (128, 168) | 137 (115, 160) | 130 (111, 150) | 130 (110, 150) |
| Median heart rate, bpm (1Q, 3Q) | 81 (70, 96) | 90 (76, 107) | 76 (67, 88) | 87 (73, 105) |
| ECG at presentation, % | ||||
| ST depression or transient elevation | 26.9 | 24.2 | 21.1 | 20.3 |
| T‐wave changes | 14.7 | 11.8 | 16.1 | 12.9 |
| No ST or T‐wave changes | 57.7 | 63.0 | 52.9 | 43.6 |
| Symptom/signs of CHF | 14.6 | 42.0 | 24.6 | 60.2 |
| Cardiogenic shock | 1.4 | 3.3 | 2.5 | 5.5 |
| Laboratory findings | ||||
| Median Cr, mg/dL (1Q, 3Q) | 1.0 (0.9, 1.3) | 1.4 (1.0, 2.0) | 1.0 (0.8, 1.2) | 1.2 (0.9, 1.8) |
| In‐hospital mortality, % | 2.1 | 11.3 | 3.2 | 18.1 |
Abbreviations: 1Q, first quartile; 3Q, third quartile; BMI, body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; Cr, creatinine; CVD, cardiovascular disease; DM, diabetes mellitus; ECG, electrocardiogram; HTN, hypertension; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
Use of Angiography and Revascularization by Risk Score, Year, and Country
The median ACTION Registry‐GWTG mortality risk score value was 27 (21 [first quartile], 34 [third quartile]) in US patients vs 29 (24, 34) in South Korean patients. Angiography and revascularization were used less frequently for higher‐risk groups both in the U.S. and South Korea, although procedure use was more frequent in South Korea within each risk group (Table 3). From 2007 to 2010, the use of angiography and revascularization for NSTEMI patients appeared to increase each year across most risk groups in South Korea, with relatively little time‐associated change across risk groups in the U.S. (Figure 1). Increasing trends in the use of angiography and revascularization were most evident in the highest‐risk group in South Korea.
Table 3.
Use of Angiography and Revascularization by Risk Subgroups
| ACTION Registry‐GWTG Risk Score Categories | United States | South Korea | |
|---|---|---|---|
| ≤20 | Proportion of the patients in the years | 22.7 | 13.2 |
| Angiography | 95.5 (95.2‐95.7) | 97.9 (96.9‐98.8) | |
| Revascularization | 73.0 (72.5‐73.5) | 85.6 (83.3‐87.9) | |
| 21–30 | Proportion of the patients in the years | 41.8 | 45.9 |
| Angiography | 88.4 (88.1‐88.7) | 96.6 (95.9‐97.2) | |
| Revascularization | 66.4 (66.0‐66.8) | 83.9 (82.6‐85.2) | |
| 31–40 | Proportion of the patients in the years | 25.4 | 30.5 |
| Angiography | 65.6 (65.1‐66.1) | 89.5 (88.2‐90.8) | |
| Revascularization | 43.5 (43.0‐44.1) | 75.1 (73.3‐77.0) | |
| >40 | Proportion of the patients in the years | 10.1 | 10.3 |
| Angiography | 42.9 (42.1‐43.8) | 78.5 (75.5‐81.6) | |
| Revascularization | 26.4 (25.6‐27.1) | 62.7 (59.1‐66.3) | |
Abbreviations: ACTION Registry‐GWTG, Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With The Guidelines; CI, confidence interval.
Data are expressed as % alone or % (95% CI).
Figure 1.
