Abstract
Background
In a prospective study, cardiac MRI (CMR) and intravascular ultrasound were performed in women with myocardial infarction (MI) and nonobstructive coronary artery disease (MINOCA). Forty participants underwent adenosine‐stress CMR (sCMR).
Hypothesis
Abnormal perfusion may co‐localize with ischemic late gadolinium enhancement (LGE) and T2‐weighted signal hyperintensity (T2+), suggesting microvascular dysfunction contributed to MI.
Methods
Qualitative perfusion analysis was performed by 2 independent readers. Abnormal myocardial perfusion reserve index (MPRI) was defined as global average ≤1.84.
Results
Abnormal rest perfusion was present in 10 patients (25%) and stress perfusion abnormalities in 25 (63%). Abnormal stress perfusion was not associated with LGE but tended to occur with T2+. Among patients with abnormal perfusion and LGE, the LGE pattern was ischemic in half. The locations of abnormal perfusion and LGE matched in 75%, T2+ in 100%. Abnormal stress perfusion was not associated with plaque disruption and matched in location in 63%. MPRI was abnormal in 10 patients (25%) and was not associated with LGE, T2+ or plaque disruption.
Conclusions
Abnormal perfusion on sCMR is common among women with MINOCA. Abnormal perfusion usually co‐localized with LGE and/or T2+ when present. Variability in LGE pattern leads to uncertainty about whether the finding of abnormal perfusion was cause or consequence of the tissue state leading to LGE. Low MPRI, possibly indicating diffuse microvascular disease, was observed with and without LGE and T2+. Multiple mechanisms may lead to abnormal perfusion on sCMR. Microvascular dysfunction may contribute to the pathogenesis of and coexist with other causes of MINOCA.
Keywords: Ischemic heart disease, myocardial infarction, Women, mri
1. INTRODUCTION
Nonobstructive coronary artery disease at angiography (ie, no stenosis ≥50%) is a frequent finding in myocardial infarction (MI) patients, occurring in 9% to 20% female and 4% to 8% of male patients.1, 2, 3 Although patients with MI with obstructive coronary artery disease (CAD) carry a worse prognosis, the rate of death and reinfarction in patients with MI and nonobstructive CAD on angiography (MINOCA) is 2% to 5%.4, 5, 6, 7, 8, 9, 10, 11, 12 Mechanisms are incompletely understood, leading to uncertainty about treatment.13 Advanced imaging modalities, including intravascular imaging and cardiac magnetic resonance imaging (CMR), may help to characterize MI etiology in these patients.14, 15, 16, 17, 18, 19
Microvascular coronary disease is a known cause of chest pain with nonobstructive CAD.20 Abnormal coronary flow reserve is a predictor of adverse outcomes, including death, in stable patients with nonobstructive CAD.21, 22 Microvascular coronary disease has been hypothesized to cause acute coronary syndrome in at least some patients with nonobstructive CAD on angiography,23 but this mechanism has not been specifically investigated.
We previously reported results of intravascular ultrasound (IVUS) and CMR in a prospective study of 50 women with MINOCA. Late gadolinium enhancement (LGE) was found in 39%, T2‐weighted signal hyperintensity (T2+) in 53%, and plaque disruption (PD; defined as rupture and/or ulceration) in 38%.14 Forty of these patients also underwent adenosine stress perfusion CMR (sCMR) to assess for microvascular coronary dysfunction as an etiologic factor.
We hypothesized that abnormal perfusion would be identified on sCMR of MINOCA patients. We further hypothesized that the location of any ischemia would correlate with areas of ischemic LGE and T2+, when present, thus supporting an etiologic role in MINOCA.
2. METHODS
2.1. Study Population
Women age ≥18 years were screened for enrollment if they presented with acute MI to NYU Langone Medical Center or Bellevue Hospital Center and were referred for coronary angiography. Myocardial infarction was defined as the combination of ischemic symptoms and elevated troponin (Tn) to ≥2× the upper limit of normal, with or without electrocardiographic changes. Patients were considered to have takotsubo cardiomyopathy if they met published criteria.24 Our patients underwent echocardiography early in their clinical course, and therefore we believe all takotsubo cases have been identified. Patients with history of angiographic obstructive CAD were excluded. Patients were instructed to abstain from caffeine‐containing products for ≥12 hours prior to sCMR. Additional exclusion criteria were use of cocaine or vasospastic agent within the past month and contraindication to CMR or IVUS. All participants provided informed consent. Those with <50% stenosis in all major epicardial arteries on coronary angiography underwent study testing, which included IVUS at the time of angiography and sCMR within 7 days. Adenosine sCMR with LGE was completed in 40 patients, all of whom had rest and stress perfusion imaging with calculation of myocardial perfusion reserve index (MPRI). Twenty‐eight of the 40 patients also underwent T2‐weighted imaging, and 32 patients underwent IVUS (Figure 1).
Figure 1.
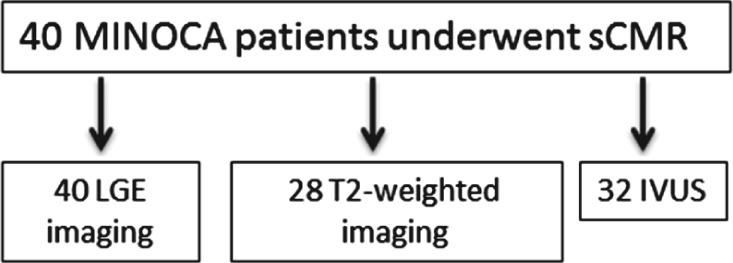
Participant flow through study. sCMR includes both rest and stress perfusion imaging. Twenty‐one patients had both IVUS and T2‐weighted imaging. Abbreviations: IVUS, intravascular ultrasound; LGE, late gadolinium enhancement; MINOCA, myocardial infarction and nonobstructive coronary artery disease; sCMR, stress cardiac magnetic resonance imaging with qualitative and quantitative analysis.
2.2. Cardiac Magnetic Resonance Technique
The CMR imaging was performed using a 1.5‐T magnetic resonance imaging system (Avanto; Siemens, Erlangen, Germany) with a phased‐array body coil and ECG monitoring. The imaging protocol consisted of the following sequences: (1) scout images, (2) black‐blood double inversion‐recovery thoracic imaging in axial planes, (3) T2‐weighted imaging, (4) cine 2D steady‐state free precession imaging of the left ventricle (LV), (5) dynamic first‐pass contrast enhancement perfusion imaging in representative short‐axis planes, and (6) inversion‐recovery late gadolinium enhancement (LGE) imaging 10 to 15 minutes after intravenous injection of a total of 0.15 mmol/kg gadolinium‐gadopentetate dimeglumine (Magnevist; Bayer HealthCare, Wayne, NJ). Imaging was performed during repeated end‐expiratory breathholds to minimize respiratory motion artifact.
Adenosine rest and stress perfusion imaging was performed following intravenous injection of 0.05 mmol/kg gadolinium‐gadopentetate dimeglumine at 5 cc/sec, followed by a saline flush of 20 mL at the same rate, using repeated image acquisitions in a T1‐weighted (saturation‐recovery) fast low‐angle shot (FLASH) sequence in 3 short‐axis imaging planes (apical, mid‐ventricular, and basal LV). Typical imaging parameters included repetition time (TR), 700; echo time (TE), 1.15; field of view (FOV), 34 cm; and integrated parallel acquisition techniques (iPAT) factor, 2. Basal resting perfusion imaging was performed initially, followed by the adenosine stress perfusion study after a delay of 10 minutes to allow for the clearance of the first contrast injection. This order was selected due to concern for possible persistent vasodilatory effects of adenosine. Adenosine (Adenocard; Astellas Pharmaceuticals, Northbrook, IL) was infused at a constant rate of 140 µg/kg/min body weight over 4 minutes, with imaging following the third minute of infusion.
Vital signs were monitored throughout the test. Details of IVUS methods and CMR image acquisition and analysis for other parameters have been previously published.14
2.3. Image Analysis
Cardiac magnetic resonance images were reviewed offline by 2 level‐3 CMR‐trained independent readers who were blinded to ECG, laboratory, angiographic, and IVUS data during interpretation. Any disagreement was resolved by consensus while the readers remained blinded to all other clinical information.
The LV was divided according to the American Heart Association (AHA) 17‐segment model.25 Presence of LGE was visually evaluated in each segment. The presence of LGE was further categorized into an ischemic (transmural, subendocardial), nonischemic (midmyocardial, subepicardial), or mixed pattern. Any LGE in a patchy, midmyocardial, nonvascular distribution was considered evidence of myocarditis. T2‐weighted imaging was added to the study protocol beginning with the thirteenth patient. T2‐weighted images were visually evaluated for the presence of increased myocardial signal in a given segment compared with adjacent liver or inhomogeneity within the myocardium cross‐referenced to other available views and to skeletal muscle at roughly similar distances from the receiver surface coil. This was done to allow for receiver coil sensitivity inhomogeneity. Visual assessment was preferred over quantitative assessment of T2‐weighted imaging because it is less susceptible to motion artifact.