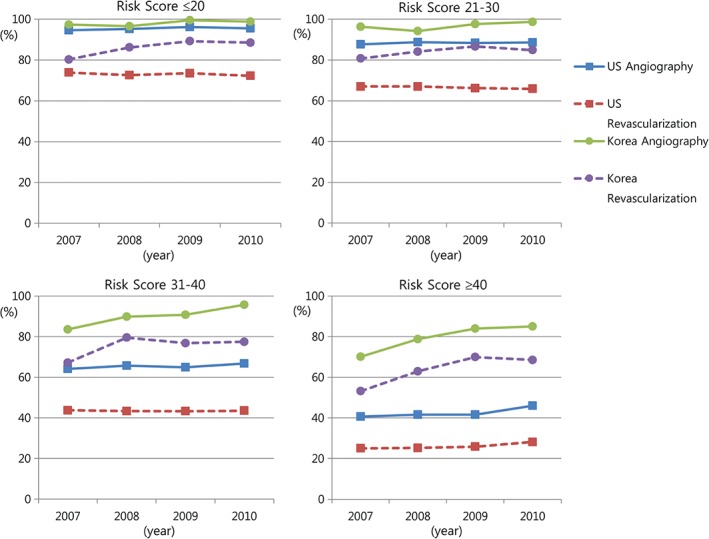
Temporal trends in the use of angiography and revascularization by predicted mortality risk. Risk scores were determined using the modified ACTION Registry‐GWTG mortality risk score, as described in the Methods. One‐sided P values for Cochran‐Armitage trend tests for yearly changes in procedure rates for the U.S. vs South Korea by risk groups are listed sequentially. For the use of angiography, risk score ≤20: P = 0.0009 for US vs P = 0.0019 for South Korea; risk score 21–30: P = 0.0374 vs P = 0.0049; risk score 31–40: P = 0.0005 vs P = 0.0117; and risk score ≥40: P < 0.0001 vs P = 0.0014. For the use of revascularization, risk score ≤20: P = 0.0382 for US vs P = 0.1130 for South Korea; risk score 21–30: P = 0.0073 vs P = 0.0081; risk score 31–40: P = 0.4074 vs P < 0.0001; and risk score ≥40: P = 0.0013 vs P = 0.0004. Abbreviations: ACTION Registry‐GWTG, Acute Coronary Treatment and Intervention Outcomes Network Registry‐Get With The Guidelines; U.S., United States.
Discussion
Guidelines recommended the use of invasive strategies in high‐risk patients with acute coronary syndrome,1, 2 and recent studies support this recommendation.6, 7 In our study, the use of angiography in NSTEMI patients rose consistently in both the U.S. and South Korea during the 4 years studied. Nevertheless, the overall use of revascularization increased in South Korea, but not in the U.S., during this time period.
International variation in the use of invasive strategies in acute coronary syndrome patients has been reported in the Global Registry of Acute Coronary Events (GRACE) Registry.8 Recent studies from Canada, Poland, Sweden, the United Kingdom, Denmark, and China have demonstrated that the use of invasive strategies for myocardial infarction (MI) patients varies by country but has consistently increased throughout the last 2 decades.9, 10, 11, 12, 13 Angiography and revascularization were used more frequently in both the U.S. and South Korea, compared with other countries.9, 10, 11, 12, 13 Additionally, in our study, we observed an inverse relationship between patient risk status measured by the ACTION Registry‐GWTG mortality risk score and the use of angiography and revascularization with a similar “risk‐treatment” paradox reported in previous studies.14, 15 Although we observed a temporal increase in the use of angiography and revascularization in the higher‐risk patients in both countries, patients with a risk score >30 were consistently less likely to undergo both angiography and revascularization. Importantly, these findings were observed in the context of routine access to invasive cardiac procedures, as all hospitals had PCI capabilities.
We found that, regardless of a patient's risk status, the use of an invasive strategy was more frequent and increased to a greater extent in South Korea compared with the U.S. Although both countries showed comparable angiography rates in the lower‐risk groups, revascularization rates were significantly higher in South Korea across risk categories. Although we did not collect detailed information regarding angiographic findings, the technical feasibility of revascularization, and patient and physician preferences, these observed differences may be due to a variety of factors. First, prior CVD, prior revascularization, diabetes mellitus (DM), and other risk factors were more commonly observed in the U.S. vs South Korea for the NSTEMI patients studies. The relatively lower burden of comorbidities and prior CV procedures and events, as well as less frequent prevalence of multivessel coronary disease, in the South Korean population may indicate that the overall technical and clinical suitability of revascularization was higher for South Korean vs US patients with NSTEMI. A second potential explanation for differences in the use of invasive strategies may be the lack of uniform population coverage of health insurance in the U.S., particularly in the case of expensive treatments such as drug‐eluting stents that were less commonly used during PCI in the U.S. vs South Korea.16 Although economic data were not collected in either registry, differences in health care reimbursement for the inpatient and subsequent outpatient treatment periods may have influenced revascularization decisions. Finally, the complex decision‐making process involved with choosing to refer a patient for angiography (with provisional use of revascularization afterwards based upon angiographic findings and other factors) likely differs substantially between the 2 countries, due to the aforementioned issues described, as well as other unmeasured constituents such as differences in cardiology training practices for interventional cardiologists and cultural factors. However, we were unable to precisely delineate potential reasons that underlie the differences in angiography and revascularization between the 2 countries in the context of 2 concurrent observational registries.