Myocardial perfusion analysis involved visual comparison of adenosine stress images with rest images, to identify segments with decreased or delayed relative signal intensity change, indicative of a perfusion defect. Patients were considered to have abnormal stress myocardial perfusion if there were ≥1 segments with poorer relative perfusion after stress as compared with the rest. The focus of the study was on investigating mechanisms of myocardial damage in the context of recent MI. Abnormal perfusion was categorized as diffuse (abnormal in all 3 coronary territories, affecting ≥12 segments) or segmental.
In addition, we calculated the semiquantitative MPRI as follows: serial images of 3 short‐axis slices of the LV were obtained during rest and stress perfusion imaging. Each slice was divided into 4 or 6 regions of interest (ROI).25 Signal intensity vs time curves were plotted for each segment, using a workstation with perfusion postprocessing capability (Multimodality Workplace; Siemens Healthcare, Germany). Slope of the curves for rising myocardial signal intensity vs time was calculated and normalized by dividing by the upslope of the corresponding LV blood pool signal in the basal slice (LV outflow tract). The MPRI was calculated as the ratio of normalized signal intensity upslopes at stress divided by those at rest, for each segment. Global MPRI was the average of MPRI values for all segments and was considered abnormal if ≤1.84.26
Location of abnormal myocardial perfusion was compared with location of LGE or T2+, when present, using 16 segments of the AHA 17‐segment model (true apex was not included in perfusion analysis). Locations were considered to match if >50% of segments with abnormal perfusion were concordant with LGE/T2 location or, for PD, if abnormal perfusion was in the affected coronary artery distribution according to the 17‐segment model.
2.4. Statistical Analysis
Continuous variables were described as mean ± SD when normally distributed and by medians and interquartile ranges (IQR) when not normally distributed. Normality assumptions were checked by Shapiro‐Wilk tests. Comparisons were performed using the Student t test or nonparametric Wilcoxon rank‐sum tests for continuous variables and χ2/Fisher exact test for noncontinuous variables. Analyses were conducted using SAS version 9.3 (SAS Institute, Inc., Cary, NC) and Microsoft Excel 2011 (Microsoft, Redmond, WA).
3. RESULTS
Participant characteristics are summarized in the Table 1 and testing in Figure 1. Two patients with takotsubo cardiomyopathy underwent stress CMR and were therefore included in the analysis. Median peak Tn was 1.86 ng/mL (IQR, 0.53–5.92). Late gadolinium enhancement was present in 16/40 patients (40%). The pattern of LGE was ischemic in 9 (22.5%), nonischemic in 3 (7.5%), and mixed in 4 (10%) participants. T2+ was present in 15/28 patients (53.6%).
3.1. Myocardial Perfusion Abnormalities and Correlation With Cardiac Magnetic Resonance Findings
Ten patients had abnormal qualitative perfusion at rest. The location of abnormal perfusion at rest matched that of LGE in 5/10, and of T2+ in 2/10.
Abnormal stress perfusion was identified in 25 participants (63%); 14/25 had diffuse subendocardial ischemia. Abnormal stress perfusion was not associated with presence of LGE (12 vs 4 patients, P = 0.18). In patients with both abnormal stress perfusion and LGE, the pattern of LGE was ischemic in only half (6/12), and the location of abnormal stress perfusion matched LGE location in most but not all patients (8/12; 67%). See Figures 2 and 3 for illustrative cases with matching and nonmatching location of LGE and abnormal stress perfusion.
Figure 2.
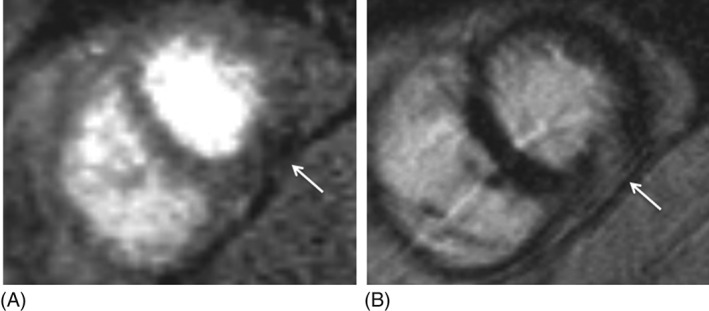
Images from an sCMR study showing matching between an area of abnormal perfusion and LGE. (A) Mid short‐axis view of stress perfusion showing area of hypoenhancement (arrow), indicating perfusion defect in inferior wall. (B) Mid short‐axis view shows area of LGE in inferior wall (arrow), matching the area of perfusion defect. Abbreviations: LGE, late gadolinium enhancement; sCMR, stress cardiac magnetic resonance imaging with qualitative and quantitative analysis.
Figure 3.
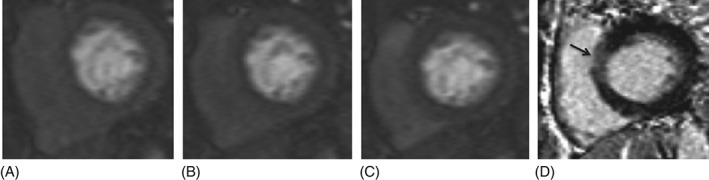
Images from an sCMR study showing lack of matching between areas of abnormal perfusion and LGE. (A–C) Three consecutive basal short‐axis views of stress perfusion showing diffuse subendocardial hypoenhancement. (D) Whereas there was diffuse subendocardial hypoperfusion at the base, there is minimal LGE at the base, localized to the anteroseptal wall (arrow). Note that the stress images illustrated were compared with the corresponding rest images for the same patient, which were acquired at the same locations with identical contrast‐agent administration and imaging protocols. The subendocardial dark band was not present in the rest images, but only in the stress images, helping to confirm the physical significance of it. This dark band appearance was also not seen in other subjects imaged with identical contrast‐agent administration and imaging protocols who did not have other indications of subendocardial ischemia. Abbreviations: LGE, late gadolinium enhancement; sCMR, stress cardiac magnetic resonance imaging with qualitative and quantitative analysis.
There was a trend toward higher likelihood of T2+ among participants with stress perfusion abnormalities (11 vs 4; P = 0.06). When both abnormal stress perfusion and T2+ were present, the locations matched in all participants.
3.2. Semiquantitative Perfusion Analysis and Correlation With Cardiac Magnetic Resonance Findings
Abnormal MPRI was present in 10 (25%) participants. Abnormal MPRI correlated with lower BMI (24.1 vs 28.7; P = 0.02). Abnormal MPRI was not associated with LGE or T2 + .
Eight of 10 participants with low MPRI (80%) had abnormal qualitative perfusion. Analysis of MPRI by segment did not improve agreement between qualitative and semiquantitative methods (data not shown). Analysis using MPRI cutoffs of 1.5, 1.6, and 1.7 did not show an association between abnormal MPRI and LGE or T2 + .
3.3. Correlation Between Stress Cardiac Magnetic Resonance and Intravascular Ultrasound Findings
Intravascular ultrasound was performed in 32/40 participants. Plaque disruption was identified in 12/32 participants (37.5%). Abnormal stress perfusion was not associated with PD on IVUS (8 vs 4 participants; P = 0.52), nor was MPRI associated with PD.
In patients with both abnormal stress perfusion and PD, the location of abnormal stress perfusion matched the territory supplied by the coronary artery with the PD in 5/8 (63%) participants. There was a trend toward more severe nonobstructive atherosclerosis on angiography (30% vs 10% diameter stenosis; P = 0.07) but not IVUS (plaque burden 41.2% vs 39.0%; P = 0.70), among those with abnormal qualitative stress perfusion. There was no relationship between abnormal MPRI and atherosclerosis severity (Table 1).
Table 1.