Another unique finding of this analysis was that the chosen method of revascularization was significantly different; drug‐eluting stent PCI was more frequently used for revascularization in South Korea, whereas CABG was more commonly used in the U.S., despite relatively similar frequencies of 2‐ and 3‐vessel disease in both countries. The increased use of CABG in the U.S. may reflect differences in the incidence of key comorbidities such as DM, the availability of CV surgeons and hospitals with CABG capabilities, differences in the prevalence of multivessel coronary disease among the 2 populations, and/or the preferences of physicians and patients in each country. Nonetheless, these findings were observed despite a much higher frequency of prior CABG (a known factor that is associated with very low likelihood of a second CABG procedure during the NSTEMI hospitalization) in US patients.17
Study Limitations
Our study had several limitations. First, there were minor differences in the selection and definition of several variables used in each registry. For example, we used the modified ACTION Registry‐GWTG mortality risk score, due to the lack of baseline Tn in the KAMIR/KorMI Registry; however, the modified ACTION Registry‐GWTG risk score reliably predicted mortality risk for each risk group in both registries. Second, precise details that could accurately profile the decision‐making process for individual patients regarding referral for angiography and revascularization could not be collected within the context of large, national, observational registries, so these findings should be considered to be hypothesis‐generating and we could not determine factors associated with the use of invasive management strategies within the 2 countries studied. Third, because long‐term outcomes were not collected, the downstream impact of these findings could not be ascertained. Finally, a subgroup analysis among the US patients with Asian and non‐Asian ethnicity vs Korean patients could be informative, as ethnic differences may have influenced our study findings; <2% of the US population in the ACTION Registry‐GWTG was classified as Asian ethnicity.
Conclusion
The use of angiography and revascularization for NSTEMI patients increased in both South Korea and the U.S. from 2007 to 2010, with higher incremental use of these procedures in South Korea. A risk‐treatment paradox was observed in both countries, but lower relative use of procedures in higher‐risk patients was more pronounced in the U.S. Therefore, our findings profile the geographical heterogeneity of NSTEMI patients and treatments in contemporary practice and underscore factors that deserve consideration for the conduct of global studies evaluating novel therapies for acute coronary syndrome patients.
Supporting information
Appendix S1. Supplemental material
Acknowledgments
The authors thank Erin Hanley, MS, for her editorial contributions to this manuscript. Ms. Hanley did not receive compensation for her contributions apart from her employment where this study was conducted.
Dr. Wang reports institutional research grant support from Eli Lilly, Daiichi‐Sankyo, Gilead Sciences, GlaxoSmithKline, and the American College of Cardiology, and honoraria from AstraZeneca and the American College of Cardiology. Dr. Alexander reports research funding from Gilead and participation on data safety monitoring board for CytRx. Dr. Jeong has no relevant disclosures to report. Dr. Kim reports research grant funding from Abbott Vascular, Medtronic, and Boston Scientific. Dr. Bates reports consulting fees/honoraria from Sanofi, Daiichi‐Sankyo, Lilly, AstraZeneca, and Janssen. Dr. Henry reports consulting fees/honoraria from Cytori, the Medicines Company, Daiichi‐Sankyo, Eli Lilly and Company, Abbott Vascular, Baxter, and Capricor. Dr. Peterson reports research funding to Duke Clinical Research Institute from the American College of Cardiology, American Heart Association, Eli Lilly and Company, and Janssen Pharmaceuticals, and consulting (including CME) for Merck & Co., Boehringer Ingelheim, Genentech, Janssen Pharmaceuticals, and Sanofi‐Aventis. Dr. Roe reports receiving research funding from Eli Lilly, Sanofi‐Aventis, Daiichi‐Sankyo, Janssen Pharmaceuticals, Ferring Pharmaceuticals, American College of Cardiology, American Heart Association, and Familial Hypercholesterolemia Foundation, and consulting or honoraria from PriMed, AstraZeneca, Boehringer‐Ingelheim, Merck, Amgen, and Elsevier Publishers. All conflicts of interest are listed at https://www.dcri.org/about‐us/conflict‐of‐interest.