Participant Characteristics
| All Patients, N = 40 | Abnormal Stress Perfusion, n = 25 | Normal Stress Perfusion, n = 15 | P Value | Abnormal MPRI, n = 10 | Normal MPRI, n = 30 | P Value | |
|---|---|---|---|---|---|---|---|
| Age, y, mean ± SD | 57.56 ± 12.9 | 58.6 ± 11.9 | 55.8 ± 14.8 | 0.54 | 58.4 ± 14.3 | 57.3 ± 12.7 | 0.81 |
| Tn, ng/mL, median (IQR) | 1.86 (0.53–5.92) | 2.35 (0.79–6.95) | 0.81 (0.30–2.75) | 0.11 | 1.52 (0.94–9.73) | 1.93 (0.19–4.13) | 0.12 |
| HTN | 27 (67.5) | 18 (72) | 9 (60) | 0.43 | 6 (60) | 21 (70) | 0.56 |
| DM | 11 (27.5) | 7 (28) | 4 (27) | 0.93 | 2 (20) | 9 (30) | 0.54 |
| Prior MI | 4 (10) | 1 (4) | 3 (20) | 0.10 | 0 (0.0) | 4 (13.3) | 0.22 |
| Smoking | 6 (15) | 5 (20) | 1 (6.7) | 0.25 | 2 (20) | 4 (13.3) | 0.61 |
| BMI, kg/m2, mean ± SD | 27.5 ± 5.5 | 26.9 ± 4.4 | 28.6 ± 7.1 | 0.41 | 24.1 ± 2.39 | 28.7 ± 5.83 | 0.02 |
| LVEF, %, mean ± SD | 54.8 ± 14.1 | 54.3 ± 11.1 | 55.6 ± 18.4 | 0 | 51.8 ± 15.6 | 55.8 ± 13.7 | 0.44 |
| ECG findings | |||||||
| ST‐segment elevation | 10 (25) | 6 (24) | 4 (26.7) | 0.85 | 4 (40) | 6 (20) | 0.21 |
| ST‐segment depression | 4 (10) | 1 (4) | 3 (20) | 0.10 | 1 (10) | 3 (10) | 1 |
| LBBB | 1 (2.5) | 0 (0.0) | 1 (6.7) | 0.19 | 0 (0.0) | 1 (3.3) | 0.56 |
| Normal ECG | 25 (62.5) | 18 (72) | 7 (46.7) | 0.11 | 5 (50) | 20 (66.7) | 0.35 |
| Catheterization findings | |||||||
| Worst angiographic stenosis, %, median (IQR) | 20 (0–40) | 30 (15–40) | 10 (0–30) | 0.07 | 17.5 (0–40) | 25 (2.5–40) | 0.74 |
| IVUS findings, n = 32 | n = 19 | n = 13 | n = 7 | n = 25 | |||
| PD | 12 (37.5) | 8 (42.1) | 4 (30.8) | 0.52 | 2 (28.6) | 10 (40) | 0.58 |
| Minimal luminal area, mm2, mean ± SD | 8.4 ± 3.2 | 7.7 ± 2.7 | 9.7 ± 3.7 | 0.17 | 10.8 ± 5.21 | 11.5 ± 4.6 | 0.59 |
| % area stenosis, mean ± SD | 23.8 ± 12.4 | 23.4 ± 14.0 | 23.4 ± 10.0 | 0.82 | 13.1 ± 11.8 | 14.6 ± 12.3 | 0.63 |
| Plaque burden, %, mean ± SD | 40.3 ± 14.3 | 41.2 ± 12.9 | 39.0 ± 16.6 | 0.70 | 29.1 ± 12.7 | 34.0 ± 13.5 | 0.15 |
| CMR findings | |||||||
| LGE, any | 16 (40) | 12 (48) | 4 (26.7) | 0.18 | 3 (30) | 13 (43.3) | 0.46 |
| Pattern of LGE if present | |||||||
| Ischemic | 9 (22.5) | 6 (24) | 3 (20) | 0.76 | 1 (10) | 8 (26.7) | 0.11 |
| Nonischemic | 3 (7.5) | 3 (12) | 0 (0.0) | 2 (20) | 1 (3.3) | ||
| Mixed | 4 (10) | 3 (12) | 1 (6.7) | 0 (0.0) | 4 (13.3) | ||
| T2 signal‐weighted hyperintensity present, n = 28 | 15 (53.6) | n = 16, 11 (69) | n = 12, 4 (33.3) | 0.06 | n = 7, 4 (57) | n = 21, 11 (52.4) | 0.83 |
Abbreviations: BMI, body mass index; CMR, cardiac magnetic resonance; DM, diabetes mellitus; ECG, electrocardiographic; HTN, hypertension; IQR, interquartile range; IVUS, intravascular ultrasound; LBBB, left bundle branch block; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MPRI, myocardial perfusion reserve index; PD, plaque disruption; SD, standard deviation; Tn, troponin.
Data are presented as n (%) unless otherwise noted.
3.4. Stress Cardiac Magnetic Resonance Findings in Patients With Takotsubo Cardiomyopathy
Neither participant with takotsubo cardiomyopathy had LGE; T2‐weighted imaging was not conducted in these participants. Stress perfusion was normal in both cases. However, one had normal MPRI and the other had low MPRI.
3.5. Number of Cases in Which Microvascular Coronary Disease May Have Been a Contributor to Pathogenesis
Among 40 participants, 25 had neither PD identified by IVUS nor CMR evidence of myocarditis. In these 25 patients, it might be hypothesized that sCMR would have the greatest potential for contributing to the etiologic diagnosis of MI. Seventeen of the 25 had abnormal qualitative stress perfusion, 6 of whom also had low MPRI.