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
This research was supported by the American College of Cardiology Foundation's National Cardiovascular Data Registry.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non–ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine [published correction appears in J Am Coll Cardiol. 2008;51:974]. J Am Coll Cardiol. 2007;50:e1–e157. [DOI] [PubMed] [Google Scholar]
- 2. Bassand JP, Hamm CW, Ardissino D, et al.; Task Force for Diagnosis and Treatment of Non–ST‐Segment Elevation Acute Coronary Syndromes of European Society of Cardiology. Guidelines for the diagnosis and treatment of non–ST‐segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. [DOI] [PubMed] [Google Scholar]
- 3. Messenger JC, Ho KK, Young CH, et al. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. [DOI] [PubMed] [Google Scholar]
- 4. Lee KH, Jeong MH, Ahn Y, et al. New horizons of acute myocardial infarction: from the Korea Acute Myocardial Infarction Registry. J Korean Med Sci. 2013;28:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chin CT, Chen AY, Wang TY, et al. Risk adjustment for in‐hospital mortality of contemporary patients with acute myocardial infarction: the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) registry–Get With The Guidelines (GWTG) acute myocardial infarction mortality model and risk score. Am Heart J. 2011;161:113.e2–122.e2. [DOI] [PubMed] [Google Scholar]
- 6. O'Donoghue ML, Vaidya A, Afsal R, et al. An invasive or conservative strategy in patients with diabetes mellitus and non–ST‐segment elevation acute coronary syndromes: a collaborative meta‐analysis of randomized trials. J Am Coll Cardiol. 2012;60:106–111. [DOI] [PubMed] [Google Scholar]
- 7. Puymirat E, Taldir G, Aissaoui N, et al. Use of invasive strategy in non–ST‐segment elevation myocardial infarction is a major determinant of improved long‐term survival: FAST‐MI (French Registry of Acute Coronary Syndrome). JACC Cardiovasc Interv. 2012;5:893–902. [DOI] [PubMed] [Google Scholar]
- 8. Fox KA, Clayton TC, Damman P, et al; FIR Collaboration. Long‐term outcome of a routine versus selective invasive strategy in patients with non–ST‐segment elevation acute coronary syndrome a meta‐analysis of individual patient data. J Am Coll Cardiol. 2010;55:2435–2445. [DOI] [PubMed] [Google Scholar]
- 9. Gale CP, Allan V, Cattle BA, et al. Trends in hospital treatments, including revascularisation, following acute myocardial infarction, 2003–2010: a multilevel and relative survival analysis for the National Institute for Cardiovascular Outcomes Research (NICOR). Heart. 2014;100:582–589. [DOI] [PubMed] [Google Scholar]
- 10. Smith LG, Herlitz J, Karlsson T, et al. International comparison of treatment and long‐term outcomes for acute myocardial infarction in the elderly: Minneapolis/St. Paul, MN, USA and Goteborg, Sweden. Eur Heart J. 2013;34:3191–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gierlotka M, Gasior M, Wilczek K, et al. Temporal trends in the treatment and outcomes of patients with non–ST‐segment elevation myocardial infarction in Poland from 2004‐2010 (from the Polish Registry of Acute Coronary Syndromes). Am J Cardiol. 2012;109:779–786. [DOI] [PubMed] [Google Scholar]
- 12. Mårtensson S, Gyrd‐Hansen D, Prescott E, et al. Trends in time to invasive examination and treatment from 2001 to 2009 in patients admitted first time with non–ST elevation myocardial infarction or unstable angina in Denmark. BMJ Open. 2014;4:e004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Fu R, Wang Z, et al. Assessing the quality of care for patients with acute myocardial infarction in China. Clin Cardiol. 2015;38:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zia MI, Goodman SG, Peterson ED, et al. Paradoxical use of invasive cardiac procedures for patients with non–ST segment elevation myocardial infarction: an international perspective from the CRUSADE Initiative and the Canadian ACS Registries I and II. Can J Cardiol. 2007;23:1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gyenes GT, Yan AT, Tan M, et al. Use and timing of coronary angiography and associated in‐hospital outcomes in Canadian non–ST‐segment elevation myocardial infarction patients: insights from the Canadian Global Registry of Acute Coronary Events. Can J Cardiol. 2013;29:1429–1435. [DOI] [PubMed] [Google Scholar]
- 16. Krone RJ, Rao SV, Dai D, et al. Acceptance, panic, and partial recovery the pattern of usage of drug‐eluting stents after introduction in the U.S. (a report from the American College of Cardiology/National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2010;3:902–910. [DOI] [PubMed] [Google Scholar]
- 17. Mehta RH, Chen AY, Pollack CV Jr, et al. Challenges in predicting the need for coronary artery bypass grafting at presentation in patients with non–ST‐segment elevation acute coronary syndromes. Am J Cardiol. 2006;98:624–627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplemental material