4. DISCUSSION
Adenosine‐induced perfusion abnormalities on sCMR are common among women with MINOCA in the early post‐MI phase. Abnormal stress perfusion was no more likely among patients with than without CMR evidence of infarction (LGE).
Microvascular disease is well known to be a cause of stable angina with nonobstructive CAD, but the role of microvascular disease in MINOCA pathogenesis has not previously been studied, to our knowledge.20, 21, 22, 27, 28, 29 Prior studies using CMR perfusion imaging in patients with acute coronary syndrome and nonobstructive CAD did not include stress testing.16, 17, 18, 19, 30, 31 Adenosine stress perfusion CMR is highly sensitive, specific, and reproducible for identification of obstructive CAD and has been reported to be superior to other stress imaging methods for this purpose.32, 33, 34, 35, 36, 37, 38 In the setting of stable, nonobstructive CAD, patients with proven microvascular disease based on invasive coronary reactivity testing had lower MPRI as compared with healthy controls.39
In our study, when stress perfusion was abnormal in patients with LGE or T2+, abnormal perfusion was typically identified in the affected myocardial segments. However, this was true of patients with either an ischemic or nonischemic LGE pattern. Microvascular disease would only be expected to play a causal role for ischemic LGE. Therefore, adenosine‐induced ischemia as identified by qualitative analysis of sCMR could be a consequence of the tissue state leading to LGE. Similarly, ischemia could have led to findings of T2+. However, T2+ indicates areas of myocardial edema, and edematous tissue could compress microvessels and cause hypoperfusion.
Stress CMR was included in the investigative protocol to search for supportive evidence of a role of microvascular coronary disease in the pathogenesis of MINOCA, such as co‐localization of abnormal perfusion and myocardial edema or LGE. However, because myocardial injury also has the potential to compromise the microvasculature, it is not possible to make a definitive conclusion about the role of microvascular disease based on this correlation.
We analyzed both qualitative perfusion abnormalities and MPRI, because the former could provide information about regionality of ischemia as it relates to MI and edema, and the latter has been correlated with invasive assessment of microvascular disease. There was surprisingly low concordance between qualitative and semiquantitative (MPRI) analysis. The reasons for this lack of overlap are unclear but may include averaging of all myocardial layers when calculating MPRI, because often only the subendocardial portion is significantly affected. Hypertension was common in our cohort and could partially explain abnormal semiquantitative perfusion.40
Greater extent of nonobstructive atherosclerosis was not associated with abnormal stress perfusion in this study. Plaque disruption was commonly located in the same coronary territory as abnormal perfusion (63%) but was not associated with abnormal stress perfusion. This suggests that if stress perfusion abnormalities represent ischemia, the ischemia was most likely microvascular in origin.
It is possible that preexisting microvascular disease as reflected by abnormal MPRI contributed to, but was not the sole cause of, MINOCA in some participants. If diffuse microvascular disease were the sole cause of MINOCA in a patient, that patient might not be expected to have localized LGE but might rather have Tn elevation in the absence of LGE, because of a broader spatial distribution of myonecrosis throughout the subendocardium.
4.1. Study Limitations
Sample size was small and some participants were unable to complete sCMR. T2‐weighted imaging was not available for all participants. The sCMR was performed at a median of 6 days after symptom onset (IQR, 4–8 days). We do not know whether perfusion abnormalities may have evolved between presentation and CMR imaging, but T2+ and LGE are known to persist over this time period.41 Fully quantitative myocardial perfusion analysis is superior to qualitative and semiquantitative methods but was not performed in this study.42 Invasive coronary flow reserve is the microvascular disease reference standard, but it was not measured in this study to avoid excessive use of invasive testing given that IVUS was performed. Thus, we are unable to determine whether some stress MRI abnormalities may be false positives. Similarly, provocative testing for coronary spasm was not performed. Caffeine was withheld for a minimum of 12 hours before sCMR, which may have resulted in attenuated diagnostic accuracy of pharmacologic stress testing for microvascular ischemia as compared with longer caffeine abstinence.43
5. CONCLUSIONS
Abnormal myocardial perfusion as measured by adenosine stress cardiac MRI is common among women with MI and nonobstructive CAD; but, based on our findings, it may not be the sole cause of infarction in all patients in whom it occurs. The results of qualitative analysis of stress CMR raise questions about whether the finding of abnormal stress perfusion was the cause or consequence of the tissue state leading to LGE. Multiple mechanisms may contribute to abnormal perfusion on adenosine stress CMR, and microvascular coronary dysfunction may coexist with other causes of MI in these patients.
Acknowledgments
The authors thank Yu Guo for her assistance with statistical analyses.
Mauricio R, Srichai MB, Axel L, Hochman JS, Reynolds HR. Stress Cardiac MRI in Women With Myocardial Infarction and Nonobstructive Coronary Artery Disease, Clin Cardiol 2016, 39, 596–602. DOI: 10.1002/clc.22571
This study was funded by the Doris Duke Charitable Foundation. Dr. Reynolds is a recipient of a Clinical Scientist Development Award. Adenosine was provided by Astellas Pharma, Inc. The funding agency and Astellas had no role in data acquisition, analysis, or reporting.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
REFERENCES
- 1. Berger JS, Elliott L, Gallup D, et al. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Halkin A, Stone GW, Grines CL, et al. Outcomes of patients consented but not randomized in a trial of primary percutaneous coronary intervention in acute myocardial infarction (the CADILLAC registry). Am J Cardiol. 2005;96:1649–1655. [DOI] [PubMed] [Google Scholar]
- 3. Hochman JS, Tamis JE, Thompson TD, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med . 1999;341:226–232. [DOI] [PubMed] [Google Scholar]
- 4. Dey S, Flather MD, Devlin G, et al; Global Registry of Acute Coronary Events Investigators. Sex‐related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart . 2009;95:20–26. [DOI] [PubMed] [Google Scholar]
- 5. Diver DJ, Bier JD, Ferreira PE, et al. Clinical and arteriographic characterization of patients with unstable angina without critical coronary arterial narrowing (from the TIMI‐IIIA Trial). Am J Cardiol . 1994;74:531–537. [DOI] [PubMed] [Google Scholar]
- 6. Roe MT, Harrington RA, Prosper DM, et al. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease. The Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Circulation . 2000;102:1101–1106. [DOI] [PubMed] [Google Scholar]
- 7. Bugiardini R, Manfrini O, De Ferrari GM. Unanswered questions for management of acute coronary syndrome: risk stratification of patients with minimal disease or normal findings on coronary angiography. Arch Intern Med . 2006;166:1391–1395. [DOI] [PubMed] [Google Scholar]
- 8. Alfredsson J, Lindbäck J, Wallentin L, et al. Similar outcome with an invasive strategy in men and women with non–ST‐elevation acute coronary syndromes: from the Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Eur Heart J . 2011;32:3128–3136. [DOI] [PubMed] [Google Scholar]
- 9. Gehrie ER, Reynolds HR, Chen AY, et al. Characterization and outcomes of women and men with non–ST‐segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J . 2009;158:688–694. [DOI] [PubMed] [Google Scholar]
- 10. De Ferrari GM, Fox KA, White JA, et al. Outcomes among non–ST‐segment elevation acute coronary syndromes patients with no angiographically obstructive coronary artery disease: observations from 37 101 patients. Eur Heart J Acute Cardiovasc Care . 2014;3:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Planer D, Mehran R, Ohman EM, et al. Prognosis of patients with non–ST‐segment‐elevation myocardial infarction and nonobstructive coronary artery disease: propensity‐matched analysis from the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circ Cardiovasc Interv 2014;7:285–293. [DOI] [PubMed] [Google Scholar]
- 12. Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA . 2014;312:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maddox TM, Ho PM, Roe M, et al. Utilization of secondary prevention therapies in patients with nonobstructive coronary artery disease identified during cardiac catheterization: insights from the National Cardiovascular Data Registry Cath‐PCI Registry. Circ Cardiovasc Qual Outcomes . 2010;3:632–641. [DOI] [PubMed] [Google Scholar]
- 14. Reynolds HR, Srichai MB, Iqbal SN, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation . 2011;124:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ouldzein H, Elbaz M, Roncalli J, et al. Plaque rupture and morphological characteristics of the culprit lesion in acute coronary syndromes without significant angiographic lesion: analysis by intravascular ultrasound. Ann Cardiol Angeiol (Paris). 2012;61:20–26. [DOI] [PubMed] [Google Scholar]
- 16. Stensaeth KH, Fossum E, Hoffmann P, et al. Clinical characteristics and role of early cardiac magnetic resonance imaging in patients with suspected ST‐elevation myocardial infarction and normal coronary arteries. Int J Cardiovasc Imaging . 2011;27:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Senthilkumar A, Majmudar MD, Shenoy C, et al. Identifying the etiology: a systematic approach using delayed‐enhancement cardiovascular magnetic resonance. Heart Fail Clin . 2009;5:349–367, vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Assomull RG, Lyne JC, Keenan N, et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J . 2007;28:1242–1249. [DOI] [PubMed] [Google Scholar]
- 19. Christiansen JP, Edwards C, Sinclair T, et al. Detection of myocardial scar by contrast‐enhanced cardiac magnetic resonance imaging in patients with troponin‐positive chest pain and minimal angiographic coronary artery disease. Am J Cardiol . 2006;97:768–771. [DOI] [PubMed] [Google Scholar]
- 20. Reis SE, Holubkov R, Conrad Smith AJ, et al; WISE Investigators. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. [DOI] [PubMed] [Google Scholar]
- 21. Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol . 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sicari R, Rigo F, Cortigiani L, et al. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near‐normal coronary arteries. Am J Cardiol . 2009;103:626–631. [DOI] [PubMed] [Google Scholar]
- 23. Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol . 2009;54:1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako‐Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J . 2008;155:408–417. [DOI] [PubMed] [Google Scholar]
- 25. Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging . 2002;18:539–542. [PubMed] [Google Scholar]
- 26. Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction: a National Heart, Lung, and Blood Institute–sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging . 2015;8:e002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lanza GA, Buffon A, Sestito A, et al. Relation between stress‐induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol . 2008;51:466–472. [DOI] [PubMed] [Google Scholar]
- 28. Buchthal SD, den Hollander JA, Merz CN, et al. Abnormal myocardial phosphorus‐31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med . 2000;342:829–835. [DOI] [PubMed] [Google Scholar]
- 29. Doyle M, Weinberg N, Pohost GM, et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI‐sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging . 2010;3:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gerbaud E, Harcaut E, Coste P, et al. Cardiac magnetic resonance imaging for the diagnosis of patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Int J Cardiovasc Imaging . 2012;28:783–794. [DOI] [PubMed] [Google Scholar]
- 31. Leurent G, Langella B, Fougerou C, et al. Diagnostic contributions of cardiac magnetic resonance imaging in patients presenting with elevated troponin, acute chest pain syndrome and unobstructed coronary arteries. Arch Cardiovasc Dis . 2011;104:161–170. [DOI] [PubMed] [Google Scholar]
- 32. Schwitter J, Wacker CM, Wilke N, et al; MR‐IMPACT Investigators. MR‐IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial: perfusion‐cardiac magnetic resonance vs. single‐photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J . 2013;34:775–781. [DOI] [PubMed] [Google Scholar]
- 33. Jaarsma C, Leiner T, Bekkers SC, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single‐photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta‐analysis. J Am Coll Cardiol. 2012;59:1719–1728. [DOI] [PubMed] [Google Scholar]
- 34. Hamon M, Fau G, Née G, et al. Meta‐analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson. 2010;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chih S, Macdonald PS, Feneley MP, et al. Reproducibility of adenosine stress cardiovascular magnetic resonance in multi‐vessel symptomatic coronary artery disease. J Cardiovasc Magn Reson. 2010;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Jong MC, Genders TS, van Geuns RJ, et al. Diagnostic performance of stress myocardial perfusion imaging for coronary artery disease: a systematic review and meta‐analysis. Eur Radiol. 2012;22:1881–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single‐photon emission computed tomography for diagnosis of coronary heart disease (CE‐MARC): a prospective trial. Lancet. 2012;379:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenwood JP, Motwani M, Maredia N, et al. Comparison of cardiovascular magnetic resonance and single‐photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE‐MARC) Trial. Circulation. 2014;129:1129–1138. [DOI] [PubMed] [Google Scholar]
- 39. Shufelt CL, Thomson LE, Goykhman P, et al. Cardiac magnetic resonance imaging myocardial perfusion reserve index assessment in women with microvascular coronary dysfunction and reference controls. Cardiovasc Diagn Ther . 2013;3:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang L, Jerosch‐Herold M, Jacobs DR Jr, et al. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol . 2006;47:565–572. [DOI] [PubMed] [Google Scholar]
- 41. Dall'Armellina E, Karia N, Lindsay AC, et al. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ Cardiovasc Imaging . 2011;4:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mordini FE, Haddad T, Hsu LY, et al. Diagnostic accuracy of stress perfusion CMR in comparison with quantitative coronary angiography: fully quantitative, semiquantitative, and qualitative assessment. JACC Cardiovasc Imaging . 2014;7:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carlsson M, Jögi J, Bloch KM, et al. Submaximal adenosine‐induced coronary hyperaemia with 12 h caffeine abstinence: implications for clinical adenosine perfusion imaging tests. Clin Physiol Funct Imaging . 2015;35:49–56. [DOI] [PubMed] [Google Scholar]


